Abstract
In orthopaedic surgery, perioperative administration of non-steroidal anti-inflammatory drugs has been shown to reduce postoperative pain and analgesic consumption. In addition, preoperative administration of ibuprofen has proved to reduce interleukin-6 (IL-6) release, while that of ranitidine reduced postoperative IL-6-induced C-reactive protein synthesis in patients undergoing abdominal surgery. However, it has not been established whether the preoperative administration of both types of drugs may reduced the postoperative inflammatory reaction after instrumented spinal surgery. Accordingly, our objective was to investigate the effects of preoperative treatment with naproxen plus famotidine on the postoperative systemic inflammatory reaction in patients undergoing instrumented lumbar spinal surgery. Forty consecutive patients scheduled for elective instrumented spinal fusion were alternately assigned to receive either naproxen (500 mg/day, p.o.) plus famotidine (40 mg/day, p.o.) for 7 days before operation, or no adjuvant treatment. Haematological parameters, acute phase proteins, complement fractions, immunoglobulins and cytokines were determined 7 days and immediately before surgery, and on days 0, 1, 2 and 7 after surgery. Haematological parameters, clinical data, duration of surgery, blood loss, perioperative blood transfusion and postoperative complications were similar in the two groups, although pretreated patients showed lower increases in body temperature and required less analgesic medication. Compared with preoperative levels, IL-6 levels were significantly increased postoperatively in all patients with no differences between groups. C-reactive protein, α1-acid-glycoprotein and haptoglobin levels were also significantly increased postoperatively in all patients; however, they were significantly lower in pretreated patients. In conclusion, perioperative treatment with naproxen plus famotidine was well tolerated and reduced the acute phase response after instrumented spinal surgery. However, further research is needed to determine the best dose and timing of preoperative treatment administration, and to correlate these changes with long-term clinical results.
Keywords: Spinal surgery, Naproxen, Famotidine, Acute phase protein, Interleukin-6
Introduction
It is well documented that surgery may induce a variable degree of suppression of both cell-mediated and humoral host defences [7]. In response to such surgical trauma the host initiates an “acute phase response” (APR), which includes a large number of behavioural, physiological, biochemical and nutritional changes [9]. This APR is mediated by molecules, generated in the local tissue site, that are highly chemotactic for neutrophils (interleukin (IL)-8) and mononuclear cells (monocyte chemoattractant protein, MCP) or are released into the circulation (IL-1β, IL-6, tumour necrosis factor alpha) inducing a systemic inflammatory response, including fever, activation of the hypothalamus-pituitary-adrenal axis, leukocytosis, activation of the immune system and stimulation of acute phase protein (APP) synthesis [1, 3, 12]. The APPs are believed to be important in restricting tissue damage, by scavenging and neutralizing and/or removing oxygen free radicals, proteolytic enzymes, antigens and microorganisms. They also take part in promoting resolution and tissue repair and are believed to enhance both non-specific and specific components of host defence [9].
When excessive in initial magnitude or duration, however, this otherwise beneficial inflammatory process may lead to deterioration in, rather than in restoration of, homeostasis [10]. Perioperative treatment with cyclo-oxygenase inhibitors (COXI) or histamine receptor-2 antagonist (H2RA) has been shown to reduce the APR to abdominal surgery [2, 24]. In addition, H2RA avoids postoperative transfusion-induced depression of cell-mediated immunity in these patients [16]. Since, in patients undergoing elective lumbar spine surgery, preoperative circulating concentrations of proinflammatory cytokines and APPs are within the normal range and increase after the surgical trauma (anaesthesia, surgery, blood loss, blood transfusion and drugs) [25, 33], the purpose of this study was to investigate whether perioperative treatment with a COXI (naproxen) plus an H2RA (famotidine) may regulate the intensity of the APR in such patients.
