Abstract
Multipotent stem cells have been shown to be extremely useful in the field of regenerative medicine1-3. However, in order to use these cells effectively for tissue regeneration, a number of variables must be taken into account. These variables include: the total volume and surface area of the implantation site, the mechanical properties of the tissue and the tissue microenvironment, which includes the amount of vascularization and the components of the extracellular matrix. Therefore, the materials being used to deliver these cells must be biocompatible with a defined chemical composition while maintaining a mechanical strength that mimics the host tissue. These materials must also be permeable to oxygen and nutrients to provide a favorable microenvironment for cells to attach and proliferate. Chitosan, a cationic polysaccharide with excellent biocompatibility, can be easily chemically modified and has a high affinity to bind with in vivo macromolecules4-5. Chitosan mimics the glycosaminoglycan portion of the extracellular matrix, enabling it to function as a substrate for cell adhesion, migration and proliferation. In this study we utilize chitosan in the form of microspheres to deliver adipose-derived stem cells (ASC) into a collagen based three-dimensional scaffold6. An ideal cell-to-microsphere ratio was determined with respect to incubation time and cell density to achieve maximum number of cells that could be loaded. Once ASC are seeded onto the chitosan microspheres (CSM), they are embedded in a collagen scaffold and can be maintained in culture for extended periods. In summary, this study provides a method to precisely deliver stem cells within a three dimensional biomaterial scaffold.
Keywords: Bioengineering, Issue 64, Biomedical Engineering, Tissue Engineering, chitosan, microspheres, collagen, hydrogel, cell delivery, adipose-derived stem cells, ASC, CSM
Protocol
1. Isolating Adipose-Derived Stem Cells (ASC)
Note: All procedures were performed at room temperature unless otherwise noted.
Isolate rat perirenal and epididymal adipose and wash with sterile Hank's buffered salt solution (HBSS) containing 1% fetal bovine serum (FBS) as previously described6.
Mince the tissue and transfer 1-2 g into 25 mL of HBSS containing 1% FBS into a 50 mL tube and centrifuge at 500 g for 8 min at room temperature.
Collect the free-floating adipose tissue layer and transfer to 125 mL erlenmeyer flask and treat with 25 mL of collagenase type II (200 U/mL) in HBSS for 45 min at 37 °C on an orbital shaker (125 rpm).
Carefully remove the liquid fraction (below oil and adipose layer) and filter sequentially through a 100-μm and 70-μm nylon mesh filter. Centrifuge the filtrate at 500 g for 10 min at room temperature, aspirate the supernatant and wash the pellet twice with 25 mL HBSS.
Resuspend the cell pellet in 50 mL of growth medium (MesenPRO RS Basal Medium) supplemented with MesenPRO RS Growth Supplement, antibiotic-antimycotic (100 U/mL of penicillin G, 100 μg/mL streptomycin sulfate, and 0.25 μg/mL Amphotericin B), and 2 mM L-glutamine and pipet cells into 2 T75 flasks (25 mL/flask).
Culture the ASC in a 5% CO2 humidified incubator at 37 °C (Passage 2-4 ASC are used for all experiments).
2. Preparing Chitosan Microspheres (CSM)
Note: All procedures were performed at room temperature unless otherwise noted.
CSM are prepared by a water-in-oil emulsification process along with an ionic coacervation technique using our previous protocol6. Emulsify an aqueous solution of chitosan (6 mL of 3%w/v chitosan in 0.5 M acetic acid) in a 100 mL of an oil phase mixture consisting of soya oil, n-octanol (1:2 v/v) and 5% sorbitan-mono-oleate (span 80) emulsifier, using overhead (1700 rpm) and magnetic stirring (1000 rpm) simultaneously, in opposite directions. This dual method of mixing ensures that micelles formed early on before cross-linking occurs can remain in solution and not settle to the bottom. Furthermore, the magnetic stir bar aids in de-aggregating chitosan during micelle formation and rigidization.
The mixture is continuously stirred for approximately 1 hour until a stable water-in-oil emulsion is obtained. Ionic crosslinking is initiated with the addition of 1.5 mL of 1% w/v potassium hydroxide in n-octanol every 15 min for 4 h (24 mL total).
After completion of the crosslinking reaction, slowly decant the oil phase of the mixture containing CSM and immediately add the spheres to 100 mL of acetone. Wash the spheres with acetone until the oil phase is completely removed.
