Abstract
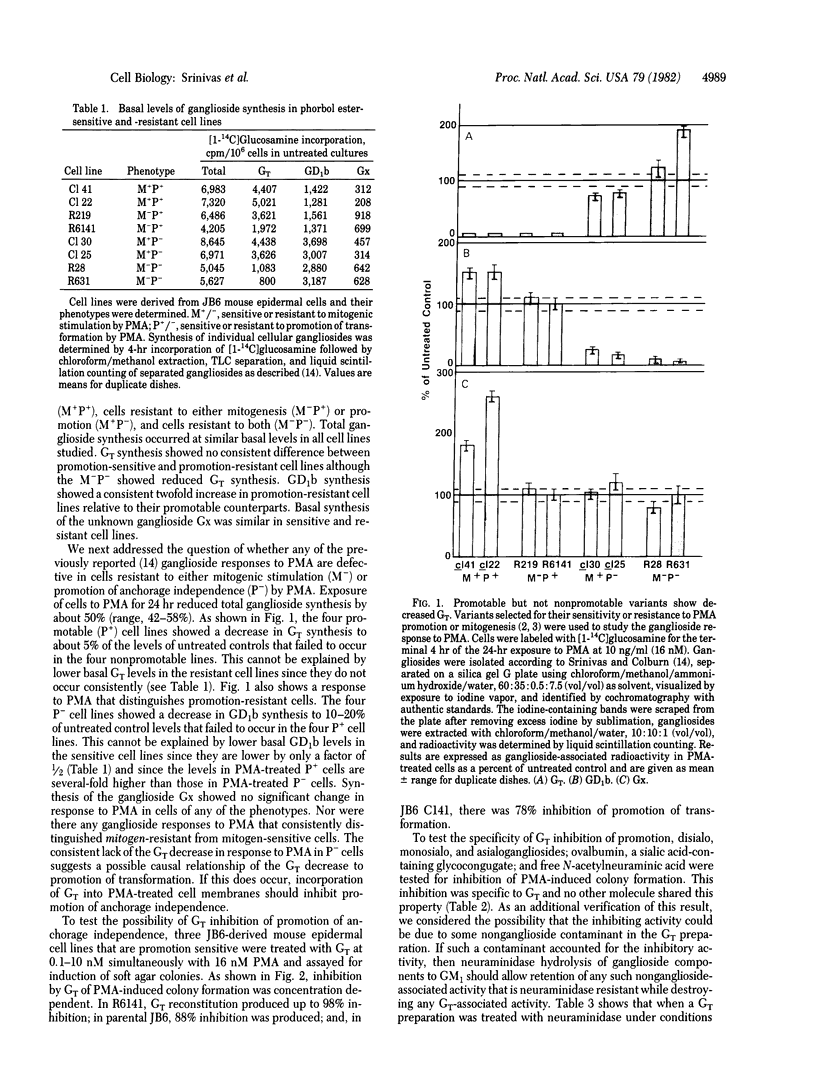
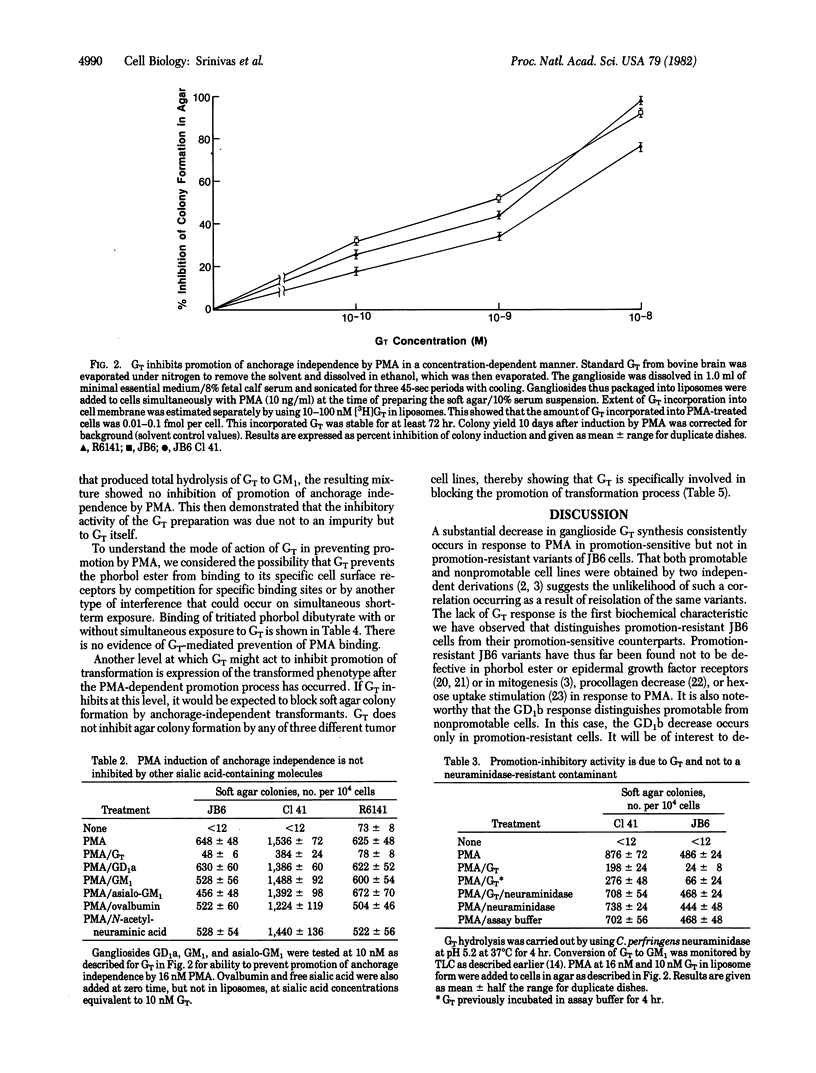
JB6 mouse epidermal cells shift irreversibly to tumor cell phenotype (anchorage independence and tumorigenicity) on treatment with phorbol esters and other tumor promoters. Exposure to phorbol 12-myristate 13-acetate (PMA) decreased the de novo synthesis of trisialoganglioside (GT) in these "promotable" JB6 cells to 5-10% of that of untreated cells. The GT decrease occurred consistently in promotion-sensitive cells and not in promotion-resistant variants. Insertion of GT into membranes of PMA-treated cells inhibited PMA promotion of transformation as measured by agar colony induction. This ability to inhibit promotion of transformation is specific to GT and is not shared by other sialoglycoconjugates, including gangliosides GM1, GD1a, and asialo-GM1. The mechanism of the blocking activity of GT must be distal to the binding of PMA to its receptors, as exogenously added GT does not inhibit specific binding of tritiated phorbol diester. GT is unable to block agar colony formation by transformed cell lines, showing that its level of action is at induction of the transformed phenotype rather than at expression of it.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R. O., Fishman P. H. Biosynthesis of glycolipids in virus-transformed cells. Biochim Biophys Acta. 1974 Sep 9;355(2):121–148. doi: 10.1016/0304-419x(74)90001-8. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Bruegge W. F., Bates J. R., Gray R. H., Rossen J. D., Kelsey W. H., Shimada T. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res. 1978 Mar;38(3):624–634. [PubMed] [Google Scholar]
- Colburn N. H., Former B. F., Nelson K. A., Yuspa S. H. Tumour promoter induces anchorage independence irreversibly. Nature. 1979 Oct 18;281(5732):589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Koehler B. A., Nelson K. J. A cell culture assay for tumor-promoter-dependent progression toward neoplastic phenotype: detection of tumor promoters and promotion inhibitors. Teratog Carcinog Mutagen. 1980;1(1):87–96. doi: 10.1002/tcm.1770010109. [DOI] [PubMed] [Google Scholar]