Patients and methods
Patients
Forty consecutive patients scheduled for elective instrumented spinal fusion (ALLOSPINE, Sultzer, Switzerland), comprising two to four levels with or without microsurgery, using a posterior-lateral approach, and performed under general anaesthesia by one surgeon (C.S.), entered the study. Patients with clinical signs of infectious disease, history of gastroduodenal ulcer, bleeding disorder, abnormal hepatic or renal function on routine laboratory analyses, being treated with known immunomodulatory agents (e.g. corticosteroids) within 2 weeks prior to the study, or having a blood transfusion prior to the operation were excluded. The patients were alternately assigned to receive either preoperative adjuvant treatment with naproxen (500 mg/day, p.o.) plus famotidine (40 mg/day, p.o.) for 7 days before operation, or no adjuvant treatment. This treatment plus ketorolac (10 mg/8 h, p.o.) was given to all patients from postoperative day 1 to postoperative day 7. The anesthesiologist, the ward and the laboratories were masked to the preoperative treatment. None of the patients was anaemic (Hb ≥13 g/dl) and all of them donated 2 units of autologous blood in the 3 weeks prior to the operation and received oral iron (120 mg Fe/day) from the third preoperative week until 1 month after the intervention.
In addition, all patients received 1.5 g of cefuroxime intraoperatively, and low molecular weight heparin (dalteparin 5000 UI/24 h, s.c., 2–3 days) and antibiotics (cefuroxime 750 mg/6 h, p.o., 3–4 days) postoperatively to prevent thromboembolic and infectious complications. Tramadol (50–100 mg/8 h, s.c.) was given for postoperative analgesia on demand. A set of clinical data, including age, gender, number of fusions, type of surgery (primary or reintervention), length of operation, perioperative blood lost and transfused, length of hospital stay, and complications, was recorded (Table 1).
Table 1.
Characteristics of patients
| Group A | Group B | P | |
| No. of patients | 19 | 18 | |
| Age (years) | 46±4 | 49±4 | NS |
| Sex (M/F) | 5/14 | 5/13 | NS |
| No. of fusions | 3.5±0.9 | 3.4±0.8 | NS |
| Reinterventions (n, %) | 3 (16%) | 4 (20%) | NS |
| Length of operation (h) | 5.2±0.9 | 5.4±0.8 | NS |
| Blood lost (ml) | |||
| Intraoperative | 641±287 | 719±314 | NS |
| Postoperative | 464±174 | 440±195 | NS |
| Total | 1105±373 | 1159±399 | NS |
| Blood transfused (units/patient) | |||
| Intraoperative | 1.37±0.74 | 1.20±0.81 | NS |
| Postoperative | 0.57±0.74 | 0.60±0.64 | NS |
| Total | 1.95±1.09 | 1.80 ± 0.97 | NS |
| Blood reposition ratio (%) | 0.74±0.39 | 0.77±0.29 | NS |
| Hb (g/dl) | |||
| Preoperative | 14.1±0.6 | 13.6±0.4 | NS |
| Postoperative day 7 | 11.0±0.7* | 11.1±0.6* | NS |
| Hospitalization (days) | 9.9±8.3 | 10.1±5.9 | NS |
| Complications | 2 | 2 | NS |
*P<0.05 with respect to preoperative values
Informed consent to the study was obtained from each patient, and the study protocol was approved by the Hospital Human Research Review Committee.
Blood samples
Six blood samples were obtained from the patients at different perioperative stages: in the preoperative period (sample 1, day −7), at the beginning (sample 2) and the end of the operation (sample 3, day 0), and on the first, second and seventh postoperative days (samples 4, 5 and 6; days +1, +2, +7). Blood samples were obtained by means of venipuncture and collected in three tubes: one Venoject-EDTA (K2) (3 ml) and two Venoject-II-AUTOSEP (4 ml) (Terumo, Belgium). Serum samples were obtained by means of centrifugation (3000 rpm/5 min), and aliquot samples were stored at −70 °C until assayed.
