Abstract
Introduction
Chronic obstructive pulmonary disease (COPD) patients present a high prevalence of cardiovascular disease. This excess of comorbidity could be related to a common pathogenic mechanism, but it could also be explained by the existence of common risk factors. The objective of this study was to determine whether COPD patients present greater cardiovascular comorbidity than control subjects and whether COPD can be considered a risk factor per se.
Methods
1200 COPD patients and 300 control subjects were recruited for this multicenter, cross-sectional, case–control study.
Results
Compared with the control group, the COPD group showed a significantly higher prevalence of ischemic heart disease (12.5% versus 4.7%; P < 0.0001), cerebrovascular disease (10% versus 2%; P < 0.0001), and peripheral vascular disease (16.4% versus 4.1%; P < 0.001). In the univariate risk analysis, COPD, hypertension, diabetes, obesity, and dyslipidemia were risk factors for ischemic heart disease. In the multivariate analysis adjusted for the remaining factors, COPD was still an independent risk factor (odds ratio: 2.23; 95% confidence interval: 1.18–4.24; P = 0.014).
Conclusion
COPD patients show a high prevalence of cardiovascular disease, higher than expected given their age and the coexistence of classic cardiovascular risk factors.
Keywords: COPD, cardiovascular risk, ischemic heart disease
Introduction
It is a well-known fact and has been seen in population studies that reduced forced expiratory volume in 1 second levels are associated with an increased risk of all-cause death.1–3 In addition, epidemiological studies of cases and controls based on patient registries have demonstrated that chronic obstructive pulmonary disease (COPD) patients present an excess of other chronic comorbidities, mainly cardiovascular.4–7 Finally, it is known that while in patients with severe and very severe disease the cause of death is determined by the respiratory disease, in patients with mild and moderate disease death due to cancer and cardiovascular disease is more frequent.8–10
In recent years, a hypothesis has been generated that a systemic inflammatory process, present in COPD patients, could be the link between this disease and different comorbidities.11,12 Inflammatory cytokines, including tumor necrosis factor-α, interleukin-6, C-reactive protein (CRP), and fibrinogen, are increased within the circulation of patients with COPD, particularly during exacerbations, probably representing an overspill of inflammatory mediators from the peripheral lung. These cytokines are common to many inflammatory diseases, and could explain their association with COPD.13,14 Other factors, however, can also explain this association. Tobacco is a common risk factor implicated in the genesis of COPD as well as cardiovascular disease. In addition, the reduced physical activity of these patients can have a bearing on increased cardiovascular disease.15 Lastly, the increase in vascular disease can be due to the higher prevalence of classic risk factors. Thus, in the recently published Cardiovascular Risk Factors in COPD study (abbreviated to ARCE in Spanish), it was observed that COPD patients presented high prevalence of hypertension, diabetes, and dyslipidemia, which were related with an increased risk for ischemic heart disease.16
However, not all the studies available confirm the association between cardiovascular disease and COPD. In a recent case–control study, COPD risk was not greater in patients with cardiovascular disease than in those that did not present it.17 The different results found depended on the different designs and populations and the diversity of possible mechanisms of association, together with the fact that the majority of the data available are from case–control studies based on quality registries that analyze the data retrospectively. Therefore, there is a need to continue research in this field.
The objective of this study was to determine whether, compared with a control group, COPD patients present increased risk for cardiovascular disease and if this fact can be justified by the presence of classic risk factors or whether, contrarily, obstruction of the airflow could be an independent risk factor.
Method
Study population and design
This cross-sectional, multicenter, case–control study, based on clinical practice situations, was done in primary and specialized care consultations. A total of 60 doctors, 20 pneumologists, and 20 general practitioners participated in the study.
The inclusion criteria for the group of cases were: age > 40 years, smoking history of more than 10 pack-years, prior diagnosis of COPD defined by the presence of a post-bronchodilator forced expiratory volume in 1 second/forced vital capacity ratio < 0.7, clinical stability for at least 8 weeks prior to inclusion, and giving written informed consent to participate in the study. Patients with chronic respiratory diseases other than COPD, including patients with sleep apnea–hypopnea syndrome, were excluded.
The inclusion criteria for the control group were: age > 40 years, smoking history of more than 10 pack-years, stable clinical condition, and giving written informed consent to participate in the study. In this group, in addition to the exclusion criteria applied to the cases, subjects previously diagnosed with COPD or with obstructive spirometry at the time of the evaluation were excluded.
COPD patients were recruited when they attended the clinic for a routine checkup, while controls were mainly recruited in tobacco consults. In order to avoid selection bias in both the cases as well as in the controls, they were selected consecutively by the physicians participating in the study during the established 1-month inclusion period until reaching the predetermined number of subjects for each doctor, which was 20 cases and five controls. The patients were recruited consecutively as long as they met all the inclusion criteria and none of the exclusion criteria.
