Abstract
Objectives
Posaconazole is an extended-spectrum triazole with proven efficacy as antifungal treatment and prophylaxis. The marketed oral suspension should be taken with food to maximize systemic absorption. A new solid oral tablet has been developed with improved bioavailability that can be administered without regard to food. The aim of this study was to evaluate rising single- and multiple-dose pharmacokinetics, safety and tolerability of the new tablet.
Methods
This was a single-centre, randomized, placebo-controlled, Phase I, rising single- and multiple-dose study of healthy subjects aged 18–65 years who received a posaconazole tablet as 200 mg once daily, 200 mg twice daily or 400 mg once daily. The 24 subjects were studied in two cohorts of 12 subjects each (9 active and 3 placebo).
Results
After single or multiple oral dose administration of posaconazole tablets (200 and 400 mg), exposure increased in a dose-related manner. Peak posaconazole concentrations were attained at a median Tmax of 4–5 h. Mean half-life was similar for 200 and 400 mg posaconazole doses (25 and 26 h). The accumulation ratio upon multiple doses over 8 days was ∼3 for 200 and 400 mg once daily and ∼5 for 200 mg twice daily. Cavg values exceeded 1300 ng/mL. The posaconazole oral tablet was safe and well tolerated, although mild, transient elevations in liver function were reported in some patients.
Conclusions
Posaconazole exposure increased in a dose-related manner. The pharmacokinetics of this new solid oral tablet of posaconazole supports the clinical evaluation of once-daily dosing regimens for fungal infections.
Keywords: exposure, absorption, once-daily dosing
Introduction
Posaconazole is an extended-spectrum triazole with demonstrated efficacy as prophylaxis for invasive fungal disease (IFD)1,2 and as treatment for refractory IFD.3,4 The bioavailability of posaconazole oral suspension is significantly enhanced when coadministered with food,5,6 and it is therefore prescribed with food to maximize systemic absorption.7 Bioavailability may also be enhanced by dividing doses or by administering the drug with a liquid nutritional supplement or acidic beverage.6,8
A new solid oral tablet with improved bioavailability has been developed that can be administered without regard to food. The tablet is designed to release the entire dose of solubilized posaconazole in the small intestine, maximizing systemic absorption. In an exploratory study, this new solid oral formulation significantly increased exposure to posaconazole relative to the oral suspension in fasting healthy volunteers.9 Since many patients taking posaconazole are unable to tolerate food, the ability to attain a higher exposure in a fasted state with this new solid oral formulation could reduce dose frequency in patients from two or three times a day to a once-daily regimen independent of food.
The primary objective of this study was to evaluate the rising single- and multiple-dose pharmacokinetics of posaconazole for the new tablet formulation. The safety and tolerability of the tablet formulation were also assessed.
Methods
Study design
This was a single-centre, randomized, placebo-controlled, Phase I, rising single- and multiple-dose study. The study took place between 19 March 2009 and 20 May 2009 at the Clinical Pharmacology Research Unit, University of Miami, Miami, FL, USA.
Subjects and treatments
The study protocol was approved by the University of Miami Human Subjects Research Office. The study was conducted in accordance with the principles of Good Clinical Practice. Written informed consent was obtained from each patient before enrolment.
Healthy male and female participants aged 18–65 years with a body mass index of 19–35 kg/m2 were enrolled. Initially, 24 subjects were planned to participate. It was decided, after consultation between the sponsor and principal investigator, that enrolment could be extended to include replacement of subjects who discontinued while on study. Subjects were required to have clinical laboratory test results (haematology, blood chemistry and urinalysis) within normal limits or clinically acceptable to the investigator/sponsor. Female subjects were either post-menopausal, surgically sterilized (with a negative pregnancy test at screening and at each admission to the study centre), or premenopausal, unsterilized using a medically accepted method of contraception or abstaining from sexual intercourse from 2 months prior to study entry to 2 months after stopping study medication. Male subjects agreed to use a medically accepted method of contraception or agreed to abstain from sexual intercourse during the study and for 1 month after stopping the study drug. Subjects were required not to have had any surgical or medical condition that might significantly alter the absorption, distribution, metabolism or excretion of any drug. Subjects were excluded if they had a history of any infectious disease within 4 weeks prior to drug administration, a positive result for hepatitis B surface antigen, hepatitis C antibodies, HIV or drug use with a high potential for abuse. Subjects were excluded if they had a history of alcohol or drug abuse in the past 2 years, donated blood in the past 60 days, previously received posaconazole, participated in a clinical study within 30 days, or smoked >10 cigarettes (or equivalent tobacco use) per day. Subjects were excluded if they had received any prescription drug within 14 days before the start of the study, or any over-the-counter medicines, vitamin supplements or herbal remedies within the previous 7 days.