Dry the recovered spheres in a vacuum desiccator and analyze without further processing. The average CSM particle size, surface area per milligram and the unit cubic volume was determined using particle size analyzer.
For subsequent experiments, wash CSM three times with sterile water to remove residual salts and sterilize with absolute alcohol.
3. Determining the Number of Free Amino Groups in CSM
Note: All procedures were performed at room temperature unless otherwise noted.
Determine the number of free amino groups present in CSM after ionic crosslinking by using the trinitro benzenesulfonic (TNBS) acid assay of Bubins and Ofner7. Incubate 5 mg of microspheres with 1 mL of 0.5% TNBS solution in a 50 mL glass tube for 4 h at 40 °C and hydrolyze with the addition of 3 mL of 6N HCl at 60 °C for 2h.
Cool the samples to room temperature, and extract the free TNBS by adding 5 mL of deionized water and 10 mL of ethyl ether.
Warm a 5 mL aliquot of the aqueous phase to 40 °C in a water bath for 15 min to evaporate any residual ether, cool to room temperature, and dilute with 15 mL of water.
Measure the absorbance at 345 nm with a spectrophotometer using TNBS solution without chitosan as blank and the chitosan used for CSM preparation to determine the total number of amino groups. Estimate the number of free amino groups of the CSM relative to chitosan.
4. Loading ASC in CSM
Note: All procedures were performed at room temperature unless otherwise noted.
Equilibrate 5 mg of sterilized CSM from section 2.5 in sterile HBSS overnight and add to an 8-μm pore size membrane culture plate insert (24-well plate).
After the CSM have settled onto the membrane, carefully aspirate the HBSS and add 300 μL of growth medium to the inside of the insert and 700 μL of growth media to the outside of the insert.
Resuspend ASC at the appropriate concentration (1 x 104 to 4 x 104) in 200 μL of growth medium and seed over the CSM inside the culture plate insert. The final volume of medium within the culture insert, after seeding, is 500 μL.
Incubate the ASC seeded on CSM for 24 h in a 5% CO2 humidified incubator at 37 °C.
5. Determining the Percentage of ASC Loading and Cell Viability in CSM
Note: All procedures were performed at room temperature unless otherwise noted.
After incubation, collect the ASC-loaded CSM in a sterile 1.5- ml microcentrifuge tube without disturbing the cells that have migrated into the insert membrane.
Remove the residual medium and add 250 μL of fresh growth medium to the tube.
To each tube add 25 μL MTT [3-(4,5-dimethylthiozole-2-yl)-2,5-diphenyltetrazolium bromide] solution (5 mg/mL) and incubate for 4 h in a 5% CO2 humidified incubator at 37 °C.
After incubation, remove the medium, add 250 μL of dimethyl sulfoxide, and vortex the mixture for 2-5 min to solubilize the formazan complex.
Centrifuge the CSM at 2700 x g for 5 min, and determine the supernatant absorbance measured at 570 nm using 630 nm as reference.
Determine the cell number associated with the CSM relative to the absorbance value obtained from known numbers of viable ASC.
6. Characterizing ASC-CSM-Embedded Collagen Gel
Note: All procedures were performed at room temperature unless otherwise noted.
Mix ASC-loaded CSM (5 mg containing ≈ 2 x 104 cells) with type 1 collagen (7.5 mg/mL) extracted from rat tail tendon according the method of Bornstein8 and fibrillate after adjusting the pH to 6.8 using 2 N NaOH.
Add the fibrillated collagen-ASC-CSM mixture to a 12-well plate and incubate for 30 min at in a 5% CO2 humidified incubator at 37 °C.
After complete fibrillation, incubate the collagen-ASC-CSM gels for up to 14 days in a 5% CO2 humidified incubator at 37 °C.
Release and migration of cells from CSM into the gel were observed using standard microscopy techniques.
7. Representative Results
In the present study, we have developed an in vitro strategy to deliver stem cells from chitosan microspheres (CSM) into a collagen gel scaffold. Porous CSM of uniform size (175-225 μm in diameter) and composition were prepared and used as cell carriers (Figure 2). Upon incubating ASC with the CSM, the cells attached at a concentration of 2 x 104 cells/5mg of CSM. The cells were capable of spreading over the microsphere, while extending filopodia into the porous crevices of the microsphere (Figure 3). Once the cell-loaded CSM were mixed with the collagen gel, the cells immediately began migrating into the gels (Figure 4).
