Abstract
One major obstacle in current diabetic wound research is a lack of an ischemic wound model that can be safely used in diabetic animals. Drugs that work well in non-ischemic wounds may not work in human diabetic wounds because vasculopathy is one major factor that hinders healing of these wounds. We published an article in 2007 describing a rabbit ear ischemic wound model created by a minimally invasive surgical technique. Since then, we have further simplified the procedure for easier operation. On one ear, three small skin incisions were made on the vascular pedicles, 1-2 cm from the ear base. The central artery was ligated and cut along with the nerve. The whole cranial bundle was cut and ligated, leaving only the caudal branch intact. A circumferential subcutaneous tunnel was made through the incisions, to cut subcutaneous tissues, muscles, nerves, and small vessels. The other ear was used as a non-ischemic control. Four wounds were made on the ventral side of each ear. This technique produces 4 ischemic wounds and 4 non-ischemic wounds in one animal for paired comparisons. After surgery, the ischemic ear was cool and cyanotic, and showed reduced movement and a lack of pulse in the ear artery. Skin temperature of the ischemic ear was 1-10 °C lower than that on the normal ear and this difference was maintained for more than one month. Ear tissue high-energy phosphate contents were lower in the ischemic ear than the control ear. Wound healing times were longer in the ischemic ear than in the non-ischemic ear when the same treatment was used. The technique has now been used on more than 80 rabbits in which 23 were diabetic (diabetes time ranging from 2 weeks to 2 years). No single rabbit has developed any surgical complications such as bleeding, infection, or rupture in the skin incisions. The model has many advantages, such as little skin disruption, longer ischemic time, and higher success rate, when compared to many other models. It can be safely used in animals with reduced resistance, and can also be modified to meet different testing requirements.
Keywords: Medicine, Issue 63, Wound, ischemia, rabbit, minimally invasive, model, diabetes, physiology
Protocol
1. Anatomic Basis
The rabbit ear has been a favored study material for wound care since the early 20th century. The ear is supplied by three vascular bundles (central, cranial, and caudal); the central bundle is the largest, while the vessels in the cranial and caudal bundles vary in size. Occasionally, the caudal artery may be absent.1 All of these vessels are easily identified on the dorsal side after the ear is shaved (Fig. 1). On the ventral side, the distal skin does not receive blood supply from the proximal vessels. The three vessel bundles on the dorsal ear have numerous branches that penetrate through the cartilage to provide blood supply to the distal ventral skin (Fig. 2). In some rabbits, a very small subcutaneous vessel also occurs on the ventral side, which supplies blood to no more than 1/3 of the ventral skin. In an open circumferential skin incision, there is essentially no bleeding from a ventral incision.
2. Surgical Procedure
The rabbit is weighed and the ears are shaved, along with the back of the neck.
A premedication of Buprenorphine (0.01 mg/kg) is given IM one hour before surgery.
Anesthesia is induced by a mixture of ketamine (50 mg/kg) and Xylazine (5 mg/kg) IM.
The rabbit is protected with a heating pad during surgery, the eyes are protected with ointment, and its vital signs are monitored during surgery.
The surgical field is prepared aseptically with a surgical scrub and draped so that the two ears are exposed.
One ear is rendered ischemic, while the other ear serves as a paired non-ischemic control.
The three vascular pedicles are identified by the arteries and veins.
Using a #15 blade, three small vertical incisions (1-1.5 cm) are made on the vascular pedicles, about 1-2 cm from the ear base.
The central vein is isolated from the surrounding tissues and a suture is used to encircle it for retraction. The central artery is ligated with 4-0 sutures and divided.
The accompanying central nerve is also cut.
The whole cranial pedicle is ligated and divided.
The caudal pedicle is preserved.
A subcutaneous tunnel is made through the 3 incisions. This is accomplished by blunt dissection with a clamp and sharp dissections with a knife and scissors. All of the subcutaneous tissues, muscles, nerves, and small vascular branches are discontinued. The small vasculatures underneath the dermis are totally divided. All of the subcutaneous tissues are cut to the level of the cartilage with the blade.
Further sharp and blunt dissections are used to clean up the base, leaving a bare cartilage about 5-10 mm wide.
