Abstract
Laminoplasty is a common surgical technique used to treat cervical myelopathy. Both voids and contradictory information exist in the literature with regard to the initial and long-term biomechanical consequences of cervical laminoplasty. In order to clarify the existing literature, as well as provide clinically useful information, we identified three specific aims: (1) to measure the long-term differences in kinetics between the open door laminoplasty (ODL) and French door laminoplasty (FDL) techniques; (2) to delineate differences in primary and long-term cervical motion after laminoplasty; and (3) to determine whether inclusion of additional levels in the laminoplasty procedure results in a change in immediate cervical biomechanics. The study design involved both an animal (caprine) model and in vitro surgical simulation. We kinematically evaluated the cervical spine specimens (C2–C7) by applying pure bending moment loads to the cephalad vertebra (C2), while constraining the caudal vertebra (C7). Resultant intervertebral rotations (C3–C6) were determined via stereophotogrammetry. Overall, the data indicate that both FDL and ODL significantly reduce range of motion 6 months postoperatively, compared with the un-operated spine. There were no significant differences between the two techniques after 6 months. We also showed that ODL produces a significant reduction in motion 6 months postoperatively compared with the immediate postoperative condition. Finally, the data indicated that extending the laminoplasty from two to four levels did not significantly change range of motion. The choice of technique should be based upon the surgeon’s experience with these technically demanding procedures. In addition, initial stability considerations should not affect the decision to extend the laminoplasty to adjacent levels. Finally, the data also suggest that early changes in biomechanics should not be a major factor when considering whether immobilization of the cervical spine is necessary after laminoplasty. In fact, our temporal study, as well as previously reported clinical data, indicates that one should expect significantly decreased intervertebral motion 6 months after laminoplasty. Therefore, early physical therapy should be considered to preserve a more physiologic pattern of cervical range of motion.
Keywords: Biomechanics, Laminoplasty, Cervical spine
Introduction
Cervical myelopathy is often a manifestation of chronic spinal cord compression from degenerative conditions such as spondylosis or ossification of the posterior longitudinal ligament (OPLL). The predominant choice of surgical intervention usually involves a relatively wide decompression in order to prevent symptomatic progression and to improve the clinical outcome [13, 27, 41]. Decompression can be achieved using either anterior (multilevel corpectomy) or posterior (via laminectomy or laminoplasty) approaches. Clinical follow-up studies using both approaches report excellent or good results [6, 45, 48]. Multilevel corpectomies usually allow for direct decompression of the spinal cord. However, this method has been associated with complications such as pseudoarthrosis or loss of solid fixation. Many of these complications are probably attributable to the inherent instability associated with these techniques [5, 8, 9, 10, 32, 42, 55]. Decompression via laminectomy is a commonly employed surgical intervention, although many clinicians have reported that the longitudinal results can be less than ideal. Deleterious outcomes associated with laminectomy include development of progressive cervical kyphosis, instability, perineural adhesions and late neurologic deterioration [1, 15, 16, 19, 20, 24, 30, 33, 34, 40]. Laminoplasty eliminates many of these complications and achieves decompression of the spinal cord by allowing the cord to migrate posteriorly away from the compressive lesion, while preserving the posterior bone stock and architecture [22, 23]. Clinical follow-up studies have shown that laminoplasty generally results in greater patient satisfaction and lower complication rates than laminectomy [2, 18, 20, 24, 26, 36, 37, 49, 50].
Several laminoplasty techniques have been devised to achieve adequate decompression. “Open door laminoplasty” (ODL), sometimes referred to as “en-bloc laminoplasty,” involves bilateral scoring of the cervical lamina with one side remaining attached to the remainder of the spine’s posterior elements, while the other side is detached and hinged open. A bone block or plate is used to reconnect the separated laminae [22, 23]. Excellent long-term results have been reported using this technique [26, 43]. However, due to the asymmetry created by the procedure, certain muscle groups may lose as much as 80% of their cross-sectional area due to disuse atrophy [12]. Kurokawa et al. [31] later published a technique, “French door laminoplasty” (FDL), whereby the spinous process is removed and the lamina is split in the midline and symmetrically hinged open. A bone block is placed between the two halves of the split lamina in order to keep the expanded canal patent [50]. The purported advantage of this new technique over the ODL method is maintenance of bone–tissue symmetry, which, theoretically, produces a return to more normal biomechanics of the cervical spine [7, 17, 46]. However, this hypothesis has not been tested, and we are not aware of any study that has attempted to establish whether differences in cervical motion exist between the ODL and FDL techniques.
