Abstract
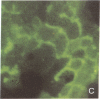
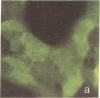
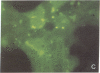
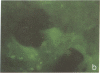
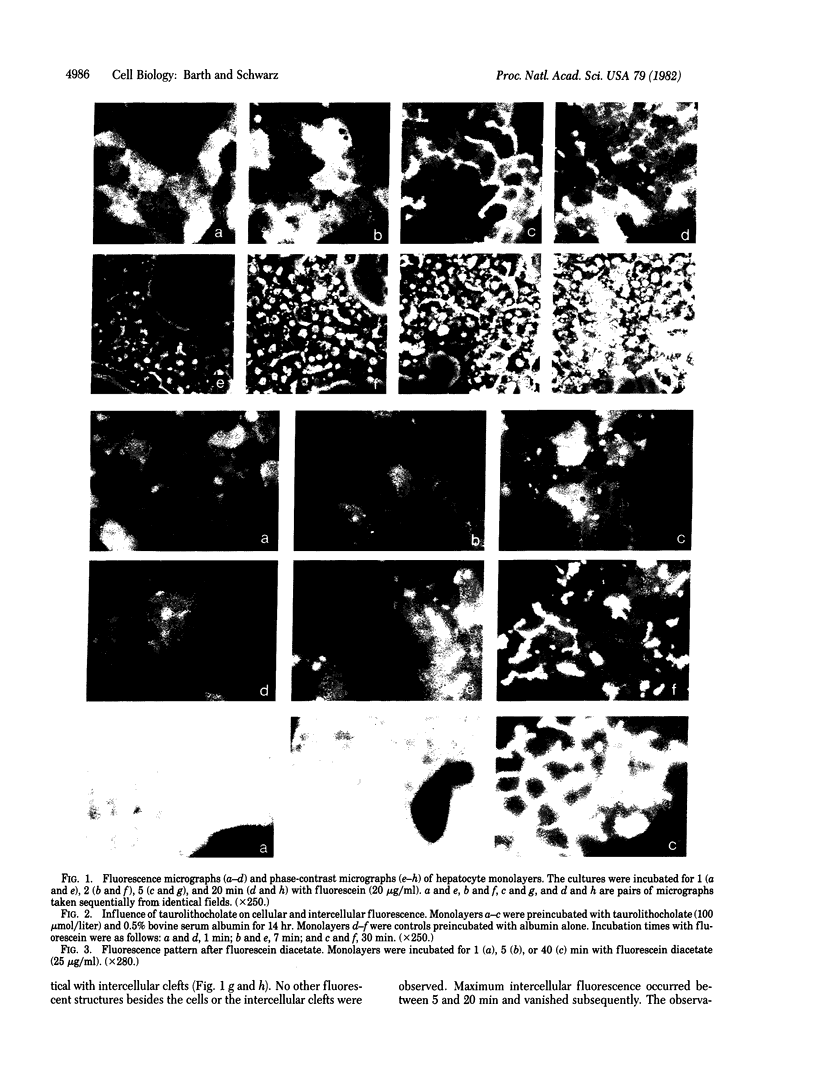
The rat liver in vivo transfers bile salts, proteins, and dyes from blood into bile. It is the purpose of this communication to demonstrate the maintenance of this transcellular transport in cultured adult rat hepatocytes. Two minutes after adding fluorescein (20 microgram/ml) to the culture medium, maximal cellular fluorescence was observed through the fluorescence microscope. Subsequently, intercellular clefts showed a steadily increasing fluorescence with a maximum between 5 and 20 min, resulting in a brightly fluorescent network of intercellular gaps. The following observations are taken as evidence that these findings reflect cellular uptake and canalicular secretion of the dye. First, the same sequence of observations was made upon addition of fluorescein diacetate (a nonfluorescent precursor of fluorescein), proving that the compound had been taken up and metabolized in the cells to fluorescein before secretion into intercellular clefts. Second, preincubation of the monolayers with the cholestatic bile salt taurolithocholate (100 mumol/liter) suppressed almost completely intercellular but not cellular fluorescence. It is concluded that hepatocytes in culture show a functional polarity permitting the transcellular transport of substances bound for biliary secretion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwen J., Gallhai-Atchard J. J. A method of maintaining parenchymal cells from adult rat liver in vitro. J Cell Sci. 1972 Jul;11(1):249–260. doi: 10.1242/jcs.11.1.249. [DOI] [PubMed] [Google Scholar]
- Barth C. A., Willershausen B., Walther B., Weis E. E. Morphology and metabolism of adult rat hepatocytes in primary culture. Hoppe Seylers Z Physiol Chem. 1980 Jul;361(7):1017–1027. doi: 10.1515/bchm2.1980.361.2.1017. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E., Meyer U. A. Parenchymal cells from adult rat liver in nonproliferating monolayer culture. I. Functional studies. J Cell Biol. 1973 Dec;59(3):722–734. doi: 10.1083/jcb.59.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney R. J., Becker J. E., Walker P. R., Potter V. R. Primary monolayer cultures of adult rat liver parenchymal cells suitable for study of the regulation of enzyme synthesis. In Vitro. 1974 May-Jun;9(6):399–413. doi: 10.1007/BF02615992. [DOI] [PubMed] [Google Scholar]
- Boyer J. L. New concepts of mechanisms of hepatocyte bile formation. Physiol Rev. 1980 Apr;60(2):303–326. doi: 10.1152/physrev.1980.60.2.303. [DOI] [PubMed] [Google Scholar]
