Abstract
Objectives
The aims of this work were to study the epidemiological profiles, differences in echinocandin susceptibilities and clinical relevance of the Candida parapsilosis sensu lato species isolated from proven fungaemia cases at La Fe University Hospital of Valencia (Spain) from 1995 to 2007.
Results
The prevalence of these species was: C. parapsilosis sensu stricto, 74.4%; Candida orthopsilosis, 23.54%; and Candida metapsilosis, 2.05%. The incidence of the species complex as agents of fungaemia remained stationary until 2005 and doubled in 2006. The incidence of C. orthopsilosis showed an increasing trend during the study period, while C. parapsilosis sensu stricto incidence diminished. Also, an important epidemiological change was observed starting in 2004, when 86.5% of the C. parapsilosis sensu lato strains were found in adult patients, while before that year only 13.5% of the isolates were found in this population.
Conclusions
Echinocandin drug susceptibility testing using the CLSI M27-A3 document showed a wide range of MIC values (0.015–4 mg/L), with micafungin being the most potent in vitro inhibitor followed by anidulafungin and caspofungin (MIC geometric mean of 0.68, 0.74 and 0.87 mg/L, respectively). C. metapsilosis was the most susceptible species of the complex to anidulafungin and micafungin in vitro (MIC50 for anidulafungin and micafungin: 0.06 mg/L), while there were no differences between C. parapsilosis sensu lato species when caspofungin MIC50s were compared (MIC50 1.00 mg/L). Differences in caspofungin in vitro susceptibility were observed between the different clinical service departments of La Fe Hospital.
Keywords: FKS, C. parapsilosis, C. orthopsilosis, C. metapsilosis
Introduction
Candida-associated invasive infections are a leading cause of mycosis-associated mortality.1 Candida parapsilosis is the second most commonly isolated Candida species from blood in Southern Europe, Latin America and Asia and the second leading cause of candidaemia in children.1–9 Genotypic differences allowed taxonomic division of C. parapsilosis into three groups10–16 that were replaced by C. parapsilosis sensu stricto (formerly group 1), Candida orthopsilosis (group 2) and Candida metapsilosis (group 3).17 Multiple studies have demonstrated that these species have different virulence and antifungal susceptibilities.18–28 Also, several retrospective analyses showed that the prevalence of C. orthopsilosis and C. metapsilosis ranged from 2.3% to 9% and from 0.9% to 6.9%, respectively, depending on the country and on the specimen type studied,19,24,25,27,29–33 and that the prevalence of C. orthopsilosis has been increasing since 2004.24
The aims of this work were to study the epidemiological profiles, differences in echinocandin susceptibilities and clinical relevance of the C. parapsilosis sensu lato species isolated from proven fungaemia cases at La Fe University Hospital of Valencia (Spain) from 1995 to 2007.
Materials and methods
Strains
During the study period (January 1995 to July 2007), 756 cases of proven fungaemia due to Candida spp. were identified at La Fe University Hospital. The incidence of candidaemia at our centre was 1.06 cases per 1000 hospital admissions (755 candidaemias/715 066 admissions). Among these cases, 293 strains were identified as C. parapsilosis sensu lato (38.8% of all candidaemias) and were isolated from 288 patients.
Strain identification
The isolates were phenotypically identified as C. parapsilosis sensu lato using VITEK® (bioMérieux, Spain) and AuxaColor™ (Bio-Rad, Spain) kits following the manufacturers’ instructions. Molecular identification was performed by sequencing the 5.8S RNA gene and adjacent internal transcribed spacer 1 (ITS1) and ITS2 regions.17,34 Phylogenetic and Clustal analyses were done using the BioEdit sequence alignment editor (Ibis Therapeutics, Carlsbad, CA, USA). Maximum parsimony clustering was performed by using the ITS1 and ITS2 sequences from C. parapsilosis sensu stricto, C. orthopsilosis and C. metapsilosis (GenBank accession numbers AB109275, EU557373 and EU557369, respectively).
C. parapsilosis sensu lato FKS1 sequence analysis
The regions of the FKS1 gene linked with reduced echinocandin susceptibility of C. parapsilosis sensu stricto, C. orthopsilosis and C. metapsilosis were sequenced, as described previously.35 The sequences obtained were compared with those deposited in GenBank under the numbers EU221325, EU350513 and EU350514 (C. parapsilosis FKS1, C. orthopsilosis FKS1 and C. metapsilosis FKS1, respectively).
Antifungal susceptibility testing and compounds
Echinocandin drug susceptibility testing was performed in triplicate in accordance with the CLSI document M27-A3 guidelines.36 C. parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as control strains. The drugs used were anidulafungin (Pfizer, New York, NY, USA), caspofungin (Merck & Co. Inc., Rahway, NJ, USA) and micafungin (Astellas Pharma USA, Inc., Deerfield, IL, USA). The drugs were obtained as standard powders from their respective manufacturers. Caspofungin and micafungin were dissolved in sterile distilled water and anidulafungin was dissolved in 100% DMSO (Sigma-Aldrich). Stock solutions of each drug were kept at −86°C. The solutions were discarded if they were not used within 3 months.
DNA extraction and sequencing
Genomic DNA was extracted with a Q-Biogene (Irvine, CA, USA) FastDNA kit following the manufacturer's instruction. DNA sequencing was performed with a CEQ Dye Terminator Cycle Sequencing Quick Start Kit (Beckman Coulter, Fullerton, CA, USA) according to the manufacturer's recommendations. Sequence analysis was performed with CEQ 8000 genetic analysis system software (Beckman Coulter).
Variables and statistical analysis
In order to study the relationship between each of the C. parapsilosis sensu lato species and the epidemiological data, the following variables were recorded: patient's age and sex, isolation date, underlying illnesses and care unit where the strain was isolated. The patients were divided into the following categories depending on their age: newborn (≤1 month old), infant (between 1 month and 1 year old), child (between 1 year and 15 years old) and adults (≥15 years old). The term ‘paediatric’ was used to group newborn, infant and child categories. The hospital-specific incidence of C. parapsilosis sensu lato fungaemias was calculated using denominator data obtained for the total number of fungaemias (n = 756) and hospital admissions at La Fe University Hospital of Valencia from January 1995 to July 2007. Echinocandin use was quantified as doses per 100 patients per day and was obtained from the pharmacy department of La Fe University Hospital.
Statistical analyses were performed with the Statistical Package for the Social Sciences software (version 17; IBM SPSS Statistics Inc., Chicago, IL, USA). Continuous variables are expressed as means ± SD, medians and ranges, while categorical variables are expressed as percentages (proportions). The unpaired Student's t-test was used to analyse continuous variables, and the χ2 test or Fisher's exact test (two-tailed) was used to compare categorical variables.