Most of the available biomechanical literature involving laminoplasty has compared resultant cervical motion and alignment patterns that are obtained using the ODL technique or laminectomy. Nowinski et al. demonstrated that laminoplasty (ODL technique) does not significantly affect cervical spine motion in the immediate postoperative period [38]. Baisden et al., using a goat model, reported a significant increase in both postsurgical kyphosis and slack motion at 6 months postoperatively in animals that had laminectomies with laminoplasty (ODL technique) and intact groups having equivalent spinal curvature and kinetics [4]. In contrast, Fields et al., using a rabbit model, reported that the laminoplasty and laminectomy groups had stiffer cervical spines than the normal group at 3 months, postoperatively [11]. There seems to be contradictory information from these studies concerning the temporal effects laminoplasty has on cervical motion patterns.
The number of intervertebral levels involved in a given laminoplasty procedure is dependent on the degree of lesion involvement. Typically, anywhere from two to six cervical levels are involved. For example, Yue et al [51] retrospectively reviewed 43 patients and reported that they operated on an average of 4.7 levels (range: three to six levels). The most frequently involved levels were C3–C7. However, we are not aware of any study that has attempted to correlate the number of levels to biomechanical performance. This is problematic in that, at least in our own clinical experience, we do not have a solid basis on which to decide whether extending the laminoplasty, either prophylactically or to be certain that the myelopathic lesion has been addressed, will deliver undue instability to the cervical spine.
Clearly there exist both voids and contradictory information in the literature with regard to the primary and long-term biomechanical consequences of cervical laminoplasty. In order to clarify the existing literature, as well as provide clinically useful information, we identified three specific aims: (1) to measure the long-term differences in kinetics between the ODL and FDL techniques; (2) to delineate differences in initial and long-term cervical motion after laminoplasty; and (3) to determine whether inclusion of additional levels in the laminoplasty procedure results in a change in immediate, postoperative cervical biomechanics.
Materials and methods
We chose to use a caprine model because such models have been used extensively by previous investigators to model human cervical biomechanics. The cervical curvature (lordosis) and biomechanical characteristics of the goat neck have been shown to closely approximate those of the human spine [4, 39, 44, 52, 53, 54, 56].
In order to satisfy the objectives of this study, we partitioned the animals into in vitro and in vivo surgical groups. Our first objective was studied by performing ODL on one group of animals and FDL on another group. The animals were sacrificed 6 months postoperatively, their spines harvested and biomechanically tested, and these data were compared to determine whether the laminoplasty technique leads to subsequent differences in range of motion across the affected area. In addition, we investigated the temporal effects of laminoplasty on cervical kinetics. We did this by comparing data obtained from biomechanical testing of in vitro-performed ODL with data obtained from the animals sacrificed 6 months postoperatively (temporal effects of ODL). Finally, we investigated the effect that longitudinal distance has on cervical mechanics (longitudinal effects of ODL). This was determined by performing a two-level ODL on harvested spines, mechanically evaluating the spines, and then extending the laminoplasty to the next level and repeating the mechanical testing. The following describes these procedures in greater detail. The mechanical testing protocol was the same for all three studies and is also described below.
Surgical techniques
Open door laminoplasty versus French door laminoplasty
The ODL versus FDL investigation includes three different animal groups (n=7 for each group): INT, ODL, and FDL. The University of California, Davis Institutional Animal Care and Use Committee approved all animal surgery and care protocols described herein. All animal care and surgeries were performed at a fully accredited animal care facility (Animal Resources Service, School of Veterinary Medicine, University of California, Davis). We did not encounter any significant surgical complications or observe neurologic changes after any of the surgeries.
Intact group
We tested seven normal caprine spines in order to determine the normal load-displacement characteristics of the caprine cervical spine. Animals were sacrificed and their cervical spines immediately harvested, placed in sealed double plastic bags, and frozen at −20°C until ready for mechanical testing.
Open door laminoplasty
General anesthesia was administered with the goats in a prone position. An incision was made from C2 to T1. The nuchal ligament was preserved, and the peri-spinous muscles were sub-periosteally dissected from the spinous processes to the lateral margins of the facets. Care was taken not to detach the paraspinal muscle from C2 in order to maintain an effective extensor force and preserve the overall neck alignment. Midline excision of ligamentum flavum was performed to expose the dura and canal above and below the intended level of the laminoplasty. A high-speed drill was utilized to create unicortical troughs bilaterally at the intersection of the lamina with the facet joints, without damaging the integrity of the facet joints, from C3 to C6. On one side the troughs were deepened immediately through the second cortex using a Kerrison rongeur, allowing the hinge to open. After excision of the inner cortex on one side of the spinous processes, the laminae were pushed laterally towards the other side to achieve the open door. Iliac crest bone graft was interposed in the space created and sutured in place, preventing potential closure of the “open door.” The paraspinous muscles were allowed to fall back into anatomic position, and the wounds were closed in regular fashion. All animals were sacrificed 6 months postoperatively via injection of sodium pentobarbital. The spines were immediately harvested, wrapped in saline-soaked gauze, sealed in double plastic bags and stored at −20°C until ready for testing.