Haematological parameters
Red cell count (RBC), haematocrit, haemoglobin (Hb), total and differential white cell count (WBC) and platelet count were determined using a Technicon III cell counter (Bayer, USA) in blood samples collected in EDTA. The cell counter is calibrated daily and is included in a quality control program by the Spanish Association for Haematology and Haemotherapy (AEHH), according to the International Committee for Standardization in Haematology (ICSH).
Serum proteins
Nephelometric measurements of serum levels of α1-acid glycoprotein (AGP), C-reactive protein (CRP), caeruloplasmin (CER), haptoglobin (HPT), transferrin (TRF), pre-albumin (PAL), complement C3 and C4 fractions, and IgA, IgG and IgM were performed using a nephelometer NB-II (Behring, Germany). The nephelometer is calibrated daily and is included in a quality control program by the Spanish Society of Clinical Chemistry (SEQC), according to the National Committee for Clinical Laboratory Standards (NCLLS). Results were corrected for haemodilution using the total protein concentration of the preoperative blood sample as control (Hitachi 747 Multianalyzer, Japan) [25].
Enzyme immunoassays for cytokines
The levels of serum immunoreactive interleukin-1β (IL-1β), IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, interferon γ (IFN-γ) and tumour necrosis factor alpha (TNF-α) were determined by means of an enzyme immunoassay in solid phase (ELISA) according to the manufacturer’s procedure (Bender Med Systems, Austria). The detection limits (pg/ml) were <1 for IL-1β and IL-12, 1.4 for IL-6 and IFN-γ, 2 for IL-4 and IL-10, <5 for IL-5 and TNF-α, and 15 for IL-2. To calculate cytokine concentration, a computerized standard curve was created by plotting the mean absorbance for each of the seven standard concentrations used on the ordinate against the corresponding cytokine concentration on the abscissa. Control samples of known cytokine concentration (high and low) were used in each run. Overall intra-assay and inter-assay coefficients of variation were between 3% and 10%. All samples were processed in duplicate. If the difference between duplicate determinations was >10%, the sample was re-analysed. Results were corrected for haemodilution using the total protein concentration of the preoperative blood sample as control [25].
Statistical analysis
All results are presented as the mean±SD (n) and statistical analysis was carried out using a repeated measures MANOVA test with a within-factor (up to six levels) and one between-factor (group). An unpaired Student’s t-test was used for comparison of non-repeated measures between groups. P values <0.05 were considered as statistically significant. All statistical analyses were performed with an SPSS 10.0 package (Licensed to the University of Málaga, Spain).
Results
Clinical data
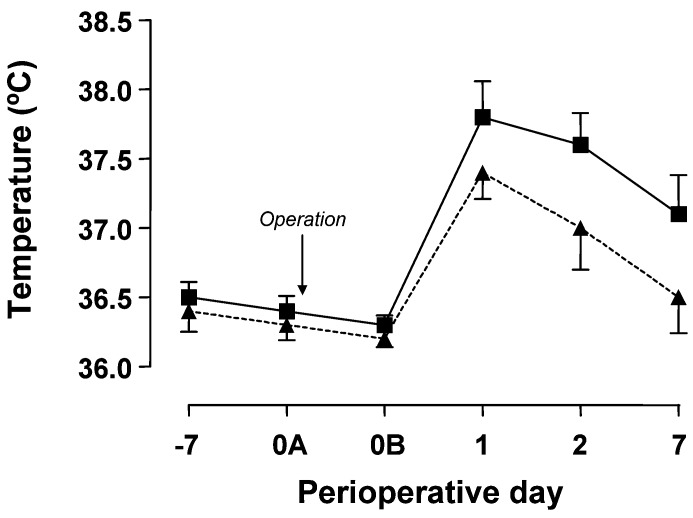
Twenty patients receiving adjuvant treatment with naproxen plus famotidine (group B) and 20 patients without adjuvant treatment (group A) undergoing elective instrumented lumbar spinal fusion, entered the study. No side effects of adjuvant treatment were observed, but one patient in group A and two patients in group B were excluded from data analysis because of protocol violation. Clinical data are given in Table 1 and there were no significant differences between groups in the recorded parameters. Patients in group B had significantly lower postoperative body temperature levels (Fig. 1) and requested less analgesic medication (tramadol) than those of group A (255±56 vs114±42 mg during the first 24 postoperative hours and 183±52 vs 72±25 mg from the 24th to the 48th postoperative hour, for groups A and B respectively; P<0.01). There were two postoperative complications in group A (one seroma and one hypovolemia) and two in group B (one seroma and one skin wound caused by the prosthetic material), but no early infectious complications.