Posaconazole tablets contained 100 mg of posaconazole in a solid dispersion formed by dissolving posaconazole in a pH-sensitive polymer matrix using hot-melt extrusion technology. The drug substance was mixed with hypromellose acetate succinate and ascorbic acid (1 : 3 : 0.08, w/w/w) for the hot-melt extrusion process. Tablets were made from the resulting solid dispersion powder and mixed with small amounts of excipients.
All subjects were confined to the study centre at least 12 h before day −1 (baseline) procedures; subjects were discharged after dosing on day 1 and returned to the centre on each dosing day. There were two cohorts (12 subjects each) based on dose; within each cohort, subjects were randomized according to a computer-generated sponsor-provided randomization code to receive single and multiple doses of either posaconazole (9 subjects) or matching placebo (3 subjects) (Figure 1). Treatment was prepared according to the randomization schedule and dispensed in a blinded fashion by a third party who was not involved in any study procedures, assessments or data recording. Placebo tablets were identical to the active tablet in colour, shape, size and taste. In Cohort 1, subjects received 200 mg of posaconazole (2 × 100 mg tablets) or matching placebo as follows: a single dose in the morning on day 1, twice on day 6, once daily on days 7–14, and twice daily on days 15–22. In Cohort 2, subjects received 400 mg of posaconazole (4 × 100 mg tablets) or matching placebo as follows: a single dose in the morning on day 1, twice on day 6, and once daily on days 7–14. Cohort 2 did not include 400 mg twice-daily dosing on days 15–22 because pharmacokinetic modelling suggested that the exposure range previously found to be safe may be exceeded with multiple daily dosing of 400 mg twice daily. In both cohorts, subjects received posaconazole tablets on day 1 after an overnight fast. On other dosing days, subjects took posaconazole without regard to food.
Figure 1.
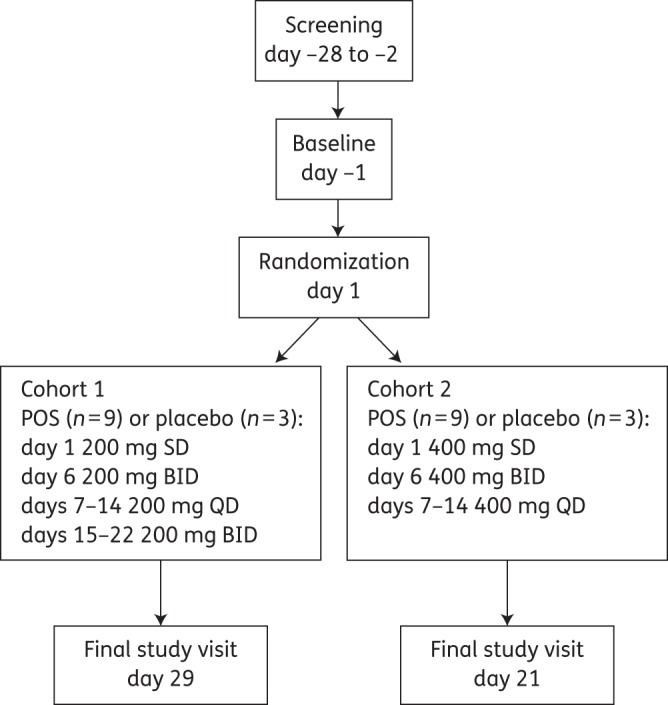
Study design. BID, twice daily; QD, once daily; POS, posaconazole; SD, single dose.