The echinocandin MIC data are the result of experiments performed in triplicate. Geometric means (GMs) were used to statistically compare MIC results. The significance levels of MIC differences were determined by a one-way ANOVA test with Bonferroni's correction for multiple comparisons; a P value ≤0.01 was considered significant. In order to approximate a normal distribution, the MICs were transformed to log2 values to establish susceptibility differences between strains. Both on-scale and off-scale results were included in the analysis. The off-scale MICs were converted to the next concentration up or down.
Results
Demographic distribution and incidence of C. parapsilosis sensu lato species
The 293 C. parapsilosis sensu lato strains were isolated from 287 patients; 179 males (62.4%) and 108 females, who were 24.4 ± 28.9 and 13.6 ± 25.3 years old, respectively. Although the majority of the patients having C. parapsilosis sensu lato fungaemias were males, there was no statistical difference between sexes (P = 0.181).
Among the C. parapsilosis sensu lato isolates, 218 (74.4%) were identified as C. parapsilosis sensu stricto, 69 (23.54%) as C. orthopsilosis and 6 (2.05%) as C. metapsilosis by sequence analyses of the 5.8S RNA gene and adjacent ITS1 and ITS2 regions17,34 (Table 1). Six patients showed two positive blood cultures. Out of these six patients, four showed C. parapsilosis sensu stricto and one patient showed C. orthopsilosis in both episodes. The sixth patient showed a bloodstream infection due to C. parapsilosis sensu stricto followed by a positive blood culture caused by C. orthopsilosis. Out of these six cases, only the last one was considered as two separate, single episodes since there was a 3 month interval between episodes and different species were isolated. Thus, 288 individual C. parapsilosis sensu lato fungaemias were studied.
Table 1.
Incidence of C. parapsilosis sensu lato species fungaemia stratified by patient age and sex
| Species | Incidence by age category per 10 000 hospital admissions (number of isolates/number of male patients) |
Total | |||||
|---|---|---|---|---|---|---|---|
| paediatrics |
adults |
||||||
| newborna | infanta | childa | 15–40 years old | 40–65 years old | >65 years old | ||
| C. parapsilosis sensu stricto | 0.36 (26/16) | 0.24 (17/6) | 0.24 (17/11) | 0.69 (49/30) | 0.83 (59/41) | 0.64 (46/22) | 2.99 (214/126) |
| C. orthopsilosis | 0.15 (11/8) | 0.10 (7/3) | 0.10 (5/1) | 0.11 (8/6) | 0.29 (21/13) | 0.22 (16/15) | 0.95 (68/46) |
| C. metapsilosis | 0 | 0 | 0.01 (1/1) | 0 (0) | 0.03 (2/1) | 0.04 (3/3) | 0.08 (6/5) |
| Total | 0.52 (37/24) | 0.34 (24/9) | 0.32 (23/13) | 0.80 (57/36) | 1.15 (82/55) | 0.91 (65/40) | 4.03 (288/177) |
aNewborn, ≤1 month old; infant, between 1 month and 1 year old; child, between 1 year and 15 years old. The patient's sex was not recorded for one patient in each of the paediatric age categories.
Table 1 outlines the distributions of C. parapsilosis sensu lato species stratified by patient's age. Overall, 35.4% of the C. parapsilosis sensu lato strains were isolated from very young (<1 year old) or elderly patients (>65 years old). In this study the median ages of patients with C. parapsilosis sensu stricto, C. orthopsilosis and C. metapsilosis were 41, 50 and 63 years, respectively. Moreover, 70.8% of the C. parapsilosis sensu lato strains were isolated from adult patients (>15 years old) and no statistical differences were observed between species and patient age (P = 0.930). Despite the low number of isolates identified as C. metapsilosis (six representing only 2% of the isolates of this study), it should be noted that all but one of the strains were isolated from adult patients and half of them from elderly patients (70–79 years old).
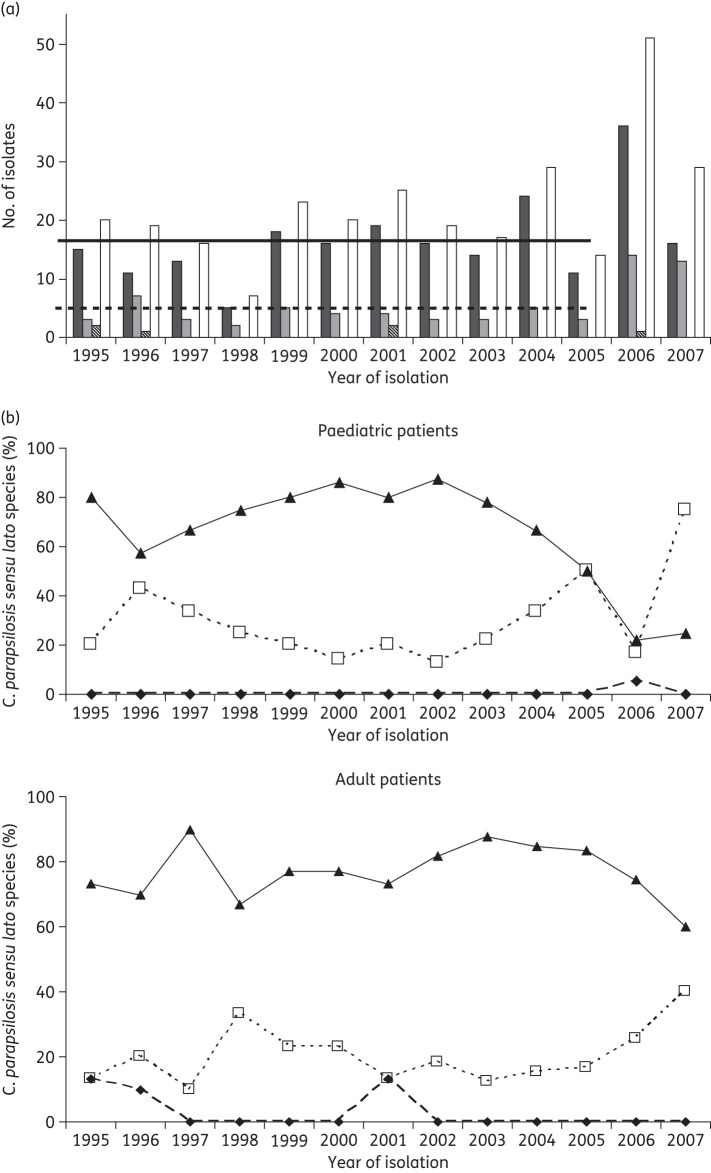
The incidence of C. parapsilosis sensu lato isolates remained almost constant between 1995 and 2005 [average number of isolates per year (1995–2005): 19.4, P = 0.31, and an average incidence of 0.35 C. parapsilosis sensu lato fungaemias/1000 admissions, P = 0.33]. In 2006, the incidence of these species increased 2.97-fold when compared with other years (2006: 51 isolates and an incidence of 1.03 C. parapsilosis sensu lato fungaemias/1000 admissions, P < 0.05). This phenomenon is attributable to a 2.69- and 4.11-fold increase in the incidence of C. parapsilosis sensu stricto and C. orthopsilosis candidaemia, respectively (1995–2005: average incidence of 0.27 and 0.07 cases/1000 admissions; 2006: 0.72 and 0.28 candidaemias/1000 admissions for C. parapsilosis sensu stricto and C. orthopsilosis, respectively). Moreover, the increasing trend in the number of C. parapsilosis sensu lato isolates observed in 2006 continued in the first 7 months of 2007 with a total of 29 C. parapsilosis sensu lato strains isolated. However, in 2007 the incidence of C. parapsilosis sensu stricto candidaemia decreased when compared with the 10 year period 1995–2005 (0.19 candidaemias/1000 admissions). On the other hand, 44.8% (13/29) of the C. parapsilosis sensu lato fungaemias diagnosed in 2007 were due to C. orthopsilosis. This fact showed that the increased incidence of C. orthopsilosis fungaemias that started in 2006 remained in 2007 (incidence of C. orthopsilosis fungaemia in 2007: 0.16 cases/1000 admissions). The six C. metapsilosis were isolated in 1995 (two strains), 1996 (one strain), 2001 (two strains) and 2006 (one strain) (Figure 1a).