Fig. 1.
Perioperative evolution of body temperature in patients receiving either preoperative adjuvant treatment with naproxen plus famotidine (triangles) or no adjuvant treatment (squares). Data are the mean±SD of measurements in 18 or 19 patients (group A vs group B, P<0.001) (−7, preoperative day 7; 0A, beginning of surgery; 0B, end of surgery; 1, postoperative day 1; 2, postoperative day 2; 7, postoperative day 7)
Blood haematology
In all patients, despite packed RBC transfusion ( ≈2 units per patient), perioperative blood loss induced postoperative anaemia, Hb values on the seventh postoperative day being significantly lower than those of the preoperative sample, and without differences between groups (Table 1). As expected, total WBC counts were raised at the end of surgery due to an increase in neutrophil counts, while those of lymphocytes decreased. However, most of the WBC counts returned to preoperative values on the second postoperative day, and there were no differences between groups. Platelets counts remained within the normal range in most patients (data not shown).
Serum cytokine levels
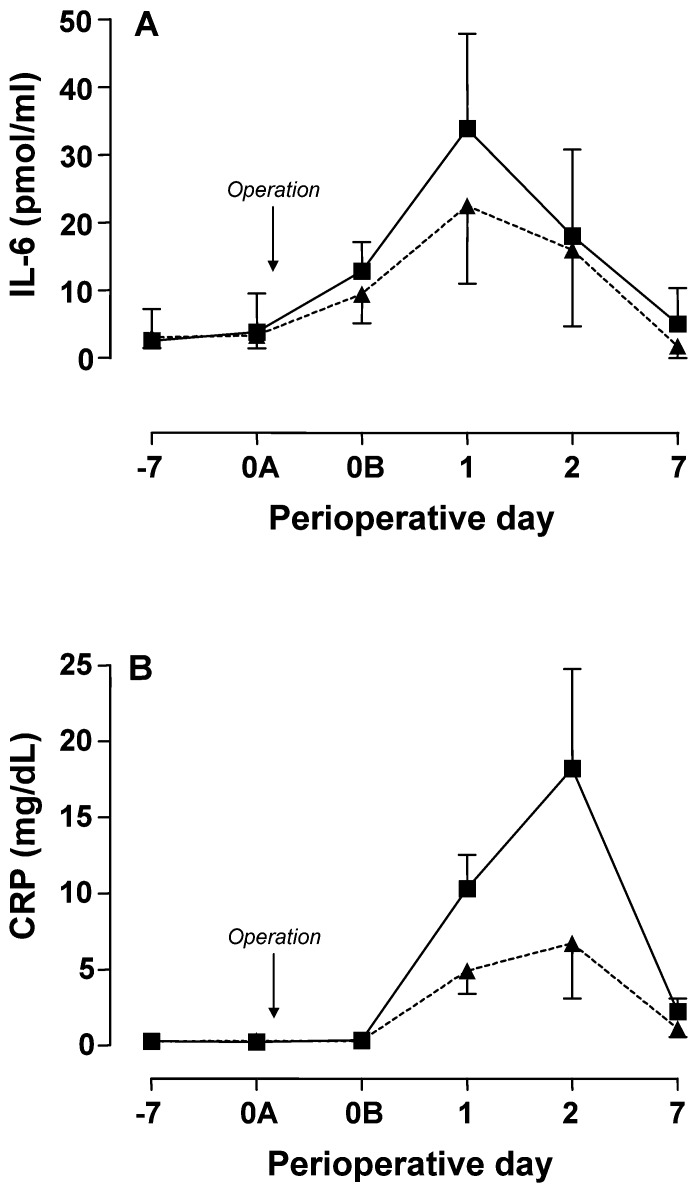
Preoperative IL-6 levels were within the normal range in all patients (<14 pg/ml). Serum IL-6 levels increased at the end of surgery and remained elevated after 24 and 48 h (P<0.01). Furthermore, IL-6 serum levels were largely normalized on the seventh postoperative day in both groups (Fig. 2A). Serum levels of the remaining cytokines did not change from baseline throughout the perioperative period (IL-1β, 4±2 pg/ml; IL-5, 15±8 pg/ml; IL-8, 132±108 pg/ml; IL-12, 41±9 pg/ml) or were below the detection limit of the assay (IL-2, IL-4, IL-10, TNF-α, IFN-γ).
