Abstract
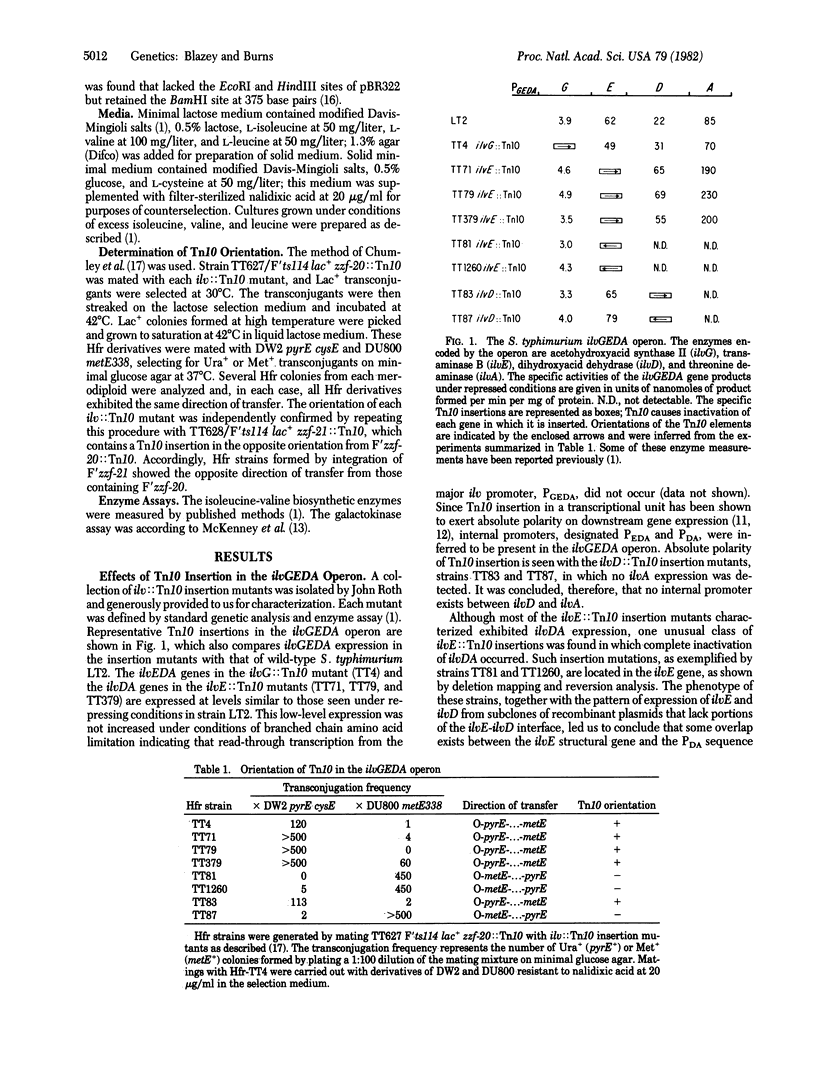
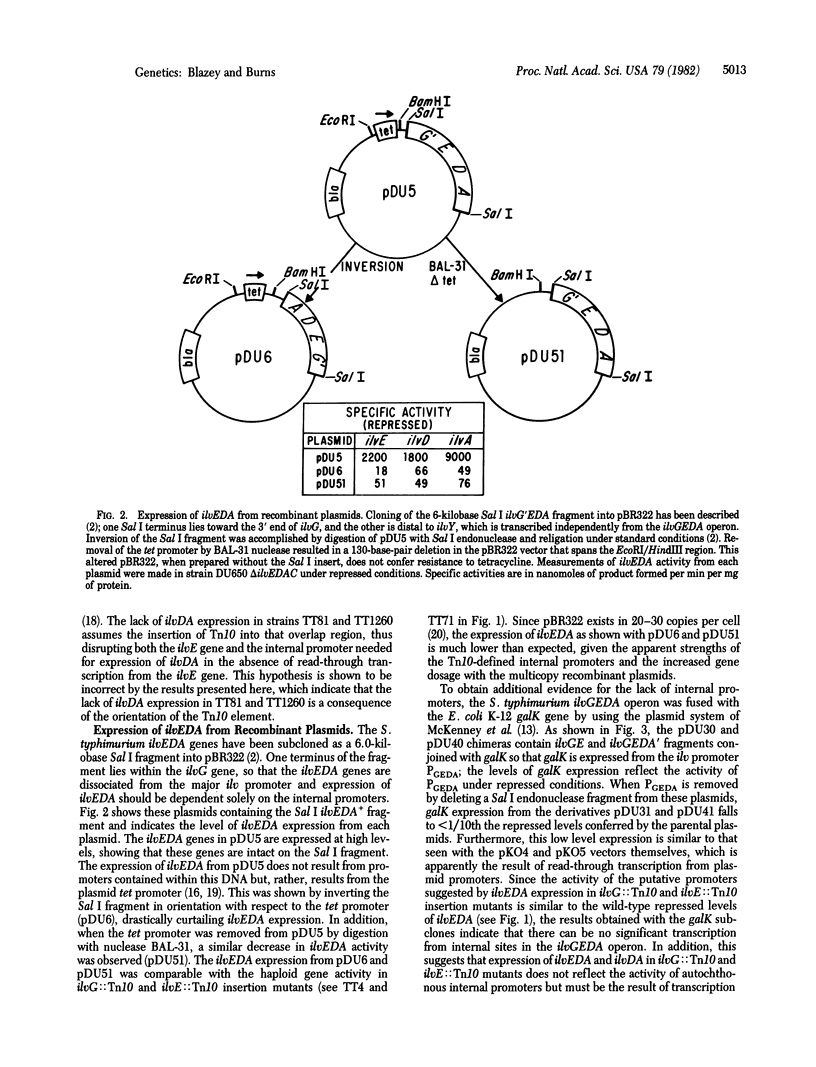
Polarity of Tn10 insertion mutations in the Salmonella typhimurium ilvGEDA operon depends on both the location and the orientation of the Tn10 element. One orientation of Tn10 insertions in ilvG and ilvE permits low-level expression of the downstream ilvEDA and ilvDA genes, respectively. Our analysis of Salmonella ilv recombinant plasmids shows that this residual ilv expression must result from Tn10-directed transcription and does not reflect the presence of internal promoters in the ilvGEDA operon, as was previously suggested. The opposite orientation of Tn10 insertion in ilvE prevents ilvDA expression, indicating that only one end of Tn10 is normally active in transcribing adjacent genes. Both orientations of Tn10 insertion in ilvD exert absolute polarity on ilvA expression. Expression of ilvA is known to be dependent on effective translation of ilvD, perhaps reflecting the lack of a ribosome binding site proximal to the ilvA sequence. Therefore, recognition of the ability of Tn10 to promote transcription of contiguous genes in the ilvGEDA operon apparently requires the presence of associated ribosome binding sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Genetic organization of the Salmonella typhimurium ilv gene cluster. Mol Gen Genet. 1979;177(1):1–11. doi: 10.1007/BF00267247. [DOI] [PubMed] [Google Scholar]
- Blazey D. L., Kim R., Burns R. O. Molecular cloning and expression of the ilvGEDAY genes from Salmonella typhimurium. J Bacteriol. 1981 Aug;147(2):452–462. doi: 10.1128/jb.147.2.452-462.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi M. S., Schmid M. B., Roth J. R. Transposon Tn10 provides a promoter for transcription of adjacent sequences. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5016–5020. doi: 10.1073/pnas.79.16.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Davis M. A., Roberts D. E., Takeshita K., Kleckner N. Genetic organization of transposon Tn10. Cell. 1981 Jan;23(1):201–213. doi: 10.1016/0092-8674(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Gemmill R. M., Wessler S. R., Keller E. B., Calvo J. M. leu operon of Salmonella typhimurium is controlled by an attenuation mechanism. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4941–4945. doi: 10.1073/pnas.76.10.