French door laminoplasty procedure
The same anesthesia protocol was followed as with the open door technique, and an incision was created from C2 to T1. The spinous processes of C3–C6 were amputated at their bases, leaving the nuchal, supraspinous and interspinous ligaments intact and the contralateral extensor muscles stripped to the lateral margins of the facets. As in the ODL technique, ligamentum flavum was excised such that the dura and spinal canal were exposed. Bilateral unicortical troughs were created at the lamina-facet intersection. Next, a T-saw was passed along the epidural space and used to create a midline sagittal section of the remnants of the spinous processes. The split hemi-laminae were hinged open using a lamina spreader. Separation of the hemi-laminae was maintained with a sutured bone block (iliac crest autograft). The spinous processes were allowed to return to their anatomic positions, and fixed to the stumps composed of split spinous processes and iliac crest bone blocks. Regular wound closure followed. All animals were sacrificed 6 months postoperatively via injection of sodium pentobarbital. The spines were immediately harvested, wrapped in saline-soaked gauze, sealed in double plastic bags and stored at −20°C until ready for testing.
Longitudinal effects of open door laminoplasty
We tested seven normal caprine spines in order to determine the normal load-displacement characteristics of the goat cervical spine (INT group). The ODL technique (as described above) was performed in vitro for all of these procedures, where one side of the lamina was hinged open just medial to the facet. Foam blocks— representing bone grafts— with suture wiring were used to ensure that the hinge remained open and patent (Fig. 1). Each specimen was tested in the following order:
- a)
INT: intact without laminoplasty
- b)
2L: two-level laminoplasty (C3–C4)
- c)
3L: three-level laminoplasty (C3–C5)
- d)
4L: four-level laminoplasty (C3–C6)
Fig. 1.

Two-level caprine laminoplasty model depicting suturing of the foam blocks in the open door to maintain patency of the laminoplasty and the exposed spinal cord
Temporal effects of open door laminoplasty
We investigated the temporal effect that open door laminoplasty has on the cervical range of motion by comparing data obtained with the two studies described above. Specifically, we compared the 4L data from the longitudinal effects of ODL with the data obtained from mechanically testing the 6-month, postoperative-goat cervical spines with ODL.
Biomechanical testing protocol
At testing, cervical spines (C2–T1) were denuded of all peri-spinous musculature, leaving the appropriate ligamentous connections intact. C2 and the lower portion of T1 were potted in polymethylmethacrylate (PMMA). Kinetic biomechanical evaluations involved application of pure bending moments to the potted cephalad vertebra (C2), with the caudal vertebra (T1) constrained (Fig. 2). Each test included pure moment loading in flexion and extension, right and left lateral bending, and right and left axial rotation. Loads were applied as pure moments through a system of weights and pulleys [14] transmitted by nylon strings, oriented perpendicular to the ends of the threaded rods in C2. Moments in four-step increments (0.25 Nm/step) up to 1.0 Nm were applied to the specimen after appropriate pre-conditioning (three full cycles). Three optoelectronic markers were attached to the anterior aspect of each vertebral body. Three cameras (Motion Analysis System, Santa Rosa, CA) detected reflected light by the marker triads, and their respective coordinates were determined. Intervertebral rotations (C3 with respect to C6) at 1.0 Nm loading were calculated from marker position data.
Fig. 2.

Photograph depicting the experimental apparatus and testing protocol during lateral bending loading. Pure moments were applied to C2 and the resultant intervertebral rotations calculated using marker triads and a three-camera system
Statistical analyses
For the FDL versus ODL study, statistical analysis was performed using one-way analysis of variance (ANOVA, p<0.05 significance). Individual differences between the INT, ODL, and FDL groups were delineated with Tukey’s post-hoc test (p<0.05 significance level). Level of involvement C3–C6 motion data were analyzed using a repeated-measures, one-way ANOVA. Finally, the temporal effect study data were analyzed using a one-way ANOVA with Tukey’s post hoc test (p<0.05 significance level). All statistical analyses were performed using SigmaStat software (Jandel, SPSS Science, Chicago, IL, USA) on a PC platform.
Results
The kinetic testing of the intact spine demonstrated good agreement in range of motion (ROM) between the caprine model used in this investigation and data published for the human spine [47], for both sagittal- and transverse-plane rotations. Specifically, the intact caprine model demonstrated total flexion–extension motion of 66.5°±22.2° (mean and standard deviation) across the C3–C6 segment, compared with the published value of 55°. The same close agreement was seen in axial rotation, with the caprine spine rotating 33.4°±14.4° with the published value at approximately 38°. The caprine C3–C6 segment demonstrated greater lateral bending ROM (83.0°±27.0°) than would be expected in a human spine (about 60°).
