Abstract
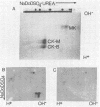
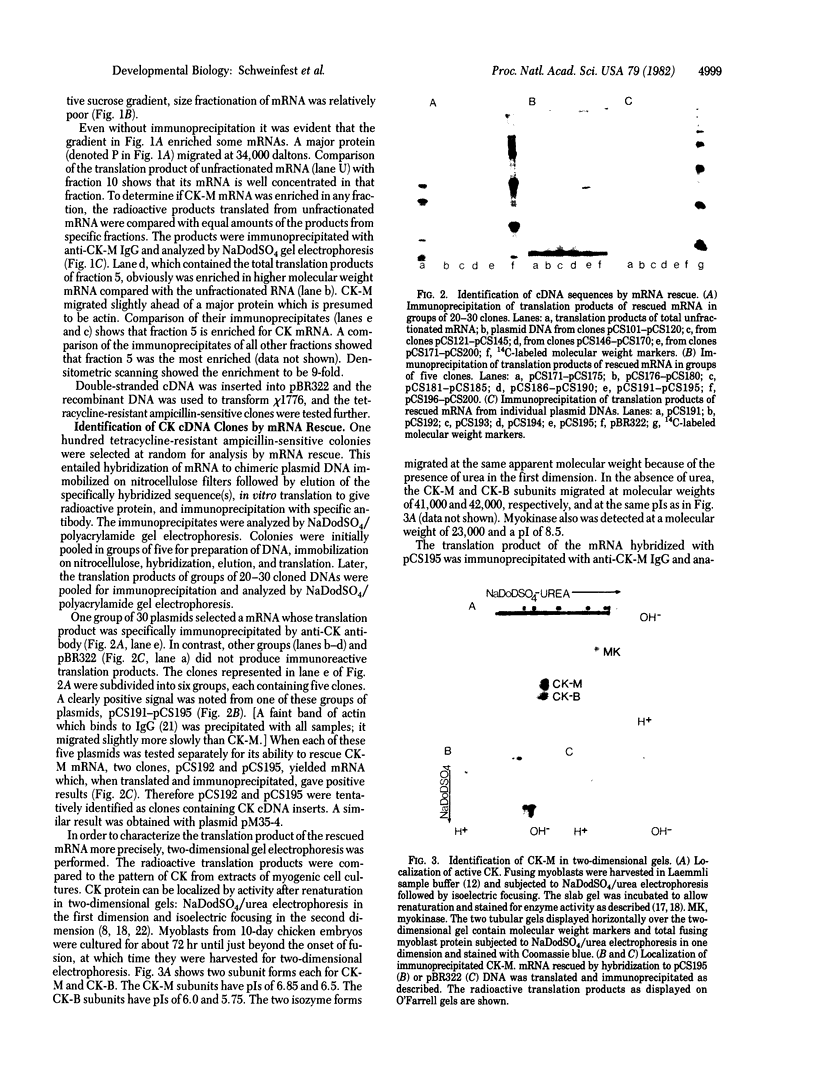
We have cloned and identified a DNA sequence complementary to the mRNA of creatine kinase (CK) isozyme M, although the mRNA is a minor species of the total mRNA in developing myoblasts. Poly(A)+RNA from breast and thigh muscle of 5-week-old chicks was enriched for CK mRNA by a novel procedure of sucrose gradient centrifugation in the presence of methylmercuric hydroxide. DNA complementary to this mRNA was inserted into pBR322, and colonies containing the recombinant plasmids were screened for the ability of the plasmid DNA to hybridize with and rescue CK mRNA from total muscle mRNA. Three plasmids, pCS195, pCS192, and pM35-4, could specifically rescue CK-M mRNA. CK-M mRNA was detected by in vitro translation and specific immunoprecipitation. The identity of the in vitro translation product was further confirmed by its migration in two-dimensional gels at the isoelectric point and molecular weight of CK-M. The heterogeneity of CK-M observed in vivo also was found upon translation of the CK-M mRNA which hybridizes to the plasmid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Wang N., Reddy A., Weinberg E., Sofer W. Alcohol dehydrogenase in Drosophila: isolation and characterization of messenger RNA and cDNA clone. Nucleic Acids Res. 1980 Dec 11;8(23):5649–5667. doi: 10.1093/nar/8.23.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravatti M., Perriard J. C., Eppenberger H. M. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Biosynthesis of creatine kinase subunits M and B. J Biol Chem. 1979 Feb 25;254(4):1388–1394. [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Dottin R. P., Manrow R. E., Fishel B. R., Aukerman S. L., Culleton J. L. Localization of enzymes in denaturing polyacrylamide gels. Methods Enzymol. 1979;68:513–527. doi: 10.1016/0076-6879(79)68040-0. [DOI] [PubMed] [Google Scholar]
- Dym H., Yaffe D. Expression of creatine kinase isoenzymes in myogenic cell lines. Dev Biol. 1979 Feb;68(2):592–599. doi: 10.1016/0012-1606(79)90229-x. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jones P. P. Analysis of H-2 and Ia molecules by two-dimensional gel electrophoresis. J Exp Med. 1977 Nov 1;146(5):1261–1279. doi: 10.1084/jem.146.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller L. R., Emerson C. P., Jr Synthesis of adult myosin light chains by embryonic muscle cultures. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1020–1024. doi: 10.1073/pnas.77.2.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacLeod A. R. Construction of bacterial plasmids containing sequences complementary to chicken alpha-tropomyosin mRNA. Nucleic Acids Res. 1981 Jun 25;9(12):2675–2689. doi: 10.1093/nar/9.12.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrow R. E., Dottin R. P. Demonstration, by renaturation in O'Farrell gels, of heterogeneity in Dictyostelium uridine diphosphoglucose pyrophosphorylase. Anal Biochem. 1982 Feb;120(1):181–188. doi: 10.1016/0003-2697(82)90334-7. [DOI] [PubMed] [Google Scholar]
- Manrow R. E., Dottin R. P. Renaturation and localization of enzymes in polyacrylamide gels: studies with UDPglucose pyrophosphorylase of Dictyostelium. Proc Natl Acad Sci U S A. 1980 Feb;77(2):730–734. doi: 10.1073/pnas.77.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford R. M., Wydro R. M., Nguyen H. T., Nadal-Ginard B. Cytoplasmic processing of myosin heavy chain messenger RNA: evidence provided by using a recombinant DNA plasmid. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5749–5753. doi: 10.1073/pnas.77.10.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Emigholz K., Monahan J. J. Increased amplification of pBR322 plasmid deoxyribonucleic acid in Escherichia coli K-12 strains RR1 and chi1776 grown in the presence of high concentrations of nucleoside. J Bacteriol. 1979 Apr;138(1):270–272. doi: 10.1128/jb.138.1.270-272.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriard J. C., Caravatti M., Perriard E. R., Eppenberger H. M. Quantitation of creatine kinase isoenzyme transition in differentiating chicken embryonic breast muscle and myogenic cell cultures by immunoadsorption. Arch Biochem Biophys. 1978 Nov;191(1):90–100. doi: 10.1016/0003-9861(78)90070-x. [DOI] [PubMed] [Google Scholar]
- Perriard J. C., Perriard E. R., Eppenberger H. M. Detection and relative quantitation of mRNA for creatine kinase isoenzymes in mRNA from myogenic cell cultures and embryonic chicken tissues. J Biol Chem. 1978 Sep 25;253(18):6529–6535. [PubMed] [Google Scholar]
- Rosenberg U. B., Eppenberger H. M., Perriard J. C. Occurrence of heterogenous forms of the subunits of creatine kinase in various muscle and nonmuscle tissues and their behaviour during myogenesis. Eur J Biochem. 1981 May;116(1):87–92. doi: 10.1111/j.1432-1033.1981.tb05304.x. [DOI] [PubMed] [Google Scholar]