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H. B., Jr, Ostrander D. A., Hodnett J. L., Legerski R. J., Robberson D. L. Extracellular nucleases of Pseudomonas BAL 31. I. Characterization of single strand-specific deoxyriboendonuclease and double-strand deoxyriboexonuclease activities. Nucleic Acids Res. 1975 Sep;2(9):1459–1492. doi: 10.1093/nar/2.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Simons R. W., Way J. C., Walsh R. B., Kleckner N. DNA sequence organization of IS10-right of Tn10 and comparison with IS10-left. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2608–2612. doi: 10.1073/pnas.79.8.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Housley P. R., Leavitt A. D., Whitfield H. J. Genetic analysis of a temperature-sensitive Salmonella typhimurium rho mutant with an altered rho-associated polycytidylate-dependent adenosine triphosphatase activity. J Bacteriol. 1981 Jul;147(1):13–24. doi: 10.1128/jb.147.1.13-24.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Steele D. A., Reichardt K., Botstein D. Specificity of insertion by the translocatable tetracycline-resistance element Tn10. Genetics. 1979 Aug;92(4):1023–1040. doi: 10.1093/genetics/92.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Multivalent translational control of transcription termination at attenuator of ilvGEDA operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1862–1866. doi: 10.1073/pnas.77.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Hodnett J. L., Gray H. B., Jr Extracellular nucleases of pseudomonas BAL 31. III. Use of the double-strand deoxyriboexonuclease activity as the basis of a convenient method for the mapping of fragments of DNA produced by cleavage with restriction enzymes. Nucleic Acids Res. 1978 May;5(5):1445–1464. doi: 10.1093/nar/5.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Nargang F. E., Subrahmanyam C. S., Umbarger H. E. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1823–1827. doi: 10.1073/pnas.77.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C., Heffron F., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: independence from host rec functions and orientation of insertion. J Bacteriol. 1976 Oct;128(1):425–434. doi: 10.1128/jb.128.1.425-434.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Taillon M. P., Gotto D. A., Lawther R. P. The DNA sequence of the promoter-attenuator of the ilvGEDA operon of Salmonella typhimurium. Nucleic Acids Res. 1981 Jul 24;9(14):3419–3432. doi: 10.1093/nar/9.14.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]