Figure 1.
(a) Number of C. parapsilosis sensu lato species isolated by year. White bars represent C. parapsilosis sensu lato species. Black, grey and striped bars show the number of C. parapsilosis sensu stricto, C. orthopsilosis and C. metapsilosis isolated during each of the years, respectively. Continuous and broken horizontal lines represent the average number of C. parapsilosis sensu stricto (14.73) and C. orthopsilosis (3.82) isolated between 1995 and 2005, respectively. (b) C. parapsilosis sensu stricto (black triangles), C. orthopsilosis (white squares) and C. metapsilosis (black diamonds) isolated per year from paediatric (upper graph) and adult (lower graph) patients.
When the year of isolation was related to the patient's age, it was noted that the 79.8% of C. parapsilosis sensu lato strains isolated from paediatric patients (<15 years old) were obtained from 1995 to 2003. Moreover, 86.5% of the C. parapsilosis sensu lato fungaemias diagnosed from 2004 to July 2007 were found in adult patients (P = 0.01), demonstrating an important epidemiological change. When the distribution of the different C. parapsilosis sensu lato species during the years was analysed, there was an increasing trend in the percentage of C. orthopsilosis isolates in detriment of C. parapsilosis sensu stricto in both paediatric and adult populations (Figure 1b).
The clinical departments where each of the C. parapsilosis sensu lato strains was isolated were recorded. Overall, 67.2% of the strains were isolated in one of the intensive care units (ICUs) of La Fe University Hospital, showing no statistical differences between species (P = 0.98). C. parapsilosis sensu lato species were isolated most frequently from patients with fungaemia in the following departments: Surgical ICU (93 strains, 32.29%), Internal Medicine ICU (47, 16.32%), Newborn ICU (36, 12.50%), Internal Medicine (34, 11.81%), Surgery (26, 9.03%) and Paediatric ICU (20, 6.94%). C. parapsilosis sensu stricto was the most common species in all the departments included in the study. On the other hand, Paediatric ICU and Surgery were the departments where the highest proportions of C. orthopsilosis were isolated [7/20 (35%) and 8/26 (30.8%), respectively].
The underlying conditions of the patients with C. parapsilosis sensu lato fungaemia are shown in Table 2. Neurological (24 fungaemias, 23 in adults) and cardiovascular diseases (23 cases, 14 adults) were the most common conditions associated with C. parapsilosis sensu lato fungaemias. Among these groups of diseases, stroke and congenital heart diseases were the most commonly recorded syndromes. Also, it was found that 14 patients having C. parapsilosis fungaemias (11 C. parapsilosis sensu stricto and 3 C. orthopsilosis) had idiopathic thrombocytopenic purpura and all but one of these patients were adults.
Table 2.
Underlying conditions associated with C. parapsilosis sensu lato fungaemias
| Underlying condition | Number (%) of fungaemias due to |
||
|---|---|---|---|
| C. parapsilosis sensu stricto | C. orthopsilosis | C. metapsilosis | |
| Sepsisa | 20 (9.17) | 5 (7.25) | 0 |
| Neurological conditionsb | 19 (8.72) | 4 (5.80) | 1 (16.67) |
| Cardiovascular diseasesc | 14 (6.42) | 8 (11.59) | 1 (16.67) |
| Premature birth | 15 (6.88) | 7 (10.14) | 0 |
| Gastrointestinal diseasesd | 13 (5.96) | 5 (7.25) | 0 |
| Solid organ transplantatione | 11 (5.05) | 4 (5.80) | 0 |
| Severe traumaf | 12 (5.50) | 2 (2.90) | 1 (16.67) |
| Fever of unknown origing | 14 (6.42) | 1 (1.45) | 0 |
| Onco-haematological diseasesh | 14 (6.42) | 1 (1.45) | 0 |
| Cancer | 6 (2.75) | 7 (10.14) | 1 (16.67) |
| Idiopathic thrombocytopenic purpura | 11 (5.05) | 3 (4.35) | 0 |
| Pulmonary diseasesi | 7 (3.21) | 3 (4.35) | 0 |
| Kidney diseasesj | 6 (2.75) | 4 (5.80) | 0 |
| Bacterial pneumonia | 6 (2.75) | 1 (1.45) | 1 (16.67) |
| Burn patients | 3 (1.38) | 3 (4.35) | 0 |
| AIDS and other immunosuppressionk | 6 (2.75) | 0 | 0 |
| Autoimmune disordersl | 3 (1.38) | 0 | 1 (16.67) |
| Urinary tract infections | 3 (1.38) | 2 (2.90) | 0 |
| Extracorporeal surgery | 3 (1.38) | 1 (1.45) | 0 |
| No available data | 32 (14.68) | 8 (11.59) | 0 |
| Total | 218 (100.00) | 69 (100.00) | 6 (100.00) |
aNo other signs or symptoms were recorded.
bStroke, tetraplegia, hydrocephalus, hepatic encephalopathy subdural haematoma and cerebral haemorrhage.
cTetralogy of Fallot, valvulopathies and other congenital heart defects.
dAcute pancreatitis, peritonitis, short bowel syndrome and enteric fistulas.
eHeart, lung and renal transplant.
fPolytrauma and cranioencephalic trauma.
gFever was the only recorded sign.
hAcute and chronic lymphoid leukaemia, acute myeloid leukaemia, multiple myeloma and Hodgkin's lymphoma.
iCystic fibrosis, acute respiratory distress, acute respiratory failure and chronic obstructive pulmonary disease.
jAcute and chronic renal failure and patients on dialysis for other renal diseases.
kAIDS, corticotherapy and congenital immunosuppression.
lEvan's syndrome, systemic erythematous lupus and Graves–Basedow disease.