- Colburn N. H. Tumor promoter produces anchorage independence in mouse epidermal cells by an induction mechanism. Carcinogenesis. 1980;1(11):951–954. doi: 10.1093/carcin/1.11.951. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Wendel E. J., Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Dion L. D., De Luca L. M., Colburn N. H. Phorbol ester-induced anchorage independence and its antagonism by retinoic acid correlates with altered expression of specific glycoproteins. Carcinogenesis. 1981;2(10):951–958. doi: 10.1093/carcin/2.10.951. [DOI] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
- Fisher P. B., Cogan U., Horowitz A. D., Schachter D., Weinstein I. B. TPA-resistance in Friend erythroleukemia cells: role of membrane lipid fluidity. Biochem Biophys Res Commun. 1981 May 15;100(1):370–376. doi: 10.1016/s0006-291x(81)80106-4. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Structures and organization of cell surface glycolipids dependency on cell growth and malignant transformation. Biochim Biophys Acta. 1975 Mar 20;417(1):55–89. doi: 10.1016/0304-419x(75)90008-6. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Langenbach R., Kennedy S. Gangliosides and their cell density-dependent changes in control and chemically transformed C3H/10T1/2 cells. Exp Cell Res. 1978 Mar 15;112(2):361–372. doi: 10.1016/0014-4827(78)90219-7. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Membrane effects of tumor promoters: stimulation of sugar uptake in mammalian cell cultures. J Cell Physiol. 1979 Jun;99(3):451–460. doi: 10.1002/jcp.1040990319. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978 Oct 20;202(4365):313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- Moss J., Fishman P. H., Manganiello V. C., Vaughan M., Brady R. O. Functional incorporation of ganglioside into intact cells: induction of choleragen responsiveness. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1034–1037. doi: 10.1073/pnas.73.4.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Robfogel M. W., Spataro A. C., Bosmann H. B., Morgan H. R. Neuraminidase activity and cell surface sialic acid turnover in Rous sarcoma virus transformed chick embryo fibroblasts. Cancer Biochem Biophys. 1981;5(3):141–146. [PubMed] [Google Scholar]