By protocol, all the patients had had a previous follow-up of more than 1 year; therefore, all the data related with the study, except the dyspnea questionnaire, were found in the medical files of the participating centers. Nevertheless, these data were confirmed at the moment when the patient was recruited and a clinical questionnaire was fulfilled. Confirmation of the COPD diagnosis was required with the use of lung function testing, which allowed for the patients to be classified into stages. No explicit criteria were established for the diagnosis of risk factors and/or comorbidity other than those used by each of the participating physicians.
Procedure
At the moment when all the subjects were included in the study, a detailed medical history was compiled for both the control group as well as the case group with a specifically designed questionnaire. The data collected included: age, sex, tobacco habit, weight, height, associated risk factors (obesity, arterial hypertension, diabetes, dyslipidemia), presence of chronic associated comorbidity (anemia, malnutrition, osteoporosis, cancer, and cardiovascular, cerebrovascular, and peripheral disease), dyspnea evaluated according to the modified Medical Research Council scale, and pharmacological treatments related with COPD and the cardiovascular sphere that the patients were taking when they were seen at the office visit. The existence of comorbidities was established in agreement with the previous clinical history and records, but no additional explorations were carried out. Likewise, the number of exacerbations and consumption of health care resources as evaluated by unscheduled visits to the doctor, visits to the emergency department, and hospitalizations over the course of the previous year were noted. In addition, all the subjects filled out quality-of-life and physical activity questionnaires. Lastly, all the subjects underwent spirometry with a bronchodilator test in order to ensure that they met the established diagnostic criteria.
Ethical aspects
The study was approved by the Clinical Research Ethics Committee of Hospital General Universitario Gregorio Maranon (Madrid, Spain). All of the patients were informed of the characteristics and objectives of the study and gave their written consent for participation.
Statistical analysis
The calculation of the sample size was done using a case/control ratio of 4:1. Taking the prevalence of ischemic heart disease in the general population to be 7%,18 and in order to detect a difference between groups of 6% in absolute terms, 300 subjects without COPD and 1200 patients with COPD were needed, accounting for an α risk of 5% and a β risk of 10% in a bilateral comparison.
The results of the continuous variables are presented by mean and standard deviation. The numerical variables with nonnormal distribution are expressed by their median and interquartile range (percentile 25; percentile 75). In the categorical variables, the results are expressed by frequency and percentage. The Kolmogorov–Smirnov test was used to study the normality of the numerical variables.
In order to analyze the differences between the groups in the quantitative variables, Student’s t-test was used in those with normal distribution and the Mann–Whitney U test if the distribution was not normal. For the comparison of the means of three or more groups, the analysis of variance test was used with the Bonferroni correction. The association between qualitative variables was studied by means of Pearson’s Chi-squared test or Fisher’s exact test, with the Bonferroni correction when needed.
The estimation of ischemic heart disease risk, acute cerebrovascular accident, and peripheral vascular disease in COPD patients was done with univariate and multivariate logistic regression analyses adjusted for possible confounding variables (eg, age, sex, smoking). The statistical analysis was done with SPSS® version 18.0 software (IBM Corporation, Armonk, NY). Results with P < 0.05 were considered statistically significant.
Results
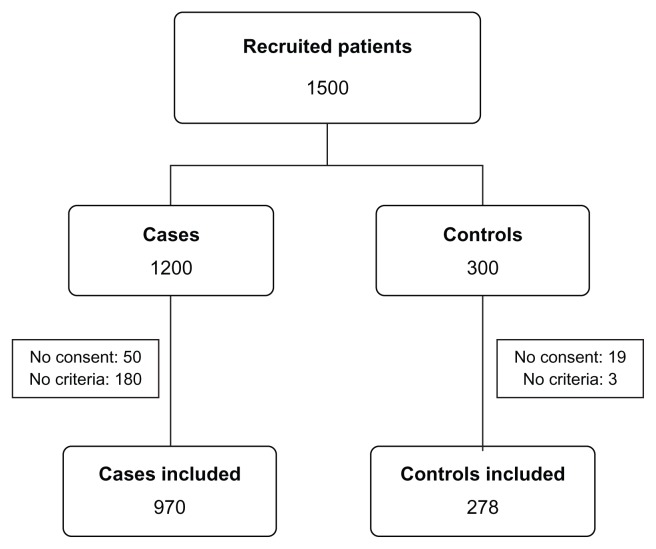
Of the 1500 subjects (1200 cases and 300 controls) recruited, 1248 (970 cases and 278 controls) were valid for study in the end. Figure 1 summarizes the patient flow.
Figure 1.
Patient flowchart of the study.