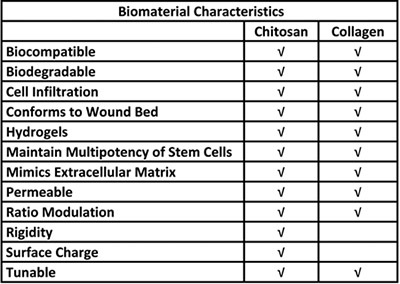 Table 1. Biological advantages for the use of chitosan and collagen in a stem cell delivery system.
Table 1. Biological advantages for the use of chitosan and collagen in a stem cell delivery system.
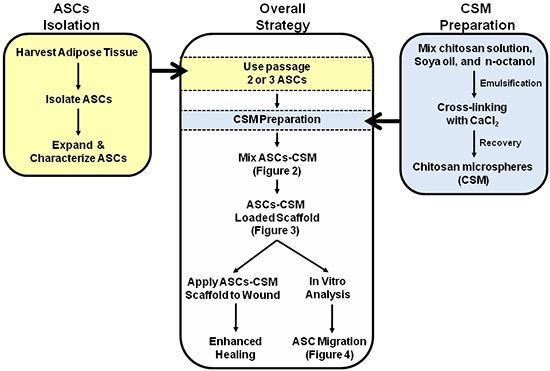 Figure 1. Schematic depicting the overall strategy for the dual use of ASC-CSM loaded collagen scaffolds. Figures 2, 3, and 4 are annotated within the schematic to assist with interpretation of the images.
Figure 1. Schematic depicting the overall strategy for the dual use of ASC-CSM loaded collagen scaffolds. Figures 2, 3, and 4 are annotated within the schematic to assist with interpretation of the images.
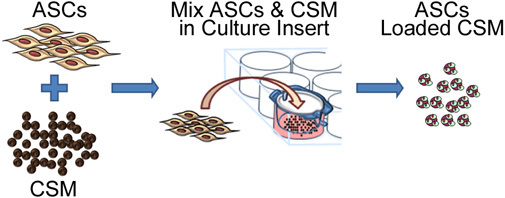 Figure 2. Schematic representation depicting the process of seeding stem cells onto chitosan microspheres. The process involves co-culturing ASC with CSM in an 8- μm pore size membrane culture plate insert. After 24 hours, the microspheres are removed from the insert and are ready for embedding into a biomaterial matrix.
Figure 2. Schematic representation depicting the process of seeding stem cells onto chitosan microspheres. The process involves co-culturing ASC with CSM in an 8- μm pore size membrane culture plate insert. After 24 hours, the microspheres are removed from the insert and are ready for embedding into a biomaterial matrix.
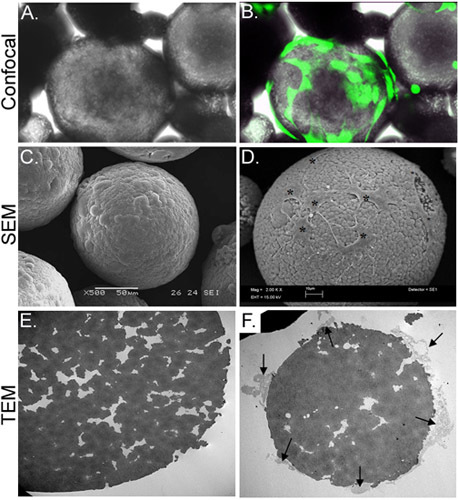 Figure 3. Morphological characterization of CSM-loaded with ASC. Panel A depicts a light micrograph of ASC-loaded CSM, while panel B shows the same field of view superimposed with an image obtained by confocal fluorescence microscopy. ASC were preloaded with calcein AM (green). Panel C depicts an image of an SEM image of an unloaded microsphere, while panel D shows cells loaded onto the microsphere (asterisks). The TEM image in panel E shows a cross-section of an unloaded microsphere. A multitude of pores and crevices are located throughout the microsphere. Panel F shows a cross-sectioned microsphere with cells (arrows) attached and extending filopodia into the crevices. Original magnifications: A & B= 70X; C= 500x , D= 2,000x; E&F= 2,500x.
Figure 3. Morphological characterization of CSM-loaded with ASC. Panel A depicts a light micrograph of ASC-loaded CSM, while panel B shows the same field of view superimposed with an image obtained by confocal fluorescence microscopy. ASC were preloaded with calcein AM (green). Panel C depicts an image of an SEM image of an unloaded microsphere, while panel D shows cells loaded onto the microsphere (asterisks). The TEM image in panel E shows a cross-section of an unloaded microsphere. A multitude of pores and crevices are located throughout the microsphere. Panel F shows a cross-sectioned microsphere with cells (arrows) attached and extending filopodia into the crevices. Original magnifications: A & B= 70X; C= 500x , D= 2,000x; E&F= 2,500x.