Fig. 2A, B.
Perioperative evolution of serum cytokine and acute phase protein levels in patients receiving either preoperative adjuvant treatment with naproxen plus famotidine (triangles) or no adjuvant treatment (squares). Data are the mean±SD of 18 or 19 determinations and results were corrected for haemodilution using the total protein concentration of the preoperative blood sample as control. Serum concentrations of interleukin 6 (IL-6) were measured by ELISA (A group A vs group B: p=NS) and those of C-reactive protein (CRP) by nephelometry (B group A vs group B: p<0.001). (−7, preoperative day 7; 0A, beginning of surgery; 0B, end of surgery; 1, postoperative day 1; 2, postoperative day 2; 7, postoperative day 7)
Serum proteins
Preoperative serum levels of CRP were within normal range (0–0.8 mg/dl) in all patients. Compared with preoperative values, postoperative serum levels of CRP increased significantly after 24–48 h, and decreased thereafter although remaining above the normal range on the seventh postoperative day (P<0.001) (Fig. 2B). The peak values were observed at 48 h with a concentration of 18.2±6.5 mg/dl in group A and 6.7±3.6 mg/dl in group B (Fig. 2B). Serum levels of AGP showed a continuous increase from postoperative day 1 until postoperative day 7, with significantly higher levels being observed in group A (P<0.01) (Table 2). Those of HPT showed a slight decrease at the end of surgery, which was followed by a postoperative time-course similar to that of AGP, whereas those of CER did not change significantly throughout the perioperative period (Table 2).
Table 2.
Perioperative changes in positive and negative acute phase proteins in patients undergoing elective lumbar spinal fusion. All serum APPs levels (mg/dl) were corrected for haemodilution using the total protein concentration of the preoperative blood sample as control. Data are the mean±SD of 18 or 19 determinations. Reference values (mg/dl): α1-acid glycoprotein (AGP), 50–140; haptoglobin (HPT), 36–195; caeruloplasmin (CER), 22–58; pre-albumin (PAL), 18–38; transferrin (TRF), 220–400 (1, preoperative; 2, beginning of surgery; 3, end of surgery; 4, postoperative day 1; 5, postoperative day 2; 6, postoperative day 7)
| Sample | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Positive acute phase proteins | ||||||
| AGP* | ||||||
| Group A | 97±25 | 96±23 | 77±17 | 110±21 | 177±27 | 190±13 |
| Group B | 82±15 | 82±15 | 68±16 | 95±14 | 127±22 | 136±27 |
| HPT* | ||||||
| Group A | 164±72 | 167±62 | 141±55 | 178±62 | 356±70 | 401±68 |
| Group B | 130±42 | 138±48 | 107±46 | 114±40 | 261±73 | 246±83 |
| CER | ||||||
| Group A | 23±5 | 28±4 | 28±4 | 33±4 | 37±4 | 29±5 |
| Group B | 26±7 | 28±9 | 24±8 | 29±6 | 29±6 | 32±8 |
| Negative acute phase proteins | ||||||
| PAL | ||||||
| Group A | 39±10 | 41±8 | 39±9 | 40±13 | 32±7 | 35±3 |
| Group B | 34±5 | 40±7 | 40±6 | 37±5 | 34±9 | 37±4 |
| TRF# | ||||||
| Group A | 309±54 | 320±62 | 342±72 | 345±114 | 314±82 | 268±79 |
| Group B | 337±64 | 354±94 | 347±67 | 350±66 | 351±68 | 358±71 |
Group A versus group B: *p<0.001, # p=0.117
On postoperative day 7, there was a trend to higher TRF levels in group B, although the differences did not reach statistical significance (p=0.117) (Table 2). Finally, once corrected for haemodilution, serum levels of PAL (Table 2), IgA, IgG, IgM, C3 and C4 did not change significantly throughout the perioperative period, and there were no differences between groups (data not shown).