The demographic and functional characteristics of the patients are shown in Table 1. Apart from the differences in the lung function tests, age and intensity of tobacco habit were significantly greater in the COPD subjects than in the controls. In contrast, there were no differences between groups in the distribution by sex. Most patients were in moderate or severe stage.
Table 1.
Clinical characteristics of the population
| Characteristics | Cases | Controls | P |
|---|---|---|---|
| Sex | |||
| Males | 681 (70.4%) | 205 (73.7%) | 0.282 |
| Females | 286 (29.6%) | 73 (26.3%) | |
| Age (years) | 64.1 (8.8) | 59 (8.5) | <0.001 |
| BMI | 28 (6) | 28.7 (4.3) | 0.041 |
| Pack-years | 65.8 (34.8) | 34.5 (17.9) | <0.001 |
| FEV1 (%) | 47 (13.9) | 97.1 (16.1) | <0.001 |
| FVC (%) | 66.1 (16.5) | 97.5 (16.6) | <0.001 |
| FEV1/FVC (%) | 57.9 (8.5) | 78.9 (5.8) | <0.001 |
| GOLD stage | |||
| I | 13 (1.3%) | ||
| II | 370 (38%) | ||
| III | 325 (33.5%) | ||
| IV | 260 (27%) | ||
Notes: Sex and Global Initiative for Obstructive Lung Disease stage expressed in absolute numbers and percentage of the sample; the remaining values are expressed by mean and standard deviation; t-test was used for qualitative variables; Pearson’s Chi-squared test was used for qualitative variables.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Obstructive Lung Disease.
Prevalence of comorbidity in COPD
Table 2 shows the prevalence of comorbidity found in both groups. Except for lung cancer, the prevalence of all the conditions contemplated was significantly greater in the subjects of the COPD group than in the controls.
Table 2.
Prevalence of comorbidities
| Comorbidities | Cases | Controls | P |
|---|---|---|---|
| AHT | 499 (51.8%) | 100 (36%) | <0.001 |
| Dyslipidemia | 462 (48.3%) | 88 (31.7%) | <0.001 |
| Diabetes | 377 (39.5%) | 27 (9.7%) | <0.001 |
| Obesity | 337 (35.2%) | 94 (33.8%) | 0.666 |
| Anemia | 132 (13.6%) | 4 (1.4%) | <0.001 |
| Lung cancer | 1 (0.1%) | 1 (0.4%) | 0.346 |
| Malnutrition | 33 (3.4%) | 0 (0%) | 0.002 |
| Osteoporosis | 159 (16.6%) | 10 (3.6%) | <0.001 |
| Ischemic heart disease | 121 (12.5%) | 13 (4.7%) | 0.000 |
| Arrhythmia | 153 (16.1%) | 13 (4.7%) | <0.001 |
| Heart failure | 238 (24.7%) | 4 (1.4%) | <0.001 |
| ACVA | 96 (10%) | 8 (2.9%) | 0.000 |
| Peripheral vascular disease | 157 (16.4%) | 11 (4.1%) | <0.001 |
Notes: Data expressed in absolute numbers and percentages; Pearson Chi-squared test was used.
Abbreviations: ACVA, acute cerebrovascular accident; AHT, arterial hypertension.
When the patients were stratified by disease severity groups following the Global Initiative on Obstructive Lung Disease criteria, it was found that the prevalence of arterial hypertension diminished progressively as the degree of disease severity increased (P = 0.002). No significant differences were found between groups for dyslipidemia, diabetes, or obesity (defined as a body mass index ≥ 30). The prevalence of ischemic heart disease in stage IV was significantly greater than in stage III (P = 0.028), but there were no differences with other groups. Nor were there significant differences found between groups for cerebrovascular or peripheral vascular disease (Table 3).
Table 3.
Prevalence of comorbidity in patients with chronic obstructive pulmonary disease stratified by degrees of severity
| Comorbidities | I: FEV1 ≥ 80% | II: FEV1 = 50–80 | III: FEV1 = 30–50 | IV: FEV1 < 30 |
|---|---|---|---|---|
| AHT | 9 (69.2%) | 213 (58%) | 164 (50.5%) | 113 (43.6%)† |
| Dyslipidemia | 2 (15.4%) | 177 (48.8%) | 150 (46.9%) | 133 (51.2%) |
| Diabetes | 6 (46.2%) | 138 (38.2%) | 122 (38%) | 111 (42.9%) |
| Obesity | 2 (15.4%) | 135 (36.9%) | 110 (34.2%) | 90 (35.2%) |
| Ischemic heart disease | 1 (7.7%) | 51 (13.8%) | 27 (8.3%) | 42 (16%)‡ |
| Arrhythmia | 0 (0%) | 58 (16.1%) | 61 (19.1%) | 34 (13.2%) |
| Heart failure | 1 (7.7%) | 81 (22%) | 82 (25.5%) | 74 (28.6%) |
| Stroke | 1 (7.7%) | 39 (10.5%) | 33 (10.2%) | 23 (8.9%) |
| Peripheral vascular disease | 1 (7.7%) | 65 (17.8%) | 54 (16.9%) | 37 (14.3%) |
Notes: Results expressed in absolute numbers and percentage; Pearson’s Chi-squared test with Bonferroni correction was used;
sex- and age-adjusted difference statistically significant (P = 0.002) with group II;
sex- and age-adjusted difference statistically significant (P < 0.028) with group III.