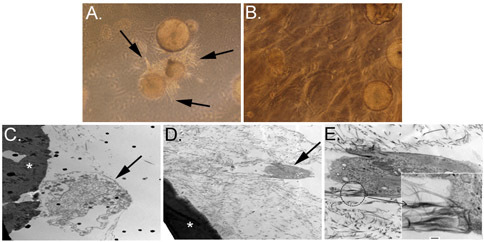 Figure 4. Migration of ASC from the CSM into the three-dimensional collagen scaffold. Panels A and B depict the CSM with cells migrating away from microsphere and into the collagen matrix on day 3 (A, arrows). Panel B shows a similar culture after 12 days. Transmission electron microscopy (TEM) images are depicted in C, D, and E. Asterisks in C and D show a microsphere that has been cross-sectioned with cells migrating away from the microsphere (arrows). Higher magnification of panel D is depicted in panel E, and shows cell filopodia attached to the collagen fibrils (inset). Original magnification: A&B = 100x; C&D = 6,000x; E = 20,000x, inset = 150,000x.
Figure 4. Migration of ASC from the CSM into the three-dimensional collagen scaffold. Panels A and B depict the CSM with cells migrating away from microsphere and into the collagen matrix on day 3 (A, arrows). Panel B shows a similar culture after 12 days. Transmission electron microscopy (TEM) images are depicted in C, D, and E. Asterisks in C and D show a microsphere that has been cross-sectioned with cells migrating away from the microsphere (arrows). Higher magnification of panel D is depicted in panel E, and shows cell filopodia attached to the collagen fibrils (inset). Original magnification: A&B = 100x; C&D = 6,000x; E = 20,000x, inset = 150,000x.
 Figure 5. Schematic depicting the vast uses of CSM in regenerative medicine and drug delivery.
Figure 5. Schematic depicting the vast uses of CSM in regenerative medicine and drug delivery.
Discussion
A major hurdle in stem cell-based therapy is developing efficient methods for delivery of cells to the specified regions for repair. Due to patient to patient variability, the tissue type, injury size and depth; the methodology of delivering stem cells must be determined on a case-by-case basis. Although embedding stem cells within a matrix and delivering them to the wound site appears to be a next logical approach for tissue engineering, some technical hurdles remain. This includes the ability of the embedded cells to attach to the matrix and provide a biocompatible surrounding in which the cells can attach, proliferate and reestablish a suitable microenvironment before anoikis 9-10. This process requires the cells to upregulate their matrix remodeling machinery, a process that can take days to accomplish prior to the desired cell processes of proliferation, migration and differentiation of the stem cells. To circumvent these issues, we have developed a method to preload stem cells onto chitosan microspheres prior to embedding them into an appropriate matrix to repair of the damaged tissue. Using our unique ionic cross-linking methods to prepare the microspheres, the protocol is able to prepare >90% of the microspheres to be within 175-225 μm in diameter, thus generating an excellent micro-sized carrier for cells. More importantly, after cross-linking, the microspheres remain positively charged, which is highly conducive for cell attachment. These microsphere characteristics allow stem cells to attach quickly onto the CSM. Once attached, these stem cell loaded microspheres are stable and able to attain spatial arrangements within the scaffold, such as collagen hydrogels, to mimic the injured tissue. In sum, this protocol provides a new method to load ASC onto CSM and illustrates that cells on the microspheres can migrate while preserving their stem cell phenotype. The porous CSM described provide an excellent microenvironment for ASC attachment and still allow the cells to migrate into the surrounding matrix.
Summary:
Critical Steps
Use uniformly sized microspheres since variation in diameter directly influences surface area available for cell attachment per milligram of microspheres.
Prepare a uniform bed of microspheres within the culture insert to optimal uniform attachment of cells to the CSM.
Ensure that the volume of media added to the outer well of the cell culture insert is higher than the meniscus of the inner well. Typically, an inner:outer volume ratio of 1:2 is appropriate, as this facilitates cells attaching to the microspheres.
Distribute the cell loaded microspheres evenly within the scaffold. If the distribution of ASC-CSM is too dense, the cells will not migrate from their microspheres.