Pharmacokinetic evaluations
Blood samples were collected at the following pre-determined timepoints for both cohorts: day 1: 0 h (pre-dose) and at 2, 3, 4, 5, 6, 8, 12, 24, 48, 72 and 120 h post-dose; day 6: at 2, 3, 4, 5, 6, 8, 12 and 24 h post-dose; and pre-dose (trough level) samples on days 12 and 13. Additional samples for Cohort 1 were taken on day 14: 0 h (pre-dose) and at 2, 3, 4, 5, 6, 8, 12 and 24 h post-dose; and day 22: 0 h (pre-dose) and at 2, 3, 4, 5, 6, 8, 12, 24, 48, 72, 120 and 168 h post-dose. Additional samples for Cohort 2 were taken on day 14: 0 h (pre-dose) and at 2, 3, 4, 5, 6, 8, 12, 24, 48, 72, 120 and 168 h post-dose.
Plasma samples were isolated and assayed for posaconazole using validated liquid chromatography with tandem mass spectrometric detection10 with a calibrated range of 5–5000 ng/mL, precision of 2.0%–6.6% and accuracy of −11.8% to 3.3%.
The primary pharmacokinetic variables assessed were: AUC [0 to 24 h (AUC0–24), during the dosing interval (AUCtau) and from time 0 to the time of the final quantifiable sample (AUCtf)]; maximum plasma concentration (Cmax); time to Cmax (Tmax); apparent total body clearance (CL/F); and terminal-phase half-life (t1/2). For the multiple-dose part, average plasma concentration over the dosing interval (Cavg) and accumulation ratio (R) were also calculated. Summary statistics (means, standard deviations and coefficients of variation (%CV) were calculated. Derived log-transformed, dose-normalized pharmacokinetic parameters (AUCtau and Cmax) were statistically analysed for a preliminary assessment of dose proportionality across subjects. Dose-normalized AUC values were scaled from mean values for 200 and 400 mg. Assessment of dose proportionality was based on ratios (between doses) of model adjusted geometric means for AUC (Cmax) and corresponding 90% CIs using day 14 data. The determination of sample size (12 subjects per cohort) was based on empirical rather than statistical considerations.
Safety analyses
Safety assessments (vital signs, physical examination, ECGs and clinical laboratory tests) were conducted for both cohorts on days −1, 6 and 10, and additionally on days 23 and 29 (Cohort 1) or days 15 and 21 (Cohort 2). Adverse events (AEs) were assessed throughout the study.
Results
Subject demographics and disposition
Of 25 subjects enrolled in this study, 13 (including a replacement subject) were in Cohort 1 (10 posaconazole and 3 matching placebo) and 12 were in Cohort 2 (9 posaconazole and 3 matching placebo) (Table 1). All subjects were white and of Hispanic or Latino ethnicity, aged between 31 and 59 years (mean age 45.9 years), and 14 subjects (56%) were male. A total of 22 out of 25 subjects completed the study (3 subjects were discontinued due to AEs). All 19 subjects who received at least one dose of posaconazole were included in the pharmacokinetic analyses; placebo subjects were excluded from the pharmacokinetic analyses.
Table 1.
Subject demographics
| Cohort 1 (200 mg of posaconazole), n = 10 | Cohort 2 (400 mg of posaconazole), n = 9 | Placebo, n = 6 | All subjects, n = 25 |
|
|---|---|---|---|---|
| Gender, male, n (%) | 5 (50) | 6 (67) | 3 (50) | 14 (56) |
| Race, n (%) | ||||
| White | 10 (100) | 9 (100) | 6 (100) | 25 (100) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 10 (100) | 9 (100) | 6 (100) | 25 (100) |
| Age, years | ||||
| mean (SD) | 47.7 (8.2) | 43.8 (9.9) | 46.0 (9.3) | 45.9 (8.9) |
| median (range) | 46.5 (33–59) | 40.0 (31–56) | 46.5 (33–56) | 44.0 (31–59) |
| Weight, kg | ||||
| mean (SD) | 74.85 (14.13) | 72.89 (7.61) | 71.83 (9.58) | 73.42 (10.72) |
| median (range) | 71 (61–100) | 72 (61–86) | 71 (61–88) | 71 (61–100) |
| Height, cm | ||||
| mean (SD) | 165.60 (5.62) | 168.78 (7.90) | 166.17 (8.18) | 166.88 (6.98) |
| median (range) | 165 (156–175) | 168 (155–181) | 167 (153–175) | 167 (153–181) |
Pharmacokinetics
After single oral administration of posaconazole tablets (200 or 400 mg), exposure between groups differed in an approximately dose-proportional manner (Table 2). Plasma concentration–time profiles are shown for days 1 and 14 (Figure 2). Mean t1/2 values after a single administration were similar for 200 and 400 mg doses (∼25 and 26 h, respectively), while peak concentrations were attained at a median Tmax of 4 h (200 mg) and 5 h (400 mg). Based on log-transformed data, the dose-normalized Cmax and AUCtf for 400 mg were 83% and 91%, respectively, of those observed with the 200 mg dose on day 1 (Table 3).