This procedure interrupts all of the subcutaneous vascular branches to prevent them from rapidly establishing collaterals.
Bleeding is minimal. If there is any, it is easily controlled by gauze compression, without the need for electrocautery.
The skin inside the ear does not carry much blood supply and thus is not incised.
The 3 skin incisions are closed using 4-0 or 5-0 sutures, and covered with sterile gauze.
The procedure results in 3 small skin incisions and incurs much less skin damage than an open surgery.
Four circular full-thickness skin wounds are created on the ventral side of each ear with a 6-mm stainless steel punch. The distance between the wounds is at least 20-30 mm. The skin inside the punch wound is removed from the cartilage. The perichondrium is also removed, with the skin or separately. The base on which granulation and epithelization take place is the cartilage, but the cartilage is not perforated (Fig. 3).
When the wounds on both ears are completed, testing drug is applied to one side of two wounds while the control drug is applied to the wounds on the other side. An occlusive dressing (TegaDerm, 3M) is used to cover the wound site. This prevents the wounds from becoming desiccated.
The wounds at the bottom portion of the ear normally heal faster than the wounds at the top of the ear. For wound healing comparison study, it is recommended to compare the two wounds on one side with the two wounds on the other side, or to compare the top and bottom wounds separately.
3. Postoperative Care
A Duragesic patch is attached to the back skin to release Fentanyl (25 μg/hour) for 2-3 days to reduce possible pain.
The rabbits are allowed free access to food and drink.
Dressing changes are carried out daily. The old dressings are removed and the wounds are cleaned with sterile cotton swabs. Digital photos are taken for comparison. New dressings are applied, and the wounds are covered again by TegaDerm. No anesthetic is needed during dressing changes.
Skin temperature is measured daily on each ear for comparison.
Additional ear blood circulation can be measured with a transcutaneous oxygen tension meter or a laser Doppler flowmeter.
The sutures may be removed after one week.
For non-diabetic animals, most of the ear wounds usually heal within 2-3 weeks.
4. Potential Complications and How to Avoid Them
This minimally invasive surgery is safe and complications are rare. Except for a transitional postoperative mild ear edema, none of our rabbits has had any severe surgical complications. Nevertheless, there are several potential complications for this procedure:
Bleeding. Bleeding may occur in the incisions if hemostasis is incomplete or if a larger blood vessel (such as one of the main pedicle vessels) is damaged. This is easily avoided by careful dissection. In addition to the three major vascular bundles, there are many minor subcutaneous vascular branches. When the subcutaneous tunnel is made, these vessels are being cut but no tying is necessary. Hemostasis can be achieved simply by gauze pressing. This is easily done by inserting a corner of gauze into the tunnel for a few minutes. Before closing the incision, make sure no active bleeding is present.
Incision infection and dehiscence: These complications may occur if the incisions are contaminated. This can be avoided by use of stringent aseptic technique and no antibiotic is needed. However, if infection does occur, appropriate drainage and dressing changes are needed.
Ear edema: Due to vascular ligations and disruption of lymph circulation, mild ear edema may present for a few days. However, it disappears by itself and no treatment is necessary.
5. Representative Results
Since 2007, we have used this procedure in more than 70 rabbits, including 23 diabetic animals. In the diabetic group, surgery was performed at different diabetic times: seven rabbits had two weeks of diabetic time, four of them had two months, ten had 12 months, and 2 had 24 months of diabetic time.
Immediately after surgery, the ischemic ear appeared cool and cyanotic, with mild edema. The most important ear artery-the central artery-had a strong pulse in the normal ear, but this pulse was not present in the ischemic ear. Ear movement was reduced, but did not totally disappear. The skin showed clear depressions over the tunnel, but no ischemia or necrosis was found in any animal (Fig. 4).
The skin temperatures of the normal and ischemic ears were measured with a digital thermometer (Omron by Omron Healthcare, Bannockburn, IL, or a Type K infrared spot thermometer by Fisher Scientific, Pittsburgh, PA). The temperature differences between the non-ischemic and ischemic ear ranged from 1 to 10 °C, with most rabbits falling within the range of 2-6 °C. Fig. 5 shows the average temperature differences from 30 rabbits. The temperature difference was higher in the early days after surgery, decreased gradually after day 15, but was still maintained at the end of one month.