Abbreviation: AHT, arterial hypertension.
Age, cardiovascular risk factors, and vascular disease
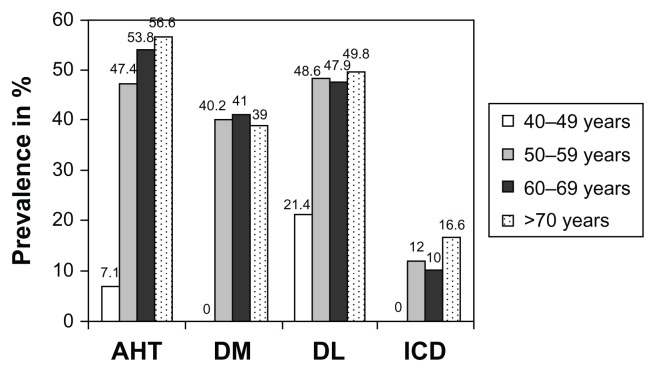
By stratifying the COPD patients by age groups, it was found that the prevalence of arterial hypertension, diabetes, and dyslipidemia significantly increased with age (Figure 2). Contrarily, when they were stratified by intensity of tobacco exposure, the differences in prevalence of cardiovascular risk factors between groups did not reach statistical significance (Table 4).
Figure 2.
Prevalence of arterial hypertension, diabetes, dyslipidemia, and ischemic heart disease stratified by age groups.
Abbreviations: AHT, arterial hypertension; DM, diabetes mellitus; DL, dyslipidemia; ICD, ischemic cardiac disease.
Table 4.
Prevalence of comorbidity associated with cardiovascular risk stratified by age and intensity of smoking in chronic obstructive pulmonary disease patients
| Risk factors | AHT† | Diabetes‡ | Obesity | Dyslipidemia |
|---|---|---|---|---|
| Age (years) | ||||
| 40–49 | 1 (7.1%) | 0 (0%) | 1 (7.1%) | 3 (21.4%) |
| 50–59 | 156 (47.4%) | 131 (40.2%) | 117 (35.3%) | 159 (48.6%) |
| 60–69 | 178 (53.8%) | 134 (41%) | 117 (35.3%) | 157 (47.9%) |
| ≥70 | 164 (56.6%) | 112 (39%) | 102 (35.8%) | 143 (49.8%) |
| Smoking | ||||
| 10–19 | 62 (41.3%) | 44 (29.5%) | 49 (32.9%) | 59 (39.6%) |
| 20–39 | 154 (47.7%) | 100 (31.3%) | 114 (35.2%) | 138 (43%) |
| 40–60 | 111 (47.4%) | 70 (30%) | 87 (37.3%) | 98 (42.1%) |
| >60 | 272 (50.8%) | 190 (35.8%) | 181 (34.2%) | 255 (48%) |
Notes: Results expressed in absolute values and percentage; Pearson’s Chi-squared test was used;
statistically significant difference between groups (P = 0.001; 40–49-year-old group was less than the rest);
statistically significant difference between groups (P < 0.023; 40–49-year-old group was less than the rest).
Abbreviation: AHT, arterial hypertension.
The prevalence of ischemic heart disease was higher in the presence of hypertension (P = 0.028), obesity (P = 0.032), and dyslipidemia (P = 0.000), as well as in the age groups > 50 years (P = 0.036) (Table 5; Figure 2). Meanwhile, no differences were observed depending on the presence or absence of diabetes and the intensity of tobacco exposure. The prevalence of peripheral vascular disease was also greater in the presence of hypertension. The Chi-squared analysis also showed differences between age groups, but in this case the Bonferroni correction demonstrated that the significance was established in the 50–59-year-old group for greater prevalence, and in the >70-year-old group for less prevalence (Table 5).
Table 5.