The desired quantity of CSM and distribution density is dependent upon application and injured tissue area.
Limitations
This method is limited to the total surface area available on the microspheres. If a higher cell number is desired, more microspheres are required to increase the surface area.
The process allows cells to be seeded on to the preformed microspheres and is not intended for cell encapsulation purposes.
The chitosan microspheres developed in this process can support anchorage-dependent cells but not other cells types.
Since chitosan microspheres are developed using an 0.5M acetic acid solution, emulsified in an oil mixture (soya oil:n-octanol) and recovered using organic solvent it does not allow active therapeutic moieties vulnerable to the above mentioned condition.
The CSM are mainly intended to deliver cells/drugs in shorter time duration (5-10 days) due to its faster degradation rate, unlike those intended for long duration availability.
Possible Modifications and Versatility (Figure 5)
Microsphere size may be modified to alter the total surface area. For example, microsphere size may be reduced to increase the surface area/mg of CSM, which in turn will increase the total surface area and the number of cells that can attach to the microspheres, resulting in an increased cell load per microsphere.
Chitosan microspheres can be used as a carrier to load other cell types (endothelial cells, pericytes, fibroblast, etc.).
Cell-loaded spheres (either single- or multiple-cell types) can be embedded within specific locations of scaffolds. This strategy will help in the construction of multicellular tissue-engineered scaffolds for tissue regeneration.
In addition to cell delivery, microspheres can also be used to deliver therapeutic drugs (i.e., growth factors, differentiation inducers, and/or antibiotics). These drug-loaded microspheres may also be used in combination with cell-loaded microspheres within a scaffold to enhance the healing and regeneration processes.
Troubleshooting
- Upon preparing the CSM, if a large variation in size occurs, then a number of items can be adjusted to yield desired CSM size 11.
- Viscosity of the chitosan solution. Freshly prepared stock is preferred to keep the solution under constant viscosity from batch-to-batch preparation
- Adjust volume of surfactant (span 80) used to prepare the emulsion.
- Ensure the speed of the overhead stirrer stays constant throughout the process (speed may vary with electrical power fluctuations
- During stirring imparting atmospheric air may cause turbulence and size variation (this can be avoided by keep the liquid ripple, which forms during stirring, just over the blades of the overhead stirrer.
To ensure optimal cell loading of the microspheres, a calorimetric MTT assay can be performed. This will give an estimated number of viable cells loaded per milligram of microspheres.
Prevent clumping of cell-loaded microspheres by suspending them in a small volume of cell culture media before mixing them within a scaffold such as a collagen hydrogel.
Disclosures
No competing financial interests exist.
Disclaimers
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the U.S. Government. The authors are employees of the U.S. Government, and this work was prepared as part of their official duties. All work was supported by the U.S. Army Medical Research and Materiel Command. This study was conducted under a protocol reviewed and approved by the US Army Medical Research and Materiel Command Institutional Review Board, and in accordance with the approved protocol.
Acknowledgments
D.O.Z. is supported by a grant awarded from The Geneva Foundation. S.N. was supported by a Postdoctoral Fellowship Grant from the Pittsburgh Tissue Engineering Initiative.