Table 2.
Mean (%CV) of posaconazole pharmacokinetic parameters after single oral tablet administration of 200 or 400 mg of posaconazole
| Dose (mg) | Day | n | Cmax (ng/mL) | Tmaxa (h) | AUCtaub (ng·h/mL) | AUCtf (ng·h/mL) | CL/F (L/h) | t1/2 (h) |
|---|---|---|---|---|---|---|---|---|
| 200 mg (Cohort 1) | 1 | 10 | 778 (29) | 4.0 (3–8) | 10 500 (23) | 23 000 (23) | 8.80 (26) | 25.1 (20) |
| 400 mg (Cohort 2) | 1 | 9 | 1290 (29) | 5.0 (3–8) | 18 900 (34) | 42 800 (35) | 9.55 (34)c | 26.1 (22)c |
aMedian (range).
btau = 24 h.
cn = 8.
Figure 2.
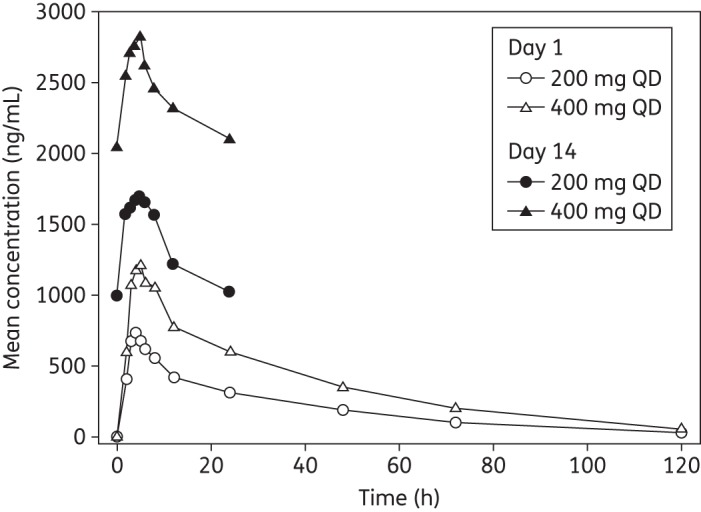
Mean plasma concentrations of posaconazole on days 1 and 14. QD, once daily.
Table 3.
Statistical analysis of dose-normalized posaconazole (400 versus 200 mg) following single and multiple oral tablet administration
| Day/parameter | Geometric mean ratio (%)a | 90% CI |
|---|---|---|
| Day 1 | ||
| Cmax | 83 | 65–106 |
| AUCtf | 91 | 72–114 |
| Day 14 | ||
| Cmax | 76 | 51–114 |
| AUCtau | 81 | 52–126 |
Comparisons were made using a one-way analysis of variance model extracting the effect due to treatment based on log-transformed, dose-normalized data.
aModel-based geometric mean ratio.
Following multiple oral administration of posaconazole tablet (200 mg once daily, 200 mg twice daily or 400 mg once daily) for 8 days, the exposure among treatment groups increased in a dose-related manner (Table 4). The accumulation ratio upon multiple doses was ∼3 for 200 and 400 mg once daily and ∼5 for 200 mg twice daily (Table 4). Accumulation appeared to be independent of dose, but as expected was dependent on the dosing regimen (once daily versus twice daily). Inter-subject AUC variability in exposure for 400 mg once daily was relatively high, compared with that for 200 mg once daily (CV, 54% and 32%, respectively).