Prevalence of ischemic heart disease, stroke, and peripheral vascular disease in patients with chronic obstructive pulmonary disease according to risk factors
| Risk factors | IHD | Stroke | PVD |
|---|---|---|---|
| AHT | |||
| Yes | 74 (14.8%)* | 56 (11.3%) | 66 (13.5%)* |
| No | 47 (10.1%) | 39 (8.5%) | 90 (19.6%) |
| Diabetes | |||
| Yes | 53 (14.1%) | 41 (11.1%) | 65 (17.4%) |
| No | 66 (11.5%) | 54 (9.4%) | 89 (15.7%) |
| Obesity | |||
| Yes | 52 (15.4%)* | 29 (8.6%) | 58 (17.3%) |
| No | 66 (10.7%) | 65 (10.6%) | 96 (15.8%) |
| Dyslipidemia | |||
| Yes | 82 (17.7%)# | 52 (11.3%) | 74 (16.3%) |
| No | 38 (7.7%) | 44 (9%) | 82 (16.8%) |
| Age (years) | |||
| 40–49 | 0 (0%) | 1 (6.7%) | 1 (6.7%) |
| 50–59 | 40 (12%) | 36 (11%) | 68 (20.7%)‡ |
| 60–69 | 33 (9.9%) | 38 (11.4%) | 56 (17.2%) |
| ≥70 | 48 (16.6%)† | 21 (7.3%) | 32 (11.1%) |
| Smoking | |||
| 10–19 | 10 (10.3%) | 11 (11.6%) | 17 (17.9%) |
| 20–39 | 29 (14.5%) | 17 (8.6%) | 33 (16.9%) |
| 40–60 | 23 (14.3%) | 15 (9.4%) | 24 15.1%) |
| >60 | 59 (11.5%) | 53 (10.4%) | 83 (16.4%) |
Notes: Results expressed in absolute values and percentage; Pearson’s Chi-squared test with Bonferroni correction was used;
P < 0.05;
P < 0.001;
P < 0.05 for differences after Bonferroni adjustment between ≥70-year-old group and 60–69-year-old group;
P < 0.05 for differences after Bonferroni adjustment between 50–59-year-old group and ≥70-year-old group.
Abbreviations: AHT, arterial hypertension; IHD, ischemic heart disease; PVD, peripheral vascular disease.
COPD, classic cardiovascular risk factors, and risk for vascular disease
The univariate risk analysis showed that the COPD subjects presented a greater risk for having hypertension (odds ratio [OR]: 1.91; 95% confidence interval [CI]: 1.45–2.52), diabetes (OR: 6.07; 95% CI: 4–9.22), and dyslipidemia (OR: 2.02; 95% CI: 1.52–2.68). A univariate logistic regression analysis demonstrated that COPD, age, obesity, hypertension, diabetes, and dyslipidemia were ischemic heart disease risk factors. In the multivariate analysis adjusted for age, smoking, and classic cardiovascular risk factors, COPD was still a risk factor for ischemic heart disease, and the male sex factor appeared as a protector (Table 6).
Table 6.
Risk factors for ischemic heart disease: results of the logistic univariate and multivariate regression analysis
| Risk factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| COPD | 2.91 | 1.61–5.24 | <0.001 | 2.23 | 1.18; 4.24 | 0.014 |
| Age (years) | ||||||
| 40–49 | 1.00 | – | – | 1.00 | – | – |
| 50–59 | 4.56 | 0.61–33.88 | 0.138 | 2.04 | 0.26–15.79 | 0.494 |
| 60–69 | 4.64 | 0.62–34.6 | 0.135 | 1.94 | 0.25–15.12 | 0.528 |
| ≥70 | 9.00 | 1.21–66.67 | 0.032 | 3.94 | 0.5–30.79 | 0.191 |
| Sex (male) | 0.81 | 0.55–1.19 | 0.283 | 0.60 | 0.39–0.93 | 0.023 |
| Smoking (pack-years) | ||||||
| 10–19 | 1.00 | – | – | 1.00 | – | – |
| 20–39 | 1.54 | 0.76–3.11 | 0.234 | 1.50 | 0.72–3.13 | 0.275 |
| 40–59 | 1.66 | 0.8–3.46 | 0.175 | 1.64 | 0.77–3.5 | 0.203 |
| ≥60 | 1.63 | 0.84–3.18 | 0.152 | 1.15 | 0.57–2.32 | 0.700 |
| AHT | 1.74 | 1.21–2.51 | 0.003 | 1.37 | 0.93–2.02 | 0.110 |
| Diabetes | 1.53 | 1.06–2.22 | 0.023 | 1.01 | 0.68–1.51 | 0.951 |
| Dyslipidemia | 2.81 | 1.92–4.11 | <0.001 | 2.43 | 1.63–3.61 | <0.001 |
| Obesity | 1.61 | 1.12–2.32 | 0.011 | 1.42 | 0.97–2.09 | 0.069 |
Abbreviations: AHT, arterial hypertension; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
COPD was also an independent risk factor for peripheral vascular disease (OR: 4.62; 95% CI: 2.37–9.03; P < 0.001) and cerebral vascular disease (OR: 3.22; 95% CI: 1.47–7.04; P = 0.003).