Discussion
In a recent paper, Hall et al. [11] found that whereas the inflammatory response to major orthopaedic surgery (hip arthroplasty), measured by the circulating IL-6 and CRP concentrations, was related to functional recovery, the classical neuroendocrine changes were relatively unimportant. Thus, according to these authors, attention should be focused on the inflammatory changes that have been implicated in functional recovery, but immunosuppressive effects induced by the surgical trauma should be not forgotten as they can also complicate the patient’s postoperative course.
The factors responsible for postoperative inflammatory reaction are known to be proinflammatory cytokines such as TNF-α, IL-1β, IL-6 and IL-8, which are produced and released by a variety of cell types, especially macrophages and monocytes, in response to tissue damage [9]. In orthopaedic surgery, patients with prostheses experience an enhanced inflammatory response as a result of these cytokines being produced by inflamed cells in contact with either the implant surface or metal ions released from the implants [17].
Non-steroidal anti-inflammatory drugs (NSAIDs) can decrease the inflammatory response to surgery and are widely used in the postoperative period. However, when used preoperatively, the results are conflicting, most probably due to the use of different surgical models and different dose regimens of the NSAIDs. In this regard, in a series of 40 patients scheduled for major orthopaedic surgery, the administration of diclofenac (intravenously 30 min before surgery followed by continuous intravenous infusion for 24 h) has been shown to reduce postoperative pain and analgesic consumption, although it did not induce changes in the postoperative CPR, AGP, HPT and CER levels during this period [4]. Similar results regarding amelioration of postoperative pain have been obtained with preoperative administration of naproxen in knee and spinal surgery [5, 6].
In abdominal surgery, perioperative treatment with ibuprofen in patients undergoing elective cholecystectomy was able to reduce the postoperative endocrine response and IL-6 release, without reducing CRP concentrations [3]. In contrast, in a series of patients undergoing elective abdominal hysterectomy perioperative treatment with ranitidine significantly reduced postoperative CRP levels but not those of IL-6 [24].
Effect of the treatment on inflammatory mediators
In the present study we measured several markers of inflammation and recorded clinical parameters to assess the efficacy of perioperative treatment with naproxen plus famotidine (N+F) in reducing the postoperative APR in patients undergoing elective instrumented lumbar spinal fusion. The results indicate that adjuvant treatment reduces the inflammatory reaction to surgery and spinal fixation devices, i.e. the levels of some APPs (CRP, AGP, HPT) showed significantly lower peaks in patients with pretreatment than in those without, whereas IL-6 levels were not significantly reduced (Fig. 2, Table 2). Moreover, the difference in CRP levels between the two groups was similar to that observed between patients undergoing instrumented and non-instrumented spinal surgery [29], supporting the role of CRP as an indicator of the intensity of the inflammatory reaction to surgery and of the efficiency of the preoperative treatment with N+F [8].