Table 4.
Mean (%CV) of posaconazole pharmacokinetic parameters after multiple oral tablet administration of posaconazole (200 mg once daily, 200 mg twice daily or 400 mg once daily)
| Dose (mg) | Day | n | Cmax (ng/mL) | Tmaxa (h) | AUCtaub (ng·h/mL) | Cavgc (ng/mL) | Rd |
|---|---|---|---|---|---|---|---|
| 200 mg QD (Cohort 1) | 14 | 8 | 1800 (31) | 5.0 (2–8) | 31 400 (32) | 1310 (32) | 3.14 (24) |
| 200 mg BID (Cohort 1) | 22 | 8 | 2980 (38) | 4.0 (2–8) | 30 600 (38) | 2550 (38) | 4.75 (28) |
| 400 mg QD (Cohort 2) | 14 | 8 | 2940 (46) | 5.0 (0–12) | 56 600 (54) | 2360 (54) | 3.16 (57) |
BID, twice daily; QD, once daily.
aMedian (range).
btau = 24 h for once daily and 12 h for twice daily.
cCavg = average concentration = AUCtau/tau.
dAccumulation ratio = ratio of AUCtau (day 14) to AUCtau (day 1).
Based on log-transformed day 14 data, the dose-normalized Cmax and AUCtau for 400 mg of posaconazole were 76% and 81%, respectively, of those observed with the 200 mg dose following multiple oral administration (Table 3). Cavg (%CV) values are presented in Table 4. Steady-state posaconazole trough concentrations appeared to be achieved after 7 days of multiple dosing in subjects from day 6 to 12 (Figure 3).
Figure 3.
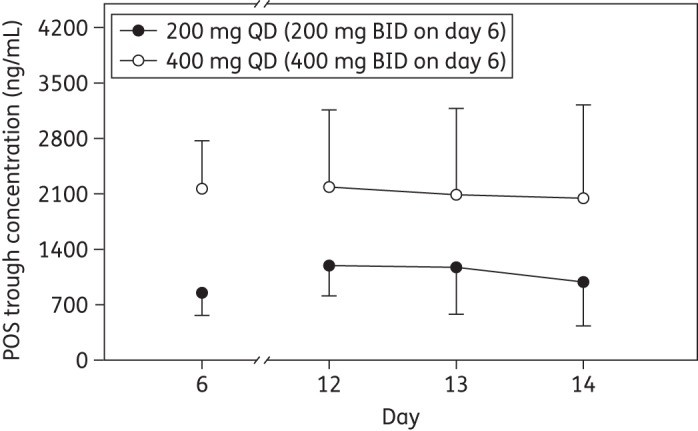
Posaconazole (POS) trough concentrations (mean ± SD) following once-daily multiple oral administration of posaconazole tablets (200 or 400 mg). BID, twice daily; QD, once daily.
Safety assessments
Posaconazole tablets were generally safe and well tolerated. No serious AEs were reported. Twelve subjects (48%) reported at least one treatment-emergent AE: 6 subjects (60%) treated with 200 mg of posaconazole once daily/twice daily; 5 subjects (56%) treated with 400 mg of posaconazole once daily/twice daily; and 1 subject (17%) in the placebo group. Eleven subjects (44%) reported treatment-related AEs (Table 5): 6 subjects (60%) treated with 200 mg of posaconazole once daily/twice daily; 4 subjects (44%) treated with 400 mg of posaconazole once daily/twice daily; and 1 subject (17%) in the placebo group. The most commonly reported treatment-related AEs were mild increase in hepatic enzyme level [6 subjects (24%)], diarrhoea [3 subjects (12%)] and headache [2 subjects (8%)]. All other treatment-related AEs occurred in only 1 subject each. The mild, transient increases in liver enzyme levels [aspartate aminotransferase (AST) and alanine aminotransferase (ALT) values] were observed in five subjects in the 200 mg of posaconazole once-daily/twice-daily group, and in one subject in the 400 mg of posaconazole once-daily/twice-daily group, after 10 days of treatment. These AEs had no clinical sequelae; however, three of these subjects discontinued treatment with posaconazole (two subjects received 200 mg of posaconazole once daily/twice daily and one subject received 400 mg of posaconazole once daily). Peak AST levels in the three subjects who discontinued treatment were 49, 66 and 73U/L (reference range, 8–40 U/L). Peak ALT levels in these subjects were 94, 81 and 138 U/L (reference range, 8–54 U/L). In all three subjects, liver enzyme elevations persisted for 1–11 days after posaconazole treatment was discontinued. AST and ALT levels had generally returned to within normal limits by the final study visit. These AEs are consistent with mild, generally reversible liver enzyme elevations previously reported for posaconazole oral suspension.7 There were no clinically significant changes in any other laboratory safety parameters, vital signs or ECG results.