C. orthopsilosis fungaemias were associated mostly with cardiovascular diseases (eight cases, 11.59% of the C. orthopsilosis fungaemias), premature birth and cancer (seven cases each, 10.14%). Moreover, cancer was associated more commonly with C. orthopsilosis than with C. parapsilosis sensu stricto (10.1% of the C. orthopsilosis cases versus 2.75% of the C. parapsilosis sensu stricto fungaemias, P < 0.01, χ2 test). Also, all but one of the cancer patients harbouring C. orthopsilosis fungaemias were adults, while the majority of the C. parapsilosis sensu stricto fungaemias in cancer patients were diagnosed in paediatric patients. On the contrary, the majority (87.5%) of the patients with a cardiovascular disease and having a C. orthopsilosis fungaemia were paediatric patients, while only 16.7% (2/12) had a C. parapsilosis sensu stricto fungaemia.
Echinocandin susceptibility of the C. parapsilosis sensu lato species
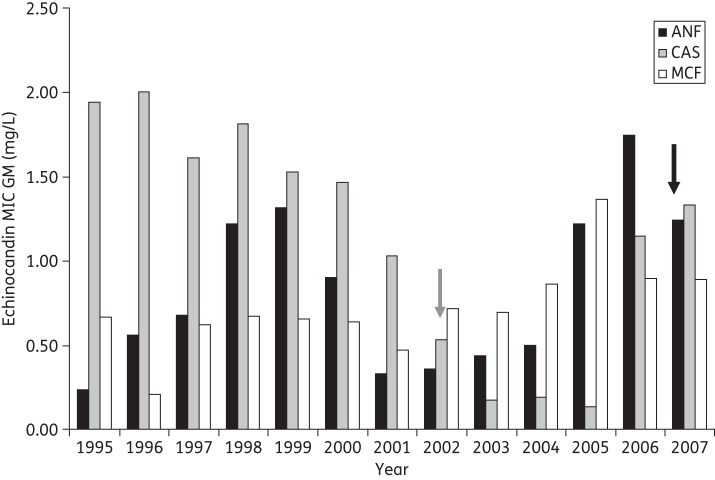
Wide MIC ranges were observed for the echinocandin-class drugs (0.06–4 mg/L for anidulafungin and caspofungin and 0.015–4 mg/L for micafungin) (Table 3). Overall, C. parapsilosis sensu lato species showed no statistically significant MIC differences between the three echinocandin drugs studied (P > 0.1). Our MIC data showed a shift in 2002, when caspofungin was introduced clinically at La Fe University Hospital (Figure 2). Prior to that year, C. parapsilosis sensu lato strains were more susceptible in vitro to micafungin than to anidulafungin and caspofungin, whereas after 2002 the isolates were more susceptible to caspofungin. When each species MIC value was analysed, it was apparent that C. metapsilosis was the species most susceptible to anidulafungin and micafungin in vitro (P < 0.001). On the other hand, there were no differences between C. parapsilosis sensu lato species when caspofungin mean MIC values were compared (P = 0.2). Similar conclusions were obtained when the MIC50 and MIC90 values for echinocandin drugs were evaluated. There were no differences between C. parapsilosis sensu lato species for caspofungin MIC50s, while C. metapsilosis showed the lowest MIC50s of anidulafungin and micafungin (P < 0.01).
Table 3.
Echinocandin MIC distribution among the C. parapsilosis sensu lato species using the CLSI M27-A3 document
| Species | Antifungal | n | GM | Isolates with an MIC (mg/L) ofa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 8.0 | ||||
| C. parapsilosis sensu lato | ANF | 287 | 0.74 | 4 | 5 | 52 | 86 | 52 | 80 | 9 | |||
| CAS | 287 | 0.87 | 1 | 50 | 3 | 20 | 94 | 118 | 1 | ||||
| MCF | 287 | 0.68 | 2 | 1 | 16 | 23 | 86 | 129 | 24 | 6 | |||
| C. parapsilosis sensu stricto | ANF | 213 | 0.844 | 2 | 33 | 64 | 38 | 68 | 8 | ||||
| CAS | 213 | 0.850 | 1 | 42 | 1 | 9 | 70 | 90 | |||||
| MCF | 213 | 0.829 | 2 | 1 | 5 | 64 | 115 | 21 | 5 | ||||
| C. metapsilosis | ANF | 6 | 0.085 | 4 | 1 | 1 | |||||||
| CAS | 6 | 1.000 | 1 | 1 | 1 | 3 | |||||||
| MCF | 6 | 0.173 | 1 | 2 | 1 | 2 | |||||||
| C. orthopsilosis | ANF | 68 | 0.604 | 2 | 18 | 22 | 14 | 12 | 1 | ||||
| CAS | 68 | 0.908 | 8 | 1 | 10 | 23 | 25 | 1 | |||||
| MCF | 68 | 0.405 | 13 | 17 | 20 | 14 | 3 | 1 | |||||
ANF, anidulafungin; CAS, caspofungin; MCF, micafungin.
aUnderlined numbers represent MIC50 and bold numbers represent MIC90.
Figure 2.
Trend of in vitro susceptibility to echinocandin drugs among C. parapsilosis sensu lato species over the study period (1995 and 2007). The graph shows the MIC GM of anidulafungin (ANF; black bars), caspofungin (CAS; grey bars) and micafungin (MCF; white bars). Arrows represent the year when caspofungin (grey arrow) and anidulafungin (black arrow) were introduced in La Fe University Hospital as therapeutic options.