- Bruni C., Porter K. R. The Fine Structure of the Parenchymal Cell of the Normal Rat Liver: I. General Observations. Am J Pathol. 1965 May;46(5):691–755. [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Ehrenfeld J., Meza I., Martínez-Palomo A. Structural and functional membrane polarity in cultured monolayers of MDCK cells. J Membr Biol. 1980;52(2):147–159. doi: 10.1007/BF01869120. [DOI] [PubMed] [Google Scholar]
- HANZON V. Liver cell secretion under normal and pathologic conditions studied by fluorescence microscopy on living rats. Acta Physiol Scand Suppl. 1952;28(101):1–268. [PubMed] [Google Scholar]
- Iype P. T. Cultures from adult rat liver cells. I. Establishment of monolayer cell-cultures from normal liver. J Cell Physiol. 1971 Oct;78(2):281–288. doi: 10.1002/jcp.1040780217. [DOI] [PubMed] [Google Scholar]
- Javitt N. B., Emerman S. Effect of sodium taurolithocholate on bile flow and bile acid exeretion. J Clin Invest. 1968 May;47(5):1002–1014. doi: 10.1172/JCI105790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakis G., Yousef I. M. Pathogenesis of lithocholate- and taurolithocholate-induced intrahepatic cholestasis in rats. Gastroenterology. 1978 Oct;75(4):595–607. [PubMed] [Google Scholar]
- King J. E., Schoenfield L. J. Cholestasis induced by sodium taurolithocholate in isolated hamster liver. J Clin Invest. 1971 Nov;50(11):2305–2312. doi: 10.1172/JCI106728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden T. J., Schwarz, Boyer J. L. Scanning electron microscopy of the rat liver. Studies of the effect of taurolithocholate and other models of cholestasis. Gastroenterology. 1975 Sep;69(3):724–738. [PubMed] [Google Scholar]
- Lin R. C., Snodgrass P. J. Primary culture of normal adult rat liver cells which maintain stable urea cycle enzymes. Biochem Biophys Res Commun. 1975 May 19;64(2):725–734. doi: 10.1016/0006-291x(75)90380-0. [DOI] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner F., Javitt N. B. Morphologic changes in hamster liver during intrahepatic cholestasis induced by taurolithocholate. Lab Invest. 1966 Nov;15(11):1783–1792. [PubMed] [Google Scholar]
- Schwarz L. R., Barth C. A. Taurocholate uptake by adult rat hepatocytes in primary culture. Hoppe Seylers Z Physiol Chem. 1979 Aug;360(8):1117–1120. [PubMed] [Google Scholar]
- Wanson J. C., Drochmans P., Mosselmans R., Ronveaux M. F. Adult rat hepatocytes in primary monolayer culture. Ultrastructural characteristics of intercellular contacts and cell membrane differentiations. J Cell Biol. 1977 Sep;74(3):858–877. doi: 10.1083/jcb.74.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]