Open door laminoplasty versus French door laminoplasty
At the time of death of the animals we did not observe any spontaneous fusions across vertebral levels for any of the surgical experimental groups. In addition, none of the laminoplasty doors (either open door or French door) closed into the canal. Finally, there was no evidence of bone-graft displacement and there was incorporation of the bone grafts across the laminoplasty sites.
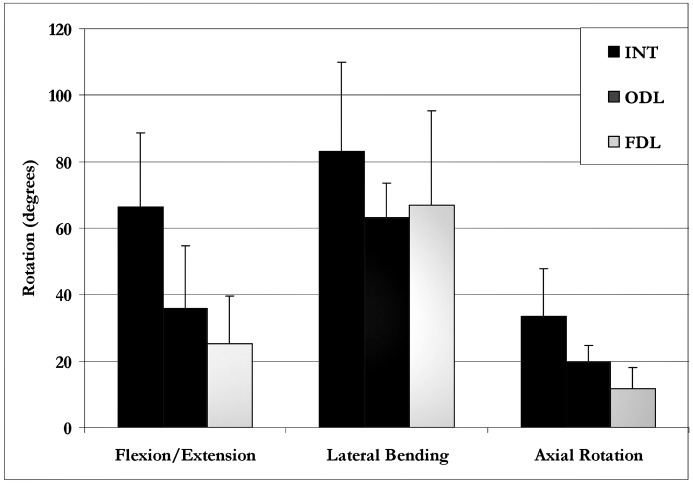
Range of motion data are shown in Fig. 3. The flexion/extension data indicate that both ODL (35.8°±18.9°) and FDL (25.3°±14.2°) significantly reduced sagittal-plane motion compared with the INT condition (66.5°±22.2°). The difference in flexion–extension motion between the ODL and FDL conditions was not statistically significant. The same general trend was seen in lateral bending, with ODL (63.3°±10.2°) and FDL (67.0°±28.3°) demonstrating reduced ROM compared with the INT spine (83.0°±27.0°). Although both laminoplasty groups demonstrated lower C3–C6 range of motion in lateral bending, this difference was not statistically significant. Finally, the axial rotation data showed that the FDL procedure (11.6°±6.4°) significantly reduced motion compared with the INT group (33.4°±14.3°) and ODL (19.5°±5.2°) group. The difference between surgical groups was not statistically significant.
Fig. 3.
ODL versus FDL: mean C3–C6 range of motion (with one standard deviation error bar) for the INT, ODL and FDL conditions for the three rotation planes
Longitudinal effects of open door laminoplasty
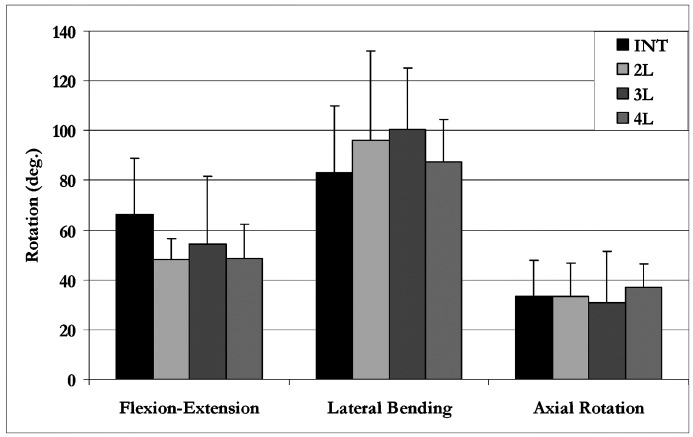
Overall, our data indicated equivalence between the intact spine and the three different laminoplasty conditions. In flexion–extension (Fig. 4), the INT condition demonstrated the greatest C3–C6 motion (66.5±22.4°) and the 2L condition allowed the least motion (48.4±3.3°). There were no statistically significant differences between any of the groups in flexion–extension. Axial rotation C3–C6 ROM ranged from 30.8° (3L condition) to 37.0° (4L condition), with each group being statistically equivalent. Finally, lateral bending demonstrated the greatest amount of motion for all groups. The INT condition showed the least amount of C3–C6 lateral bending ROM (83.0±27.0°), and the 3L condition showed the highest degree of lateral bending ROM (100.3±24.7°). Once again, repeated-measures ANOVA indicated that there were no statistically significant differences between any of these groups in lateral bending.
Fig. 4.