Table 5.
Summary of treatment-related AEs
| Number (%) of subjects with treatment-related AEs |
||||
|---|---|---|---|---|
| Cohort 1 (200 mg of posaconazole), n = 10 | Cohort 2 (400 mg of posaconazole), n = 9 | Placebo, n = 6 | All subjects, n = 25 | |
| Subjects reporting any AE | 6 (60) | 4 (44) | 1 (17) | 11 (44) |
| Hepatic enzyme increased | 5 (50) | 1 (11) | 0 | 6 (24) |
| Diarrhoea | 0 | 3 (33) | 0 | 3 (12) |
| Headache | 0 | 1 (11) | 1 (17) | 2 (8) |
| Dizziness | 1 (10) | 0 | 0 | 1 (4) |
| Dry mouth | 0 | 1 (11) | 0 | 1 (4) |
| Nausea | 0 | 1 (11) | 0 | 1 (4) |
Discussion
This is the first known report of the pharmacokinetics of a new solid oral posaconazole tablet from rising single- and multiple-dose pharmacokinetics in healthy subjects. While extensive clinical safety data for posaconazole are available, this study also evaluated the safety of this new tablet formulation. The pharmacokinetics data obtained in this study were compared with target efficacious concentrations based on an exposure–response analysis from marketed oral suspension, to help select the dose and dosing frequency for an ongoing pivotal study of a new solid oral tablet in patients.
The results of the current study show that following single and multiple doses of posaconazole solid oral tablets (200 and 400 mg) in healthy subjects, posaconazole exposure increased in a dose-related manner. When the dose was increased in a 1 : 2 ratio, exposure increased in 1 : 1.9 and 1 : 1.8 ratios for days 1 and 14, respectively. On day 1, the dose-normalized posaconazole exposure (AUCtau) value in this study was comparable to that of the tablet formulation determined in a previous study (11 400 ng·h/mL) and was substantially higher than for the oral suspension under both fasted and fed conditions (2970 and 8470 ng·h/mL, respectively).9
The half-life of ∼1 day predicted attainment of steady state by day 5–7; this was confirmed by plasma trough concentration data (Figure 3). A loading dose on day 1 can be utilized for a rapid attainment of the steady state in cases of serious infections requiring early effective treatment. The accumulation ratios for 200 and 400 mg once-daily dosing regimens were ∼3 and were not dose-dependent. As expected, the accumulation ratio for the twice-daily regimen was higher (∼5). The half-life for 200 and 400 mg once-daily doses remained unchanged. The apparent clearance also remained relatively unchanged across doses.
In this study, the posaconazole tablet formulation attained mean Cavg values >1300 ng/mL at the lowest dose (200 mg once daily). These values were above average concentration values associated with efficacy in patients.3 For example, in a salvage study of patients with refractory invasive aspergillosis, higher posaconazole plasma concentrations were associated with higher response rates; a mean posaconazole Cavg of ≥411 ng/mL was associated with a response rate of 53% (versus 26% for a historical control sample), and a 75% response rate in patients with a mean posaconazole Cavg of ≥1250 ng/mL.3 The positive association between response rate and average concentration reinforces the importance of maximizing posaconazole exposure in patients at high risk of IFD. These results further support development of this new solid oral formulation; furthermore, it does not have the food requirement that may limit the utility of the currently marketed oral suspension formulation in patients with poor food intake.