Discussion
Several population studies have related reduced forced expiratory volume in 1 second levels with an increase in cardiovascular disease and all-cause mortality.1,2,19 Centered on COPD subjects, cohort studies supported by data obtained from surveys and registries have shown a greater prevalence of ischemic heart disease and death related with cardiovascular disease. Equivalent results have been observed in retrospective, nested case–control studies.20 Although less numerous than prevalence studies, there are also longitudinal analyses that show an increase in the incidence of cardiovascular and cerebrovascular events,20–22 although these studies were not designed for this objective. In addition to the impact in the cardiovascular area, it has been seen that COPD patients present a greater prevalence of other chronic diseases, in particular lung cancer, anemia, osteoporosis, and malnutrition.6,23,24 Although there is great heterogeneity amongst the studies regarding the methodology and the COPD diagnostic criteria used, there is currently a generalized opinion that considers COPD a cardiovascular risk factor.
The current results confirm the previous data, demonstrating that patients with COPD present a prevalence of chronic diseases higher than that found in non-COPD populations, with the sole exception of lung cancer, which was reported as an antecedent in only two cases (and probably due to the characteristics of the study). As found in previous studies, the subjects with COPD had a high prevalence of arterial hypertension, diabetes, and dyslipidemia, very similar to those reported in a previous study of cases carried out in the same geographical setting, conferring high consistency to the results.16 However, the multivariate analysis, confirmed the hypothesis that COPD was an independent risk factor for ischemic cardiac disease. Numerous hypotheses have been contemplated that could explain the possible association between COPD and other chronic diseases. Firstly, the age factor must be kept in mind. The prevalence of COPD increases ostensibly over the age of 65 years, an age at which some studies done in the general population have shown that they present more than one chronic comorbidity, COPD among them.25 In the current study, the prevalence of cardiovascular as well as ischemic heart disease risk factors increased in the older age groups but, once adjusted for this confounding factor, COPD continued as a risk factor for ischemic heart disease per se. The second factor to be considered is tobacco habit, a fundamental risk factor for the development of COPD that also plays a primordial role in the genesis of ischemic heart disease. In order to minimize diagnostic errors, one of the inclusion criteria for all the participants of the study was the condition of being either a smoker or ex-smoker. This did not allow the factor of smoking intensity to be properly evaluated as a risk factor; however, there is an observed tendency towards increased hypertension, diabetes, and ischemic heart disease in the subjects with tobacco histories of more than 20 pack-years. The greater prevalence of classic cardiovascular risk factors (hypertension, diabetes, and dyslipidemia) that is found in COPD patients is a third factor that could explain the increase in vascular disease. However, after adjusting for these factors in the current study, COPD was still a risk factor for ischemic heart disease and cerebrovascular disease, as occurs in the majority of studies published in COPD.
If the existence of common risk factors does not totally explain the association between COPD and cardiovascular disease, there might be a common pathogenic process. Over the last few years, it has been proposed that COPD can produce systemic inflammation and that this inflammation could be the link between COPD and an increase in cardiovascular disease in these patients. This theory has been very attractive, especially if it is taken into account the fact that both COPD as well as arteriosclerosis are considered inflammatory diseases and that in both it is possible to observe higher levels of some inflammatory mediators, such as CRP, which could explain a common pathogenesis. Experimental studies have demonstrated the role of CRP in the development of atheromas, and this finding explains the epidemiological data showing a relationship between systemic inflammation and atherosclerosis, ischemic cardiopathy, cerebrovascular disease, and death due to coronary disease.26 On the other hand, also in COPD patients and even in moderate disease, high serum levels of inflammatory markers have been shown to exist, in particular CRP, so that this could constitute the link with cardiovascular disease.12,14 In a study done by Sin and Man, with data obtained from the subjects included in the National Health and Nutrition Examination Survey III study, it was observed that COPD patients presented an increase in ischemic heart disease that was related with CRP levels.27 Finally, although less numerous, some studies have observed that COPD patients present endothelial dysfunction.