Longitudinal effect of ODL: total C3–C6 range of motion (mean with one standard deviation bar) for all four model conditions. There was no statistical difference (p>0.05) in motion for any condition
Temporal effects of open door laminoplasty
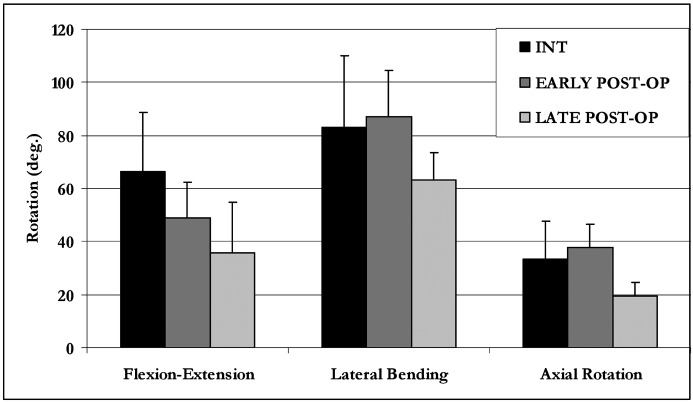
Overall, the data indicated equivalence between the intact (INT) and immediately postoperative (early post-op) groups. The 6-month postoperative group (late post-op) demonstrated reduced motion (Fig. 5) compared with both the INT and early post-op groups. Combined flexion–extension was significantly reduced for the late post-op (35.8°±19.0) group compared with both the INT (p<0.01) and early post-op (p<0.01) groups. Although the late post-op group exhibited a nearly a 25% decrease in combined lateral bending range of motion (63.3°±10.2), this difference was not statistically significant compared with the INT (83.0°±27.0) and the early post-op (87.2°±17.2°) conditions. Finally, combined right and left axial rotation was significantly reduced for the late post-op group (19.5°±5.3) compared with the INT (p<0.05) and early post-op (p<0.02) groups.
Fig. 5.
Temporal effect of ODL: mean C3–C6 ROM data (with one standard deviation error bar) for the INT, early post-op, and late post-op groups. ROM for the late post-op group is significantly decreased (p<0.05) compared with INT group for all three motions. There is no statistical difference between the INT group and the early post-op group for any motion
Discussion
The laminoplasty procedure for surgical treatment of multilevel cervical myelopathy was developed in response to the relatively high complication rates associated with both pre-existing anterior and posterior decompression techniques. Laminoplasty provides spinal cord decompression via canal expansion, thus allowing the cord to migrate posteriorly while preserving posterior bone stock and architecture. Various laminoplasty techniques have been described, and the literature is replete with follow-up studies that have demonstrated better clinical outcomes and patient satisfaction [3, 20, 21, 23, 24, 26, 35, 36, 37, 43, 48, 50]. Relatively few reports have demonstrated the biomechanical effects laminoplasty has on the cervical spine [4, 28, 29].
Most of the currently available biomechanics literature has investigated motion differences resulting from laminoplasty and laminectomy. Baisden et al. reported similar flexion–extension and axial rotation motion and greater lateral bending motion after 6 months, postoperatively, in a three-level laminoplasty (ODL technique) goat model, compared with the intact specimen [4]. Using a rabbit model, Fields et al. did not find significant differences in the load-displacement behavior of the rabbit spine after laminoplasty [11]. Kubo et al. showed that FDL, performed on cadavers, did not produce significant increases in range of motion compared with the intact cervical spine [29]. Similarly, Nowinski et al., in a cadaver study, demonstrated that a four-level ODL did not significantly affect cervical motion patterns [38]. Data that we are reporting for the early post-op and INT groups agree with the aforementioned studies. The important clinical implication is that stability and range of motion are not compromised in the immediate postoperative period. However, the data from the present study demonstrated significant decreases in flexion–extension, axial rotation and lateral bending range of motion compared with intact and immediately postoperative laminoplasty specimens after 6 months, postoperatively. A clear disparity exists between our findings and the Baisden et al. study data. This may be attributable to differences in specimen preparation. In the current study, all of the paraspinal musculature was removed, while the scar tissue covering the laminar hinges and facet joints was left undisturbed. We believe that complete removal of the scar tissue necessitates violation of the facet capsules, which most likely introduces an artifact with respect to laminoplasty’s biomechanical effect on cervical range of motion. Accordingly, we did not remove any peri-facet scar tissue before kinetically evaluating the cervical spines, and we believe this may explain the difference in findings between our studies and those published earlier (Fig. 6). Goel et al. has shown that significant violation of the facet capsule ligaments results in pronounced intervertebral motion [14]. By extrapolation, one should expect these motions to decrease if soft tissue support via scar tissue pattern is augmented in the facet-capsule area and posterior ligamentous complex. Most important, our biomechanical data reflect what has been seen clinically. Specifically, decreases in range of motion of the cervical spine after laminoplasty have been reported [35, 50]. Ishibashi, in a clinical follow-up study (mean follow-up period 33±19 months) of laminoplasty patients, reported that sagittal range of motion was decreased by approximately 50% [25]. Our group demonstrated a mean reduction in combined flexion–extension motion of approximately 60%. We feel this is an outcome of the surgery’s temporal iatrogenic effects, a complication which has not been previously reported. Clinically, one should expect that, in the long term, scar tissue formation in and around the facet joints and posterior ligamentous complex significantly limits intervertebral motion after cervical laminoplasty.