Mild, asymptomatic increases in liver enzyme values were observed in five subjects after 10 days of posaconazole treatment. Although elevations observed in the liver function test were transient and not considered serious or severe, three subjects discontinued the study due to these AEs. Such transient elevations in liver function have been previously reported. An analysis of safety data from 18 clinical pharmacology single- and multiple-dose trials of posaconazole conducted in healthy volunteers reported that while posaconazole has the potential to elevate hepatic enzymes, these changes do not appear to be exposure-dependent, and most levels returned to baseline when posaconazole was discontinued.11
Conclusions
In summary, posaconazole exposures from a new tablet formulation increased in a dose-related manner, with steady-state Cavg values at the lowest dose (200 mg once daily) exceeding those previously found to be efficacious against IFD. Accumulation after multiple dosing was dose-independent. The single- and multiple-dose pharmacokinetics of this new solid oral tablet of posaconazole support the clinical evaluation of once-daily dosing regimens for fungal infections. The posaconazole tablet at doses of 200 mg, whether administered once or twice daily, and 400 mg, administered once daily, were generally safe and well tolerated in healthy subjects.
Funding
This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. Medical writing and editorial assistance were funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA.
Transparency declarations
At the time the study was conducted, G. K. and E. O. were employees and stockholders of Merck Sharp & Dohme Corp. L. M. is a Merck Sharp & Dohme employee and stockholder. M. M. is a Merck Sharp & Dohme employee. R. A. P. has no conflicts of interest to declare.
Medical writing and editorial assistance were provided by Sheena Hunt, PhD, and Susan Quiñones, PhD, of ApotheCom, Yardley, PA, USA, of authors' original work.
Author contributions
G. K. was involved in study conception, design and planning; he also supervised analyses, interpreted results and wrote sections of the initial draft of the manuscript. L. M., M. M. and E. O. performed analyses and interpreted results. R. A. P. was the study principal investigator and was responsible for the conduct of the study, protocol review, participant recruitment, informed consent, safety and data collection and management. All authors critically reviewed the manuscript, providing suggestions for revision where necessary. All authors reviewed and approved the final version of the paper. L. M. and M. M. are guarantors for the data and have full access to the data.
Acknowledgements
These data were previously presented at the Fifty-first Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 2011 (A2–573).
We thank Dr Hetty Waskin, Merck Sharp & Dohme Corp., for her thoughtful comments and valuable discussions during the design and report preparation phases of this study.
References
- 1.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–59. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 2.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–47. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 3.Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 4.Raad II, Hachem RY, Herbrecht R, et al. Posaconazole as salvage treatment of invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin Infect Dis. 2006;42:1398–403. doi: 10.1086/503425. [DOI] [PubMed] [Google Scholar]
- 5.Courtney R, Wexler D, Radwanski E, et al. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol. 2003;57:218–22. doi: 10.1046/j.1365-2125.2003.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna G, Moton A, Ma L, et al. The pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother. 2009;53:958–66. doi: 10.1128/AAC.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schering Corporation. 2010. NOXAFIL® (Posaconazole) Oral Suspension 40 mg/mL.
- 8.Krishna G, Ma L, Vickery D, et al. Effect of varying amounts of a liquid nutritional supplement on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob Agents Chemother. 2009;53:4749–52. doi: 10.1128/AAC.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishna G, Ma L, Martinho M, et al. A single dose phase I study to evaluate the pharmacokinetics of posaconazole new tablet and capsule formulations relative to oral suspension. Antimicrob Agents Chemother. 2012 doi: 10.1128/AAC.00222-12. doi:10.1128/AAC.00222–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen JX, Krishna G, Hayes RN. A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma. J Pharm Biomed Anal. 2007;43:228–36. doi: 10.1016/j.jpba.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Moton A, Krishna G, Wang Z. Tolerability and safety profile of posaconazole: evaluation of 18 controlled studies in healthy volunteers. J Clin Pharm Ther. 2009;34:301–11. doi: 10.1111/j.1365-2710.2009.01055.x. [DOI] [PubMed] [Google Scholar]