Nevertheless, despite being a widely extended concept, it is still not known to what extent there is systemic inflammation in COPD. Even though in COPD patient populations it is possible to observe higher levels of certain inflammatory components (eg, interleukin-8, tumor necrosis factor-α, fibrinogen, CRP), this increase is only observed in specific groups of patients. Moreover, it is not known if this increase is related with the obstruction itself or rather with collateral factors. Recently, the presence of a more specific circulating biomarker – surfactant protein D – has been reported in COPD patients, providing more evidence for the inflammatory overspill hypothesis.28
Obesity, along with lower physical activity, can have an unfavorable impact on CRP values. In a study by Aronson et al, the higher CRP in COPD patients was associated with those who presented associated obesity.12 More recently, a Dutch group confirmed that COPD patients who are overweight have higher levels of CRP than patients with normal weight. The authors suggest that obesity has a very relevant role in systemic inflammation, which is observed in some COPD patients.29 Analyzing body fat in greater detail using dual-energy X-ray absorptiometry tomography, the same group has demonstrated that abdominal fat contributes significantly to the systemic inflammation that can be observed in stable patients with moderate and severe COPD.30 More recently, Furutate et al reported that COPD patients have an excess of visceral fat, even in the absence of obesity.31
One interesting aspect to be discussed is the relationship between cardiovascular disease and the degree of severity of COPD. While some studies show that the risk for cardiovascular disease increases with the severity of COPD,32 in others the results are less evident when adjusted for other possible factors such as age and classic risk factors. In a previous study of cases, this relationship could not be seen,16 nor has it been confirmed in the recently published ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) study.33 In the current study, although the prevalence of ischemic cardiopathy was greater in the stages with greater severity, the differences were established in subgroups II and IV but not in subgroup III, which detracts consistency from the findings and leads one to speculate whether there are confounding factors that have not been considered in the analysis. Looking at the behavior of peripheral vascular disease, it could be concluded that, as in the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) study, if the patients with mild disease are excluded, the severity of the disease is not associated with a greater increase in vascular disease.
One result that deserves to be commented on is the lower prevalence of arterial hypertension in the subjects with the severest COPD. This finding can be justified in the light of the study recently published by Garcia-Aymerich et al, which identified three COPD phenotypes, the third of which corresponded with subjects with less severity but with greater comorbidities with vascular risk.34 These would be along the same lines as the results of the communications by Schneider et al, which showed that although the COPD subjects showed a high prevalence of ischemic heart disease, the risk for suffering new cardiovascular events was less in the patients with greater severity than in those that presented less severe disease.20
The current study presents limitations that should be kept in mind when interpreting the results. First of all, the subjects of the control group are younger and have a lesser degree of tobacco smoke exposure than the COPD subjects. Although the adjustments made resolve this problem, it still remains a limitation, especially relevant regarding the difference in intensity of tobacco consumption. Although the obesity factor was taken into account, only body mass index was evaluated, and there is no data for abdominal fat mass or visceral fat, which – if there were differences – also could have explained a greater cardiovascular risk in the light of recent studies.
Probably the greatest limitations are derived from the study design. Although the main cardiovascular risk factors were adjusted for, its cross-sectional character meant that the characteristics and duration of previous treatments related with the cardiovascular area that have an impact on the appearance of comorbidity could not be evaluated. Nor can the impact of the degree of physical activity, a relevant factor for cardiovascular risk,35 be adequately taken into account, which in a recent study constituted the main risk factor for all-cause death in COPD subjects.36
Conclusion
In conclusion, this study confirms that COPD patients present an increase in cardiovascular comorbidity over what could be expected based solely on age and the coexistence of classic risk factors. Therefore COPD must be considered as an independent risk factor, although additional studies are necessary to elucidate the intimate mechanisms of the association.
Acknowledgments
The CONSISTE study group members were: Aurelio Arnedillo (Hospital Puerta del Mar, Cadiz, Spain); Adolfo Baloira (Hospital Montexelo, Pontevedra, Spain); Rosa Guell Rous (Hospital Santa Creu i San Pau, Barcelona, Spain); Jesus Molina Paris (Centro de Salud Francia, Madrid, Spain); Carlos Naveran (Centro de Salud de Barbastro, Zaragoza, Spain); Juan Jose Soler Cataluna (Hospital de Requena, Valencia, Spain).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–715. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schunemann HJ, Dorn J, Grant BJ, Winkelstein W, Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118(3):656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(5):388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curkendall S, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128(4):2640–2646. doi: 10.1378/chest.128.4.2640. [DOI] [PubMed] [Google Scholar]