The trends of in vitro resistance to echinocandin drugs among C. parapsilosis sensu lato over the study period were analysed and are shown in Figure 2. Overall, there were no significant differences in the micafungin MIC GMs from 1995 to 2007 (P > 0.05). On the other hand, anidulafungin MIC GMs showed a bimodal distribution, with higher MIC values in the strains isolated from 1998 to 2000 (anidulafungin MIC GMs for 1998, 1999 and 2000: 1.22, 1.31 and 0.90 mg/L, respectively) and from 2005 to 2007 (anidulafungin MIC GMs for 2005, 2006 and 2007: 1.22, 1.75 and 1.24 mg/L, respectively). However, a significant trend towards increased anidulafungin MIC values (P < 0.01) was observed from 1995 to 2007. Turning to caspofungin, a decrease in MIC values started to be evident in 2001 (caspofungin GM: 1.03 mg/L), reaching a minimum in 2005 with a GM of 0.13 mg/L. These MIC decreases were statistically significant when compared with earlier years and the 2 years following 2001 (P < 0.01).
When the echinocandin susceptibilities (MIC GMs) of the C. parapsilosis sensu lato species isolated from each of the La Fe University Hospital departments were compared, some differences were observed. For example, the caspofungin MIC GM for the strains isolated from the Oncology ward was 2.67-fold higher than the MIC GM for those isolated from the Paediatric department (caspofungin MIC GM Oncology 1.47 mg/L versus 0.55 mg/L for the Paediatric department). However, these differences were not statistically significant (P > 0.05).
At La Fe University Hospital's Medical ICU and Surgical ICU departments, caspofungin has been in use since its release to the market in 2002, and anidulafungin has been in use since 2007. Caspofungin usage at La Fe University Hospital ICU showed a 9.8- and 1.86-fold increase between 2002 and 2003 and between 2003 and 2004, respectively. On the other hand, in the Surgical ICU department caspofungin consumption increased more than 90-fold and 1.63-fold between 2002 and 2003 and between 2003 and 2004, respectively, but there was no significant increase from 2004 to 2006 (P = 0.2).
C. parapsilosis sensu lato FKS1 sequence
FKS1 gene fragments of C. parapsilosis sensu stricto, C. orthopsilosis and C. metapsilosis that include the hot spot regions were sequenced. All the C. parapsilosis sensu lato strains showed the characteristic proline to alanine substitution at Fks1p hot spot 1, linked with a reduced echinocandin susceptibility phenotype.35 Moreover, when Fks1p hot spot 2 was studied, all the C. parapsilosis sensu stricto and C. metapsilosis showed no amino acid substitutions. On the other hand, 94.2% of the C. orthopsilosis strains (65/69) showed a homozygous I758V substitution at Fks1p hot spot 2,35 while four C. orthopsilosis isolates showed a heterozygous isoleucine to valine substitution at the same position. The C. orthopsilosis isolates showing these heterozygous Fks1p substitutions showed no differences in echinocandin MIC and were not epidemiologically linked to one another.
Discussion
In 2005, the three distinct genotypic groups of C. parapsilosis15 were further separated into three different species.17 Since then, different reports have described the antifungal susceptibility and epidemiology of these newly defined species, anticipating their epidemiological importance. An important proportion of these reports included a small number of isolates obtained from different infection sites with little epidemiological data13,21,23,37,38 or selected from existing yeast collections.23,24,29,30,39 The C. parapsilosis sensu lato strains studied here were isolated consecutively from bloodstream infections during a 12 year period and represent all the C. parapsilosis sensu lato strains isolated at La Fe University Hospital. C. parapsilosis sensu lato species were the second most common Candida species isolated from bloodstream infections, as described before in Spain by other authors.6,7,25 However, the proportion of C. parapsilosis sensu lato species isolated from blood was higher in Valencia (38.8%) than in other Spanish or worldwide studies (ranging from 12.5% to 32.9%).2–7,23–25,32,37,40–47 On the other hand, the percentage of C. orthopsilosis causing bloodstream infections at La Fe University Hospital (23.54% of the C. parapsilosis sensu lato isolates) was one of the highest published so far,21,23–25,32,37,38,44,48 only surpassed by a Malaysian hospital, where 23.8% of the C. parapsilosis sensu lato species were C. orthopsilosis.39 However, in this last report, the authors studied 43 C. parapsilosis sensu lato strains selected randomly. Moreover, La Fe University Hospital showed 2.5-fold more C. orthopsilosis isolates than any of the Spanish centres analysed so far (Seville, 8.2%; Barcelona, 1.4%).25,38 Also, its incidence showed an important increase in the last 2 years of the study and in 2007 the incidence of C. orthopsilosis fungaemias reached its maximum, being comparable to that for C. parapsilosis sensu stricto. Similarly, Lockhart et al.24 reported a worldwide longitudinal increase in the incidence of C. orthopsilosis starting in 2005. In contrast, the prevalence of C. metapsilosis at La Fe University Hospital was comparable to that in previous Spanish and worldwide reports.21,23–25,32,38,39,48 This fact may be explained by the demonstrated lower virulence of this species.22,26
When the frequency of C. parapsilosis sensu lato fungaemias at each of the departments of La Fe University Hospital was analysed, it could be inferred that critically ill patients, especially those housed in the ICU, were the most susceptible to these infections. It is well known that numerous factors, such as immunosuppression, metabolic dysfunction, corticosteroids, cytotoxic and broad-spectrum antibiotic therapies, increase the risk of Candida spp. infections.1,49 Also, very old patients and individuals hospitalized in an ICU are predisposed to invasive candidiasis.2,3,8,50 The high frequency of C. parapsilosis sensu lato fungaemia in critically ill patients observed in this work could be due to the high affinity of these species for vascular devices, medical instrumentation and indwelling plastics.2,3,45,51–54
In this work there were three novel epidemiological findings to consider. The first is that almost 5% of the patients had thrombocytopenic purpura as the only symptom or sign before the fungaemia was diagnosed. It has been reported that thrombocytopenia is a marker for invasive candidiasis in low birth weight newborns.40 However, thrombocytopenic purpura has been described only rarely as a risk factor for Candida spp. fungaemia in adult patients,40,55,56 and never as a predisposing condition for C. parapsilosis fungaemia. Secondly, in 2003 an epidemiological shift was observed. Before that year, the paediatric population had the highest risk of having a C. parapsilosis sensu lato fungaemia, while the adult population had a higher chance from 2004 to 2007. Lastly, there was a differential association between C. parapsilosis sensu stricto and C. orthopsilosis in cancer and cardiology patients. Whether these differences had any association with patient management or treatment will require further studies.