Fig. 6.
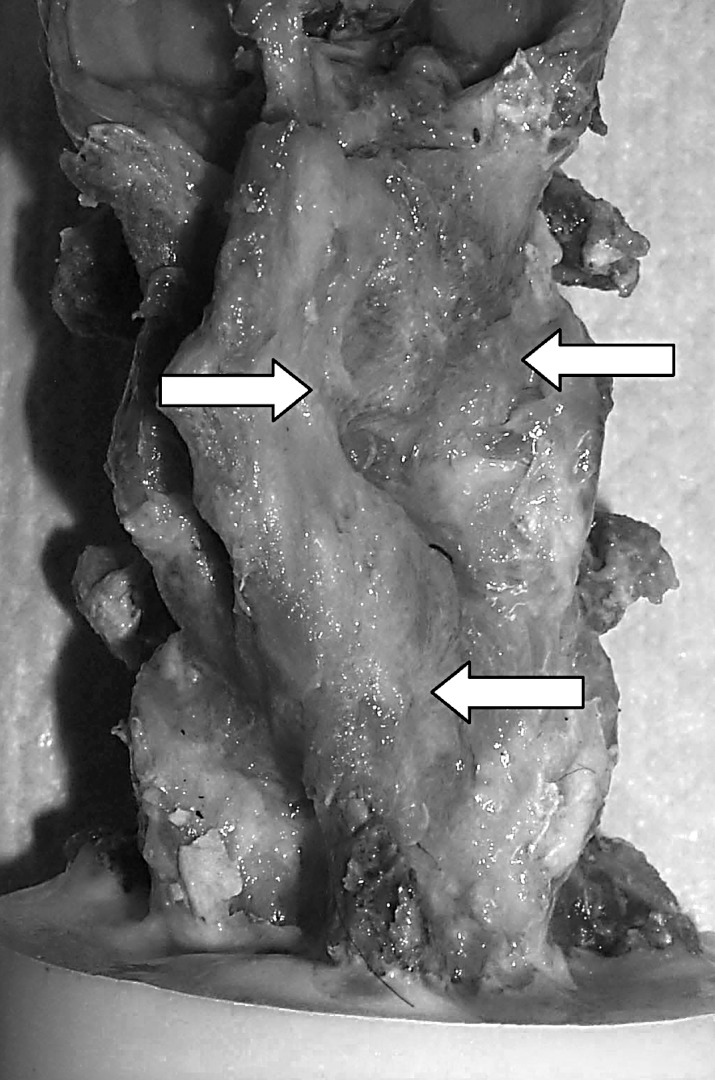
Photograph depicting appreciable abundant fibrous tissue formation (arrows) round the facet joints, 6 months postoperatively
Over the past three decades, many laminoplasty techniques have been developed. Two of the most commonly used and reported are ODL and FDL. We are not aware of any study that has investigated the differences in spine kinetics due to the choice of surgical technique. The current study has shown that cervical ODL or FDL results in significant motion reductions after 6 months with respect to the pre-operative condition. In addition, we showed that there is no difference in resultant cervical motion between the two techniques. These two common procedures allow decompression by preserving the lamina’s protective role, with different geometric configurations with respect to lamina alignment. Accordingly, either technique affords different advantages and disadvantages with respect to execution and additional neural decompression. ODL allows for performing an additional foraminotomy or facetectomy lateral to the opening. However, decompression of the nerve roots is usually impossible when FDL is performed. On the other hand, prohibitive hemorrhaging during the FDL procedure can usually be avoided, because relatively few vascular structures are located in the dorsal midline epidural space. FDL’s main disadvantage is that it usually requires a relatively wide exposure of the epidural space above and below the treated levels. This epidural exposure can also involve a dissection between levels in order to pass a sublaminar T-saw. Although there were no complications in our group related to placement and use of the T-saw, we felt that the FDL procedure was far more technically challenging to execute than the ODL. Furthermore, in the present study, the data indicated that the more technically difficult FDL technique did not result in more physiologically normal cervical spine biomechanics than did the ODL.
One of this project’s other aims was to investigate the biomechanical effect of adding progressively more levels to the laminoplasty procedure. Overall, we observed no difference in immediately postoperative kinetics between the unoperated condition (INT) and spines that had two-level (2L), three-level (3L), and four-level (4L) laminoplasties. This is consistent with our other data showing that laminoplasty does not have an effect on motion in the immediate, postoperative period. This investigation’s data demonstrate that primary changes in biomechanics should not be a major factor when considering whether a laminoplasty procedure should be extended to adjacent levels.