- Rohrschneider L. R., Boutwell R. K. The early stimulation of phospholipid metabolism by 12-0-tetradecanoyl-phorbol-13-acetate and its specificity for tumor promotion. Cancer Res. 1973 Aug;33(8):1945–1952. [PubMed] [Google Scholar]
- Seligmann B. E., Gallin J. I. Use of lipophilic probes of membrane potential to assess human neutrophil activation. Abnormality in chronic granulomatous disease. J Clin Invest. 1980 Sep;66(3):493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharom F. J., Barratt D. G., Thede A. E., Grant C. W. Glycolipids in model membranes. Spin label and freeze-etch studies. Biochim Biophys Acta. 1976 Dec 2;455(2):485–492. doi: 10.1016/0005-2736(76)90319-9. [DOI] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Solanki V., Slaga T. J., Callaham M., Huberman E. Down regulation of specific binding of [20-3H]phorbol 12,13-dibutyrate and phorbol ester-induced differentiation of human promyelocytic leukemia cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1722–1725. doi: 10.1073/pnas.78.3.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas L., Colburn N. H. Ganglioside changes induced by tumor promoters in promotable JB6 mouse epidermal cells: antagonism by an antipromoter. J Natl Cancer Inst. 1982 Mar;68(3):469–473. [PubMed] [Google Scholar]
- Süss R., Kinzel V., Kreibich G. Cocarcinogenic croton oil factor A1 stimulates lipid synthesis in cell cultures. Experientia. 1971 Jan 15;27(1):46–47. doi: 10.1007/BF02137733. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Nissley S. P., Rechler M. M. MSA and EGF receptors on sarcoma virus transformed cells and human fibrosarcoma cells in culture. Nature. 1977 Jun 9;267(5611):526–528. doi: 10.1038/267526a0. [DOI] [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]
- Yogeeswaran G. Incorporation of asialo GM2 and gangliosides in cell surface of cultured metastatic and nonmetastatic BALB/3T3 cell lines: subcutaneous tumor cell take. J Natl Cancer Inst. 1981 Feb;66(2):303–310. [PubMed] [Google Scholar]