Echinocandin MIC varied during the study period
We describe the in vitro echinocandin susceptibility of the C. parapsilosis sensu lato strains isolated at La Fe University Hospital in Valencia during a 12 year period. We confirmed that C. parapsilosis sensu lato showed a reduced echinocandin susceptibility phenotype and that all the strains studied showed the naturally occurring proline to alanine substitution at the end of the Fks1p hot spot 1, as described by our group.35 Moreover, we confirmed the existence of a characteristic isoleucine to valine amino acid substitution in hot spot 2 as a naturally occurring polymorphism in C. orthopsilosis, as previously described.35 Moreover, four C. orthopsilosis showed a heterozygous isoleucine to valine substitution at this position, which was reported earlier by Chen et al.57 These authors described the same isoleucine to valine substitution in five of their C. metapsilosis isolates, but our six strains showed no amino acid substitution when compared with C. parapsilosis sensu stricto or C. albicans Fks1p hot spot 2. Despite the similar FKS1 genotype in all the strains included in this work, a wide range of echinocandin MIC values were observed. This indicates that factors other than Fks1p affinity for echinocandin drugs may play a role in determining MIC values in C. parapsilosis sensu lato. This broad range of echinocandin MICs has been observed by other authors.21,24,25,27,32 In contrast to our MIC data, Diekema et al.,27 Gomez-Lopez et al.25 and Lockhart et al.24 showed that C. parapsilosis sensu lato is more susceptible to caspofungin than to the other echinocandins. However, when dissecting our MIC data by year, we conclude that our results are similar to those of other author's, but only after 2002 (Figure 2). During that year, caspofungin started to be used clinically at La Fe University Hospital. It is difficult to explain this phenomenon since the use of caspofungin was linked with an increased incidence of C. parapsilosis sensu lato candidaemia,58 but not with changes in MIC values. When we analysed the caspofungin consumption at the hospital, we conclude that there was no straightforward relationship between caspofungin usage and caspofungin MIC GMs, since the lowest MIC values were seen when this echinocandin drug use increased. However, we can speculate on the basis of the data in Table 4 that the consequences of increased use of caspofungin were observed after 3 years, when the caspofungin MIC GM had increased 10-fold. It is well known that increased MIC and the selection of less susceptible strains are logical consequences of the use of any antifungal drug.59
Table 4.
Echinocandin MIC and dosing variation between 1995 and 2007
| Year |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | |
| ANF | |||||||||||||
| MIC GM | 0.23 | 0.56 | 0.68 | 1.22 | 1.31 | 0.90 | 0.33 | 0.36 | 0.44 | 0.50 | 1.22 | 1.75 | 1.24 |
| DDDs | |||||||||||||
| medical ICU | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.010 |
| surgical ICU | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.000 |
| CAS | |||||||||||||
| MIC GM | 1.94 | 2.00 | 1.61 | 1.81 | 1.52 | 1.46 | 1.03 | 0.53 | 0.17 | 0.19 | 0.13 | 1.15 | 1.33 |
| DDDs | |||||||||||||
| medical ICU | NA | NA | NA | NA | NA | NA | NA | 0.236 | 2.314 | 4.298 | 4.347 | 4.845 | 1.286 |
| surgical ICU | NA | NA | NA | NA | NA | NA | NA | 0.054 | 4.981 | 8.081 | 11.457 | 4.249 | 4.985 |
| MCF | |||||||||||||
| MIC GM | 0.66 | 0.21 | 0.62 | 0.67 | 0.65 | 0.64 | 0.47 | 0.72 | 0.69 | 0.86 | 1.37 | 0.90 | 0.89 |
ANF, anidulafungin; CAS, caspofungin; MCF, micafungin; DDDs, defined daily doses (doses per 100 patients per day); NA, not available.
In conclusion, this work reinforces the notion that C. parapsilosis sensu lato is an important hospital pathogen and that C. parapsilosis sensu stricto is the predominant species of this complex. We showed that C. orthopsilosis is increasing in frequency and could become an important health problem in the near future. Also, this work provides information about the echinocandin susceptibility of C. orthopsilosis and C. metapsilosis.
Funding
This work was supported by a grant to D. S. P. from NIH (AI069397).
Transparency declarations
D. S. P. is a shareholder in Merck, has served on advisory boards for Merck, Pfizer and Astellas, has received research funding from Merck, Pfizer and Astellas, and has been an invited speaker for Merck, Pfizer, Astellas, AstraZeneca and Novartis. All other authors: none to declare.
Acknowledgements
We are grateful to Astellas, Merck and Pfizer for providing micafungin, caspofungin and anidulafungin pure substances.
References
- 1.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almirante B, Rodriguez D, Park BJ, et al. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2005;43:1829–35. doi: 10.1128/JCM.43.4.1829-1835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almirante B, Rodriguez D, Cuenca-Estrella M, et al. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2006;44:1681–5. doi: 10.1128/JCM.44.5.1681-1685.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brito LR, Guimaraes T, Nucci M, et al. Clinical and microbiological aspects of candidemia due to Candida parapsilosis in Brazilian tertiary care hospitals. Med Mycol. 2006;44:261–6. doi: 10.1080/13693780500421476. [DOI] [PubMed] [Google Scholar]
- 5.Cuenca-Estrella M, Rodero L, Garcia-Effron G, et al. Antifungal susceptibilities of Candida spp. isolated from blood in Spain and Argentina, 1996–1999. J Antimicrob Chemother. 2002;49:981–7. doi: 10.1093/jac/dkf060. [DOI] [PubMed] [Google Scholar]
- 6.Peman J, Canton E, Orero A, et al. [Epidemiology of candidemia in Spain–Multicenter study] Rev Iberoam Micol. 2002;19:30–5. [PubMed] [Google Scholar]
- 7.Peman J, Canton E, Gobernado M. Epidemiology and antifungal susceptibility of Candida species isolated from blood: results of a 2-year multicentre study in Spain. Eur J Clin Microbiol Infect Dis. 2005;24:23–30. doi: 10.1007/s10096-004-1267-5. [DOI] [PubMed] [Google Scholar]
- 8.Pfaller MA, Diekema DJ, Gibbs DL, et al. Geographic and temporal trends in isolation and antifungal susceptibility of Candida parapsilosis: a global assessment from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J Clin Microbiol. 2008;46:842–9. doi: 10.1128/JCM.02122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandven P. Epidemiology of candidemia. Rev Iberoam Micol. 2000;17:73–81. [PubMed] [Google Scholar]