- 6.Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099–2107. doi: 10.1378/chest.128.4.2099. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein J, Cha E, Scharf SM. Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int J Chron Obstruc Pulmon Dis. 2009;4:337–349. doi: 10.2147/copd.s6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J. 2003;22(5):809–814. doi: 10.1183/09031936.03.00031403. [DOI] [PubMed] [Google Scholar]
- 9.Mannino DM, Doherty DE, Buist AS. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med. 2006;100(1):115–122. doi: 10.1016/j.rmed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 11.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome. Lancet. 2007;370(9589):797–799. doi: 10.1016/S0140-6736(07)61383-X. [DOI] [PubMed] [Google Scholar]
- 12.Aronson D, Roterman I, Yigla M, et al. Inverse association between pulmonary function and C-reactive protein in apparently healthy subjects. Am J Respir Crit Care Med. 2006;174(6):626–632. doi: 10.1164/rccm.200602-243OC. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PJ. Chronic obstructive pulmonary disease: effects beyond the lung. PLoS Med. 2010;7(3):e1000220. doi: 10.1371/journal.pmed.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valvi D, Mannino DM, Mullerova H, Tal-Singer R. Fibrinogen, chronic obstructive pulmonary disease (COPD) and outcomes in two United States cohorts. Int J Chron Obstruct Pulmon Dis. 2012;7:173–182. doi: 10.2147/COPD.S29892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33(2):262–272. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 16.de Lucas-Ramos P, Izquierdo-Alonso JL, Rodriguez-Gonzalez Moro JM, et al. Cardiovascular risk factors in chronic obstructive pulmonary disease: results of the ARCE study. Arch Bronconeumol. 2008;44(5):233–238. doi: 10.1016/s1579-2129(08)60037-3. Spanish. [DOI] [PubMed] [Google Scholar]
- 17.Izquierdo JL, Martinez A, Guzman E, de Lucas P, Rodriguez JM. Lack of association of ischemic heart disease with COPD when taking into account classical cardiovascular risk factors. Int J Chron Obstruct Pulmon Dis. 2010;5:387–394. doi: 10.2147/copd.s14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medrano Alberto MJ, Boix Martinez R, Cerrato Crespan E, Ramirez Santa-Pau M. Incidence and prevalence of ischemic heart disease and cerebrovascular disease in Spain: a systematic review of the literature. Rev Esp Salud Publica. 2006;80(1):5–15. doi: 10.1590/s1135-57272006000100002. Spanish. [DOI] [PubMed] [Google Scholar]
- 19.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 20.Schneider C, Bothner U, Jick SS, Meier CR. Chronic obstructive pulmonary disease and the risk of cardiovascular diseases. Eur J Epidemiol. 2010;25(4):253–260. doi: 10.1007/s10654-010-9435-7. [DOI] [PubMed] [Google Scholar]
- 21.Johnston AK, Mannino DM, Hagan GW, Davis KJ, Kiri VA. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax. 2008;63(7):599–605. doi: 10.1136/thx.2007.088112. [DOI] [PubMed] [Google Scholar]
- 22.Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi: 10.1136/thx.2009.128082. [DOI] [PubMed] [Google Scholar]
- 23.van der Molen T. Co-morbidities of COPD in primary care: frequency, relation to COPD, and treatment consequences. Prim Care Respir J. 2010;19(4):326–334. doi: 10.4104/pcrj.2010.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharafkhaneh A, Petersen NJ, Yu HJ, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125–132. doi: 10.2147/copd.s8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Weel C, Schellevis FG. Comorbidity and guidelines: conflicting interests. Lancet. 2006;367(9510):550–551. doi: 10.1016/S0140-6736(06)68198-1. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PW, Nam BH, Pencina M, D’Agostino RB, Benjamin EJ, O’Donnell CJ. C-reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med. 2005;165(21):2473–2478. doi: 10.1001/archinte.165.21.2473. [DOI] [PubMed] [Google Scholar]
- 27.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107(11):1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 28.Sin DD, Leung R, Gan WQ, Man SF. Circulating surfactant protein D as a potential lung-specific biomarker of health outcomes in COPD: a pilot study. BMC Pulm Med. 2007;7:13. doi: 10.1186/1471-2466-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breyer MK, Spruit MA, Celis AP, Janssen PP, Wouters EF CIRO Network. Highly elevated C-reactive protein levels in obese patients with COPD: a fat chance? Clin Nutr. 2009;28(6):642–647. doi: 10.1016/j.clnu.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Rutten EP, Breyer MK, Spruit MA, et al. Abdominal fat mass contributes to the systemic inflammation in chronic obstructive pulmonary disease. Clin Nutr. 2010;29(6):756–760. doi: 10.1016/j.clnu.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Furutate R, Ishii T, Wakabayashi R, et al. Excessive visceral fat accumulation in advanced chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:423–430. doi: 10.2147/COPD.S22885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curkendall SM, Lanes S, de Luise C, et al. Chronic obstructive pulmonary disease severity and cardiovascular outcomes. Eur J Epidemiol. 2006;21(11):803–813. doi: 10.1007/s10654-006-9066-1. [DOI] [PubMed] [Google Scholar]
- 33.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Aymerich J, Gomez FP, Benet M, et al. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax. 2011;66(5):430–437. doi: 10.1136/thx.2010.154484. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 36.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with chronic obstructive pulmonary disease: a prospective cohort study. Chest. 2011;140(2):331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]