References
- Krampera M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39:678–683. doi: 10.1016/j.bone.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Patrick CW. Tissue engineering strategies for adipose tissue repair. Anat. Rec. 2001;263:361–366. doi: 10.1002/ar.1113. [DOI] [PubMed] [Google Scholar]
- Pountos I, Giannoudis PV. Biology of mesenchymal stem cells. Injury. 2005;36(Suppl 3):S8–S12. doi: 10.1016/j.injury.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Kim IY. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Shi C. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 2006;133:185–192. doi: 10.1016/j.jss.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Natesan S. Adipose-derived stem cell delivery into collagen gels using chitosan microspheres. Tissue Eng. Part. A. 2010;16:1369–1384. doi: 10.1089/ten.tea.2009.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubnis WA, Ofner CM., 3rd The determination of epsilon-amino groups in soluble and poorly soluble proteinaceous materials by a spectrophotometric method using trinitrobenzenesulfonic acid. Anal. Biochem. 1992;207:129–133. doi: 10.1016/0003-2697(92)90513-7. [DOI] [PubMed] [Google Scholar]
- Bornstein MB. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 1958;7:134–137. [PubMed] [Google Scholar]
- Benoit DS. Integrin-linked kinase production prevents anoikis in human mesenchymal stem cells. J. Biomed. Mater. Res. A. 2007;81:259–268. doi: 10.1002/jbm.a.31292. [DOI] [PubMed] [Google Scholar]
- Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005;24:208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan S. Preparation and characterization of chitosan microspheres for doxycycline delivery. Carbohydr. Polym. 2008;73:201–211. [Google Scholar]
- Haque T, Chen H, Ouyang W, Martoni C, Lawuyi B, Urbanska A, Prakash S. Investigation of a new microcapsule membrane combining alginate, chitosan, polyethylene glycol and poly-L-lysine for cell transplantation applications. Int. J. Artif. Organs. 2005;28:631–637. doi: 10.1177/039139880502800612. [DOI] [PubMed] [Google Scholar]
- Goren A, Dahan N, Goren E, Baruch L, Machluf M. Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy. FASEB J. 2010;24:22–31. doi: 10.1096/fj.09-131888. [DOI] [PubMed] [Google Scholar]
- Zielinski BA, Aebischer P. Chitosan as a matrix for mammalian cell encapsulation. Biomaterials. 1994;15:1049–1056. doi: 10.1016/0142-9612(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Girandon L, Kregar-Velikonja N, Božikov K, Barliç A. In vitro Models for Adipose Tissue Engineering with Adipose-Derived Stem Cells Using Different Scaffolds of Natural Origin. Folia Biol. (Praha) 2011;57:47–56. [PubMed] [Google Scholar]
- Baruch L, Machluf M. Alginate-chitosan complex coacervation for cell encapsulation: effect on mechanical properties and on long-term viability. Biopolymers. 2006;82:570–579. doi: 10.1002/bip.20509. [DOI] [PubMed] [Google Scholar]
- Wei Y, Gong K, Zheng Z, Wang A, Ao Q, Gong Y, Zhang X. Chitosan/silk fibroin-based tissue-engineered graft seeded with adipose-derived stem cells enhances nerve regeneration in a rat model. J. Mater. Sci. Mater. Med. 2011. [DOI] [PubMed]
- Wang Q, Jamal S, Detamore MS, Berkland C. PLGA-chitosan/PLGA-alginate nanoparticle blends as biodegradable colloidal gels for seeding human umbilical cord mesenchymal stem cells. J. Biomed. Mater. Res. A. 2011;96:520–527. doi: 10.1002/jbm.a.33000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves da Silva ML, Martins A, Costa-Pinto AR, Correlo VM, Sol P, Bhattacharya M, Faria S, Reis RL, Neves NM. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J. Tissue Eng. Regen. Med. 2010. [DOI] [PubMed]
- Kang YM, Lee BN, Ko JH, Kim GH, Kang KN, Kim da Y, Kim JH, Park YH, Chun HJ, Kim CH, Kim MS. In vivo biocompatibility study of electrospun chitosan microfiber for tissue engineering. Int. J. Mol. Sci. 2010;11:4140–4148. doi: 10.3390/ijms11104140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt G, Mothe AJ, Zahir T, Kim H, Shoichet MS, Tator CH. Chitosan channels containing spinal cord-derived stem/progenitor cells for repair of subacute spinal cord injury in the rat. Neurosurgery. 2010;67:1733–1744. doi: 10.1227/NEU.0b013e3181f9af35. [DOI] [PubMed] [Google Scholar]
- Leipzig ND, Wylie RG, Kim H, Shoichet MS. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials. 2011;32:57–64. doi: 10.1016/j.biomaterials.2010.09.031. [DOI] [PubMed] [Google Scholar]
- Altman AM, Gupta V, RÃos CN, Alt EU, Mathur AB. Adhesion, migration and mechanics of human adipose-tissue-derived stem cells on silk fibroin-chitosan matrix. Acta Biomater. 2010;6:1388–1397. doi: 10.1016/j.actbio.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Altman AM, Yan Y, Matthias N, Bai X, Rios C, Mathur AB, Song YH, Alt EU. IFATS collection: Human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2009;27:250–258. doi: 10.1634/stemcells.2008-0178. [DOI] [PubMed] [Google Scholar]
- Machado CB, Ventura JM, Lemos AF, Ferreira JM, Leite MF, Goes AM. 3D chitosan-gelatin-chondroitin porous scaffold improves osteogenic differentiation of mesenchymal stem cells. Biomed. Mater. 2007;2:124–131. doi: 10.1088/1748-6041/2/2/010. [DOI] [PubMed] [Google Scholar]