- 10.Cassone A, De Bernardis F, Pontieri E, et al. Biotype diversity of Candida parapsilosis and its relationship to the clinical source and experimental pathogenicity. J Infect Dis. 1995;171:967–75. doi: 10.1093/infdis/171.4.967. [DOI] [PubMed] [Google Scholar]
- 11.Kato M, Ozeki M, Kikuchi A, et al. Phylogenetic relationship and mode of evolution of yeast DNA topoisomerase II gene in the pathogenic Candida species. Gene. 2001;272:275–81. doi: 10.1016/s0378-1119(01)00526-1. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann PF, Lin D, Lasker BA. Genotypic identification and characterization of species and strains within the genus Candida by using random amplified polymorphic DNA. J Clin Microbiol. 1992;30:3249–54. doi: 10.1128/jcm.30.12.3249-3254.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin D, Wu LC, Rinaldi MG, et al. Three distinct genotypes within Candida parapsilosis from clinical sources. J Clin Microbiol. 1995;33:1815–21. doi: 10.1128/jcm.33.7.1815-1821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nosek J, Tomaska L, Rycovska A, et al. Mitochondrial telomeres as molecular markers for identification of the opportunistic yeast pathogen Candida parapsilosis. J Clin Microbiol. 2002;40:1283–9. doi: 10.1128/JCM.40.4.1283-1289.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy B, Meyer SA. Confirmation of the distinct genotype groups within the form species Candida parapsilosis. J Clin Microbiol. 1998;36:216–8. doi: 10.1128/jcm.36.1.216-218.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherer S, Stevens DA. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987;25:675–9. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavanti A, Davidson AD, Gow NA, et al. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J Clin Microbiol. 2005;43:284–92. doi: 10.1128/JCM.43.1.284-292.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varga I, Soczo G, Kardos G, et al. Comparison of killing activity of caspofungin against Candida parapsilosis, Candida orthopsilosis and Candida metapsilosis. J Antimicrob Chemother. 2008;62:1466–8. doi: 10.1093/jac/dkn403. [DOI] [PubMed] [Google Scholar]
- 19.van Asbeck E, Clemons KV, Martinez M, et al. Significant differences in drug susceptibility among species in the Candida parapsilosis group. Diagn Microbiol Infect Dis. 2008;62:106–9. doi: 10.1016/j.diagmicrobio.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Szabo Z, Szilagyi J, Tavanti A, et al. In vitro efficacy of 5 antifungal agents against Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis as determined by time-kill methodology. Diagn Microbiol Infect Dis. 2009;64:283–8. doi: 10.1016/j.diagmicrobio.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Silva AP, Miranda IM, Lisboa C, et al. Prevalence, distribution, and antifungal susceptibility profiles of Candida parapsilosis, C. orthopsilosis, and C. metapsilosis in a tertiary care hospital. J Clin Microbiol. 2009;47:2392–7. doi: 10.1128/JCM.02379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orsi CF, Colombari B, Blasi E. Candida metapsilosis as the least virulent member of the ‘C. parapsilosis’ complex. Med Mycol. 2010;48:1024–33. doi: 10.3109/13693786.2010.489233. [DOI] [PubMed] [Google Scholar]
- 23.Mirhendi H, Bruun B, Schonheyder HC, et al. Molecular screening for Candida orthopsilosis and Candida metapsilosis among Danish Candida parapsilosis group blood culture isolates: proposal of a new RFLP profile for differentiation. J Med Microbiol. 2010;59:414–20. doi: 10.1099/jmm.0.017293-0. [DOI] [PubMed] [Google Scholar]
- 24.Lockhart SR, Messer SA, Pfaller MA, et al. Geographic distribution and antifungal susceptibility of the newly described species Candida orthopsilosis and Candida metapsilosis in comparison to the closely related species Candida parapsilosis. J Clin Microbiol. 2008;46:2659–64. doi: 10.1128/JCM.00803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Lopez A, Alastruey-Izquierdo A, Rodriguez D, et al. Prevalence and susceptibility profile of Candida metapsilosis and Candida orthopsilosis: results from population-based surveillance of candidemia in Spain. Antimicrob Agents Chemother. 2008;52:1506–9. doi: 10.1128/AAC.01595-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gacser A, Schafer W, Nosanchuk JS, et al. Virulence of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis in reconstituted human tissue models. Fungal Genet Biol. 2007;44:1336–41. doi: 10.1016/j.fgb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Diekema DJ, Messer SA, Boyken LB, et al. In vitro activity of seven systemically active antifungal agents against a large global collection of rare Candida species as determined by CLSI broth microdilution methods. J Clin Microbiol. 2009;47:3170–7. doi: 10.1128/JCM.00942-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canton E, Espinel-Ingroff A, Peman J, et al. In vitro fungicidal activities of echinocandins against Candida metapsilosis, C. orthopsilosis, and C. parapsilosis evaluated by time-kill studies. Antimicrob Agents Chemother. 2010;54:2194–7. doi: 10.1128/AAC.01538-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavanti A, Hensgens LA, Ghelardi E, et al. Genotyping of Candida orthopsilosis clinical isolates by amplification fragment length polymorphism reveals genetic diversity among independent isolates and strain maintenance within patients. J Clin Microbiol. 2007;45:1455–62. doi: 10.1128/JCM.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asadzadeh M, Ahmad S, Al Sweih N, et al. Rapid molecular differentiation and genotypic heterogeneity among Candida parapsilosis and Candida orthopsilosis strains isolated from clinical specimens in Kuwait. J Med Microbiol. 2009;58:745–52. doi: 10.1099/jmm.0.008235-0. [DOI] [PubMed] [Google Scholar]
- 31.Sabino R, Sampaio P, Rosado L, et al. New polymorphic microsatellite markers able to distinguish among Candida parapsilosis sensu stricto isolates. J Clin Microbiol. 2010;48:1677–82. doi: 10.1128/JCM.02151-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goncalves SS, Amorim CS, Nucci M, et al. Prevalence rates and antifungal susceptibility profiles of the Candida parapsilosis species complex: results from a nationwide surveillance of candidaemia in Brazil. Clin Microbiol Infect. 2010;16:885–7. doi: 10.1111/j.1469-0691.2009.03020.x. [DOI] [PubMed] [Google Scholar]