There exists no clear consensus with respect to the use of postoperative external bracing and timing of physical therapy after laminoplasty. No clinical data supports increased instability after laminoplasty, which would require early immobilization. Our data concur with this finding of no immediate biomechanical consequences due to laminoplasty. In addition, it is commonly held that early mobilization preserves or increases the chance for resultant long-term range of motion. Therefore, based upon our data and the clinical findings, we propose that early mobilization and physical therapy should be considered following laminoplasty.
This study is not without limitations, most of them attributable to the ex vivo nature of the investigation. In this model, the evaluated motions were simulated by pure moments applied to the spine, instead of from direct muscle force activation. Given that the goal was to perform a comparative study, this is probably not a significant issue, and the intended goal was accomplished by employing this commonly used protocol. Inter-specimen variability is always a concern when performing animal studies. However, rigorous statistical analysis (and repeated-measure design, when allowable) allowed us to minimize these unavoidable effects. Another issue is the appropriateness of performing the laminoplasty procedure in animals that did not show any existing degeneration. However, we believe that inducing degeneration in the animal model probably would have created even greater variability in the baseline condition, confounding the results beyond the point where we would be able to reliably detect changes between groups. One of this study’s shortcomings was that we did not document the changes in sagittal profile with time and correlate these data with ROM. Nevertheless, others have reported that significant lordotic changes after laminoplasty do not occur at 6 months in a caprine model [4]. Finally, as we performed only static testing, we cannot comment on the fatigue behavior of any of these laminoplasty preparations beyond 6 months.
Overall, the data did not show significant kinetic differences between ODL and FDL. The choice of technique to use should be based upon the surgeon’s experience with these technically demanding procedures. In addition, we observed no change in cervical range of motion between the unoperated spine and the spine that had a two-level, three-level, and four-level laminoplasty. Therefore, the decision to extend the laminoplasty to adjacent levels should not be affected by primary stability considerations. Finally, the data also suggest that changes in early post-operative biomechanics should not be a major factor when considering whether immobilization of cervical spine is necessary after laminoplasty. In fact, our temporal study, as well as previously reported clinical data, indicates that one should expect significantly decreased intervertebral motion 6 months after laminoplasty. Therefore, early physical therapy should be considered to preserve a more physiologic pattern of cervical range of motion.
Acknowledgements
Financial support provided by the Department of Orthopaedic Surgery (UCSF) and the Dean’s Summer Research Program, School of Medicine (UCSF).
References
- 1.Albert Spine. 1998;23:2738. doi: 10.1097/00007632-199812150-00014. [DOI] [PubMed] [Google Scholar]
- 2.Baba Int Orthop. 1995;19:116. doi: 10.1007/BF00179972. [DOI] [PubMed] [Google Scholar]
- 3.Baba J Neurol. 1996;243:626. doi: 10.1007/BF00878657. [DOI] [PubMed] [Google Scholar]
- 4.Baisden Spine. 1999;24:1283. doi: 10.1097/00007632-199907010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bolesta Spine. 2000;25:2040. doi: 10.1097/00007632-200008150-00007. [DOI] [PubMed] [Google Scholar]
- 6.Carol J Spinal Disord. 1988;1:59. [PubMed] [Google Scholar]
- 7.Edwards Spine. 2000;25:1788. doi: 10.1097/00007632-200007150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Emery J Bone Joint Surg Am. 1998;80:941. doi: 10.2106/00004623-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Emery Spine. 1997;22:2622. doi: 10.1097/00007632-199711150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Farey J Bone Joint Surg Am. 1990;72:1171. [PubMed] [Google Scholar]
- 11.Fields Spine. 2000;25:2925. doi: 10.1097/00007632-200011150-00015. [DOI] [PubMed] [Google Scholar]