However, although in different types of orthopaedic surgery CRP levels remained consistently elevated (10–20 mg/dl) between 48 and 72 postoperative hours [11, 29, 31], postoperative IL-6 time-courses were variable, even for a particular surgery, both in the concentrations attained and in the time to reach peak values (4–24 h) [11, 13, 33]. Hence, since IL-6 was not determined at 6–8 postoperative hours, a possible significant difference between groups in IL-6 levels at that time can not be excluded.
On the other hand, in patients undergoing scoliosis surgery, surgical trauma is also known to produce a transient impairment of immunocompetence affecting both the cellular and humoral arms of the immune system [28]. This immunological depression is induced by several mediators, including histamine acting primarily on H2 receptors [19, 27]. In addition, patients undergoing elective spinal surgery are often transfused and this may further increase postoperative histamine concentration since histamine accumulates in stored blood in a time-dependent manner [15]. In this regard, patients receiving N+F showed significantly lower postoperative concentrations of AGP and HPT, which may lead to a lesser degree of postoperative immunosuppression [18, 26, 34].
Overall, these data seem to indicate that perioperative treatment with N+F induces a change from inflammatory acute phase protein synthesis to that of constitutive proteins, as suggested by reduced serum levels of CRP, AGP and HPT, whereas those of TRF and PAL remain unmodified. These changes may be mediated by a direct action of famotidine on hepatic H2 receptors [14] which could regulate, at least in part, IL-6-induced APP synthesis, and by a lower hepatic IL-1 receptor expression induced by naproxen, as has been seen in synovial cells and chondrocytes [22, 23]. In addition, results from experimental research suggest that granular products of mast cells, especially histamine, are involved in both acute and chronic inflammation affecting implanted biomaterial and that simultaneous administration of histamine H1 and H2 receptor antagonists (pyralamine and famotidine, respectively), greatly diminishes histamine effects [30].
Finally, an effect of tramadol can not be completely ruled out. Tramadol has lesser immunosuppressive effects than morphine, but still has a potent analgesic effect which is very useful in the treatment of moderate to severe pain, especially in patients with compromised immunity [32]. If tramadol exerts some immunosuppressive/anti-inflammatory activity, then the difference in the postoperative inflammatory response between the two groups should be even larger than that observed, as the control group received significantly more tramadol than the pretreated group.
Effects of the treatment on clinical parameters
From a clinical point of view, patients in group B experienced less postoperative pain, as suggested by a lower consumption of analgesics and a smaller increase in postoperative body temperature compared with the control group, and no adverse effects of the adjuvant treatment were observed. One of these potential adverse effects was higher perioperative blood loss due to inhibition of platelet aggregation. However, as shown in Table 1, no significant differences were observed between groups regarding perioperative blood loss or transfusion requirements, most probably due to the low dose of naproxen given to patients [20, 21]. Similarly, no alteration of renal or hepatic function was detected in routine perioperative laboratory analyses (data not shown).
Conclusion
The perioperative treatment with N+F was well tolerated, did not produce any significant side effects, and was shown to reduce the APR after instrumented spinal surgery, at both laboratory and clinical levels. However, the power of this prospective study is limited by its observational character and by the small number of patients included. Further research, preferably as a large randomized controlled trial, is needed to confirm these findings, to determine the best dose and timing of preoperative treatment administration, and to ascertain whether these short-term changes correlate with long-term clinical outcomes.
Acknowledgements
The author gratefully acknowledge the manuscript reviewing by Dr. J.H. Palmer (Department of Anaesthesiology, Clínica Santa Elena, Torremolinos, Spain) and Prof. F. Rius (Department of Statistics, School of Medicine, University of Málaga, Spain).