- 33.van Asbeck EC, Clemons KV, Markham AN, et al. Correlation of restriction fragment length polymorphism genotyping with internal transcribed spacer sequence, randomly amplified polymorphic DNA and multilocus sequence groupings for Candida parapsilosis. Mycoses. 2009;52:493–8. doi: 10.1111/j.1439-0507.2008.01649.x. [DOI] [PubMed] [Google Scholar]
- 34.White TJ, Bruns TD, Lee SB, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al., editors. PCR Protocols: A Guide to Methods and Applications. San Diego, CA, USA: Academic Press Inc.; 1990. pp. 315–22. [Google Scholar]
- 35.Garcia-Effron G, Katiyar SK, Park S, et al. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Agents Chemother. 2008;52:2305–12. doi: 10.1128/AAC.00262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard M27-A3. Wayne, PA, USA: CLSI; 2008. [Google Scholar]
- 37.Kocsube S, Toth M, Vagvolgyi C, et al. Occurrence and genetic variability of Candida parapsilosis sensu lato in Hungary. J Med Microbiol. 2007;56:190–5. doi: 10.1099/jmm.0.46838-0. [DOI] [PubMed] [Google Scholar]
- 38.de Toro M, Torres MJ, Maite R, et al. Characterization of Candida parapsilosis complex isolates. Clin Microbiol Infect. 2011;17:418–24. doi: 10.1111/j.1469-0691.2010.03302.x. [DOI] [PubMed] [Google Scholar]
- 39.Tay ST, Na SL, Chong J. Molecular differentiation and antifungal susceptibilities of Candida parapsilosis isolated from patients with bloodstream infections. J Med Microbiol. 2009;58:185–91. doi: 10.1099/jmm.0.004242-0. [DOI] [PubMed] [Google Scholar]
- 40.Torres CS, Dupla AM, Perez DR, et al. [Nosocomial Candida infections and thrombocytopenia in very low birth weight newborns] An Pediatr (Barc) 2007;67:544–7. doi: 10.1016/s1695-4033(07)70801-9. [DOI] [PubMed] [Google Scholar]
- 41.San Miguel LG, Cobo J, Otheo E, et al. Secular trends of candidemia in a large tertiary-care hospital from 1988 to 2000: emergence of Candida parapsilosis. Infect Control Hosp Epidemiol. 2005;26:548–52. doi: 10.1086/502582. [DOI] [PubMed] [Google Scholar]
- 42.Marco F, Danes C, Almela M, et al. Trends in frequency and in vitro susceptibilities to antifungal agents, including voriconazole and anidulafungin, of Candida bloodstream isolates. Results from a six-year study (1996–2001) Diagn Microbiol Infect Dis. 2003;46:259–64. doi: 10.1016/s0732-8893(03)00086-5. [DOI] [PubMed] [Google Scholar]
- 43.Lopez Sastre JB, Coto Cotallo GD, Fernandez CB. Neonatal invasive candidiasis: a prospective multicenter study of 118 cases. Am J Perinatol. 2003;20:153–63. doi: 10.1055/s-2003-40008. [DOI] [PubMed] [Google Scholar]
- 44.Thierry G, Morio F, Le Pape P, et al. [Prevalence of Candida parapsilosis, C. orthopsilosis and C. metapsilosis in candidemia over a 5-year period at Nantes hospital and in vitro susceptibility to three echinocandins by E-test®] Pathol Biol (Paris) 2011;59:52–6. doi: 10.1016/j.patbio.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Girmenia C, Martino P, De Bernardis F, et al. Rising incidence of Candida parapsilosis fungemia in patients with hematologic malignancies: clinical aspects, predisposing factors, and differential pathogenicity of the causative strains. Clin Infect Dis. 1996;23:506–14. doi: 10.1093/clinids/23.3.506. [DOI] [PubMed] [Google Scholar]
- 46.Cisterna R, Ezpeleta G, Telleria O, et al. Nationwide sentinel surveillance of bloodstream Candida infections in 40 tertiary care hospitals in Spain. J Clin Microbiol. 2010;48:4200–6. doi: 10.1128/JCM.00920-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Duran MT, Velasco D, Canle D, et al. [Antifungal susceptibility of Candida spp. isolates from blood cultures in a five-year period (1997–2001)] Enferm Infecc Microbiol Clin. 2003;21:488–92. [PubMed] [Google Scholar]
- 48.Paugam A, Baixench MT, Taieb F, et al. [Emergence of Candida parapsilosis candidemia at Cochin hospital. Characterization of isolates and search for risk factors.] Pathol Biol (Paris) 2011;59:44–7. doi: 10.1016/j.patbio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Segal BH, Kwon-Chung J, Walsh TJ, et al. Immunotherapy for fungal infections. Clin Infect Dis. 2006;42:507–15. doi: 10.1086/499811. [DOI] [PubMed] [Google Scholar]
- 50.Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3:685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 51.Kuhn DM, Mikherjee PK, Clark TA, et al. Candida parapsilosis characterization in an outbreak setting. Emerg Infect Dis. 2004;10:1074–81. doi: 10.3201/eid1006.030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levin AS, Costa SF, Mussi NS, et al. Candida parapsilosis fungemia associated with implantable and semi-implantable central venous catheters and the hands of healthcare workers. Diagn Microbiol Infect Dis. 1998;30:243–9. doi: 10.1016/s0732-8893(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 53.Safdar A, Perlin DS, Armstrong D. Hematogenous infections due to Candida parapsilosis: changing trends in fungemic patients at a comprehensive cancer center during the last four decades. Diagn Microbiol Infect Dis. 2002;44:11–6. doi: 10.1016/s0732-8893(02)00423-6. [DOI] [PubMed] [Google Scholar]
- 54.Weems JJ., Jr. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin Infect Dis. 1992;14:756–66. doi: 10.1093/clinids/14.3.756. [DOI] [PubMed] [Google Scholar]
- 55.Pavlovsky M, Weinstein R. Thrombotic thrombocytopenic purpura following coronary artery bypass graft surgery: prospective observations of an emerging syndrome. J Clin Apher. 1997;12:159–64. doi: 10.1002/(sici)1098-1101(1997)12:4<159::aid-jca1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 56.Safdar A, van Rhee F, Henslee-Downey JP, et al. Candida glabrata and Candida krusei fungemia after high-risk allogeneic marrow transplantation: no adverse effect of low-dose fluconazole prophylaxis on incidence and outcome. Bone Marrow Transplant. 2001;28:873–8. doi: 10.1038/sj.bmt.1703252. [DOI] [PubMed] [Google Scholar]
- 57.Chen YC, Lin YH, Chen KW, et al. Molecular epidemiology and antifungal susceptibility of Candida parapsilosis sensu stricto, Candida orthopsilosis, and Candida metapsilosis in Taiwan. Diagn Microbiol Infect Dis. 2010;68:284–92. doi: 10.1016/j.diagmicrobio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Forrest GN, Weekes E, Johnson JK. Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J Infect. 2008;56:126–9. doi: 10.1016/j.jinf.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 59.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]