- 12.Fujimura Arch Orthop Trauma Surg. 1996;115:203. doi: 10.1007/BF00434554. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara J Bone Joint Surg Br. 1989;71:393. doi: 10.1302/0301-620X.71B3.2722928. [DOI] [PubMed] [Google Scholar]
- 14.Goel Spine. 1988;21:673. [Google Scholar]
- 15.Guigui Spine. 1998;23:440. doi: 10.1097/00007632-199802150-00006. [DOI] [PubMed] [Google Scholar]
- 16.Guigui Rev Chir Orthop Reparatrice Appar Mot. 1998;84:17. [PubMed] [Google Scholar]
- 17.Hara Neurosurgery. 2001;48:235. doi: 10.1097/00006123-200101000-00050. [DOI] [PubMed] [Google Scholar]
- 18.Heller Spine. 2001;26:1330. doi: 10.1097/00007632-200106150-00013. [DOI] [PubMed] [Google Scholar]
- 19.Herkowitz J Spinal Disord. 1988;1:179. [PubMed] [Google Scholar]
- 20.Herkowitz Spine. 1988;13:774. doi: 10.1097/00007632-198807000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Herkowitz HN (1989) The surgical management of cervical spondylotic radiculopathy and myelopathy. Clin Orthop 94–108 [PubMed]
- 22.Hirabayashi Spine. 1988;13:870. doi: 10.1097/00007632-198807000-00032. [DOI] [PubMed] [Google Scholar]
- 23.Hirabayashi Spine. 1983;8:693. doi: 10.1097/00007632-198310000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Hukuda J Bone Joint Surg Br. 1988;70:325. doi: 10.1302/0301-620X.70B2.3346317. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi Kurume Med J. 2000;47:135. doi: 10.2739/kurumemedj.47.135. [DOI] [PubMed] [Google Scholar]
- 26.Kimura J Bone Joint Surg Br. 1995;77:956. [PubMed] [Google Scholar]
- 27.Koyanagi Spine. 1993;18:1958. doi: 10.1097/00007632-199310001-00006. [DOI] [PubMed] [Google Scholar]
- 28.Kubo J Spinal Disord Tech. 2002;15:477. doi: 10.1097/00024720-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 29.KuboSpine 200328227. 10.1097/00007632-200302010-0000512567022 [DOI] [Google Scholar]
- 30.Kumar Neurosurgery. 1999;44:771. doi: 10.1097/00006123-199904000-00046. [DOI] [PubMed] [Google Scholar]
- 31.Kurokawa Bessatsu Seikeigeka. 1982;2:234. [Google Scholar]
- 32.Lowery Spine. 1995;20:2436. doi: 10.1097/00007632-199511001-00012. [DOI] [PubMed] [Google Scholar]
- 33.Matsunaga Spinal Cord. 1999;37:20. doi: 10.1038/sj.sc.3100749. [DOI] [PubMed] [Google Scholar]
- 34.MikawaSpine 19871263576358 [Google Scholar]
- 35.Nakano Spine. 1992;17:41. [Google Scholar]
- 36.Nakano Nippon Seikeigeka Gakkai Zasshi. 1988;62:1139. [PubMed] [Google Scholar]
- 37.Nakano Spine. 1988;13:792. doi: 10.1097/00007632-198807000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Nowinski Spine. 1993;18:1995. doi: 10.1097/00007632-199310001-00012. [DOI] [PubMed] [Google Scholar]
- 39.Pintar Spine. 1994;19:2524. doi: 10.1097/00007632-199411001-00006. [DOI] [PubMed] [Google Scholar]
- 40.Saito Spine. 1991;16:494. doi: 10.1097/00007632-199105000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Sampath Spine. 2000;25:670. doi: 10.1097/00007632-200003150-00004. [DOI] [PubMed] [Google Scholar]
- 42.Sasso Spine. 2003;28:140. doi: 10.1097/00007632-200301150-00009. [DOI] [PubMed] [Google Scholar]
- 43.Satomi Spine. 1994;19:507. doi: 10.1097/00007632-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi J Neurosurg. 1999;90:224. doi: 10.3171/spi.1999.90.2.0224. [DOI] [PubMed] [Google Scholar]
- 45.TeramotoNeurosurgery 199435647936154 [Google Scholar]
- 46.Tomita Spine. 1998;23:32. doi: 10.1097/00007632-199801010-00007. [DOI] [PubMed] [Google Scholar]
- 47.White AA, Panjabi MM (1990) Clinical biomechanics of the spine. Lippincott, Philadelphia
- 48.Yonenobu Spine. 1992;17:1281. [PubMed] [Google Scholar]
- 49.Yonenobu Orthopade. 1996;25:533. doi: 10.1007/s001320050057. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida Spine. 1992;17:491. doi: 10.1097/00007632-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Yue J Spinal Disord. 2000;13:329. doi: 10.1097/00002517-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Zdeblick Spine. 1994;19:2348. doi: 10.1097/00007632-199410150-00017. [DOI] [PubMed] [Google Scholar]
- 53.Zdeblick Spine. 1993;18:1974. doi: 10.1097/00007632-199310001-00009. [DOI] [PubMed] [Google Scholar]
- 54.Zdeblick Spine. 1998;23:758. doi: 10.1097/00007632-199804010-00002. [DOI] [PubMed] [Google Scholar]
- 55.Zdeblick J Bone Joint Surg Am. 1997;79:523. doi: 10.1302/0301-620X.79B4.6940. [DOI] [PubMed] [Google Scholar]
- 56.Zdeblick Spine. 1992;17:418. [Google Scholar]