Footnotes
Supported by grants from Junta de Andalucía, Spain (I+D Group CTS 0189) and Laboratorios Smaller from ASAC Pharmaceutical International (OTRI 806/041123)
References
- 1.Baumann Immunol Today. 1994;15:74. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 2.Chambrier Ann Surg. 1996;224:178. doi: 10.1097/00000658-199608000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrousos N Engl J Med. 1995;332:1351. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 4.Claeys Acta Anaesthesiol Scand. 1992;36:270. doi: 10.1111/j.1399-6576.1992.tb03463.x. [DOI] [PubMed] [Google Scholar]
- 5.CodeCan J Anaesth 199441988131242 [Google Scholar]
- 6.Coli Minerva Anesthesiol. 1993;59:531. [PubMed] [Google Scholar]
- 7.Decker Surgery. 1996;119:316. doi: 10.1016/s0039-6060(96)80118-8. [DOI] [PubMed] [Google Scholar]
- 8.Foglar Orthopedics. 1998;21:687. doi: 10.3928/0147-7447-19980601-11. [DOI] [PubMed] [Google Scholar]
- 9.Gabay N Engl J Med. 1999;340:448. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 10.Guirao World J Surg. 1996;20:437. doi: 10.1007/s002689900069. [DOI] [PubMed] [Google Scholar]
- 11.Hall Br J Anaesth. 2001;87:537. doi: 10.1093/bja/87.4.537. [DOI] [PubMed] [Google Scholar]
- 12.Hill World J Surg. 2000;24:624. doi: 10.1007/s002689910103. [DOI] [PubMed] [Google Scholar]
- 13.Hogevold Cytokine. 2000;12:1156. doi: 10.1006/cyto.2000.0675. [DOI] [PubMed] [Google Scholar]
- 14.Meretey Agents Actions. 1991;33:189. doi: 10.1007/BF01993163. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen Br J Surg. 1996;83:259. doi: 10.1046/j.1365-2168.1996.02119.x. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen Surgery. 1989;105:711. [PubMed] [Google Scholar]
- 17.Nivbrant J Bone Joint Surg Br. 1999;81:163. doi: 10.1302/0301-620x.81b1.8664. [DOI] [PubMed] [Google Scholar]
- 18.Oh J Leukoc Biol. 1990;47:142. doi: 10.1002/jlb.47.2.142. [DOI] [PubMed] [Google Scholar]
- 19.Parsons Scand J Gastroenterol. 1991;26:46. doi: 10.3109/00365529109093177. [DOI] [PubMed] [Google Scholar]
- 20.Patrono J Clin Invest. 1986;77:590. doi: 10.1172/JCI112341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patrono Chest. 2001;199:39S. doi: 10.1378/chest.119.1_suppl.39S. [DOI] [PubMed] [Google Scholar]
- 22.Pelletier Arthritis Rheum. 1993;36:1517. doi: 10.1002/art.1780361106. [DOI] [PubMed] [Google Scholar]
- 23.Pronost Agents Actions Suppl. 1993;39:213. doi: 10.1007/978-3-0348-7442-7_24. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen J Am Coll Surg. 1995;181:138. [PubMed] [Google Scholar]
- 25.Sebastián Eur Spine J. 2000;9:458. doi: 10.1007/s005860000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiyan Glycoconj J. 1997;14:631. doi: 10.1023/A:1018544711767. [DOI] [PubMed] [Google Scholar]
- 27.Sitter Agents Actions. 1991;33:203. doi: 10.1007/BF01993168. [DOI] [PubMed] [Google Scholar]
- 28.SuzukiEur Spine J 19976399093826 [Google Scholar]
- 29.Takahashi Spine. 2001;26:1698. doi: 10.1097/00007632-200108010-00014. [DOI] [PubMed] [Google Scholar]
- 30.Tang Proc Natl Acad Sci USA. 1998;95:8841. doi: 10.1073/pnas.95.15.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thelander Spine. 1992;17:400. doi: 10.1097/00007632-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Tsai Pain. 2001;92:63. doi: 10.1016/S0304-3959(00)00472-3. [DOI] [PubMed] [Google Scholar]
- 33.Van Cytokine. 1998;10:897. doi: 10.1006/cyto.1998.0367. [DOI] [PubMed] [Google Scholar]
- 34.Wang Chin Med Sci J. 1996;11:180. [PubMed] [Google Scholar]