Tissue high-energy phosphate contents were measured in some animals after they were sacrificed. Six tissue samples were taken from each ear and high-energy phosphate contents were measured by HPLC. Both ATP and total energy concentrations were higher in the normal ears than in the ischemic ears. Fig. 6 shows an example of a rabbit sacrificed 23 days after surgery. The mean tissue ATP contents were 0.349±0.047 μMol/g (mean±SD) in the normal ear vs. 0.237±0.059 μMol/g in the ischemic ear (p<0.005). Mean total energy (ATP+ADP+AMP) was 0.882±0.137 μMol/g in the normal ear vs. 0.556±0.115 μMol/g in the ischemic ear (p<0.001).
Wound healing times were always longer for the ischemic ear than for the non-ischemic ear in all groups (non-diabetic or diabetic). Fig. 7 shows a comparison of healing between 26 pairs of wounds on ischemic and non-ischemic ears. Healing times ranged from 13 to 18 days (mean 14.9±1.6 days) on the normal ears vs. 17 to 27 days (mean 20.5±3.4 days) on the ischemic ears (p=0.001). Wound healing times in aged and diabetic rabbits were much longer, especially when diabetic time was more than 12 months.
In these rabbits, all the incisions healed normally without any complications, including the rabbits with 24 months of diabetic time. Figure 8 is a histology sample showing the healed subcutaneous tunnel in which muscle is absent and is replaced by fibrous tissues. However, the skin is intact.
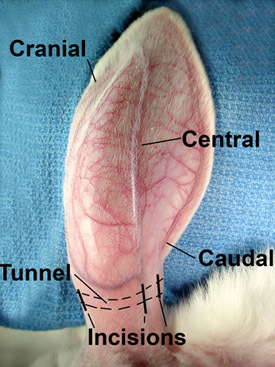 Figure 1. The rabbit ear is supplied by three vascular bundles. The central is the largest, the cranial is smaller, and the caudal bundle is the smallest. These vessels are easily seen on the surface after the ear is shaved.
Figure 1. The rabbit ear is supplied by three vascular bundles. The central is the largest, the cranial is smaller, and the caudal bundle is the smallest. These vessels are easily seen on the surface after the ear is shaved.
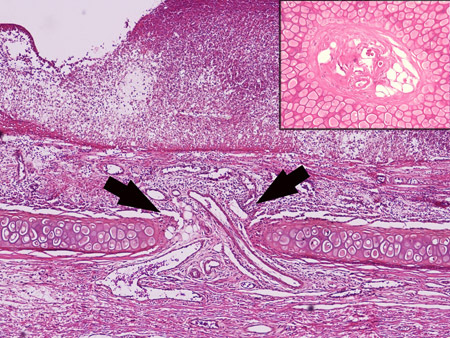 Figure 2. The three vessel bundles on the dorsal ear supply blood not only to the dorsal skin, but also to the ventral skin through penetrating vessels. The arrow shows the penetrating vessels through the cartilage. The inset shows cross section of these vessels.
Figure 2. The three vessel bundles on the dorsal ear supply blood not only to the dorsal skin, but also to the ventral skin through penetrating vessels. The arrow shows the penetrating vessels through the cartilage. The inset shows cross section of these vessels.
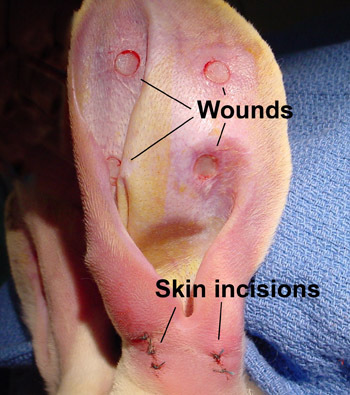 Figure 3. Four 6-mm wounds are created on the ventral side of the ear, and the perichondrium is also removed. The base on which granulation and epithelization take place is the cartilage, but the cartilage is not perforated. The 3 small skin incisions are closed with very little skin disruption.
Figure 3. Four 6-mm wounds are created on the ventral side of the ear, and the perichondrium is also removed. The base on which granulation and epithelization take place is the cartilage, but the cartilage is not perforated. The 3 small skin incisions are closed with very little skin disruption.
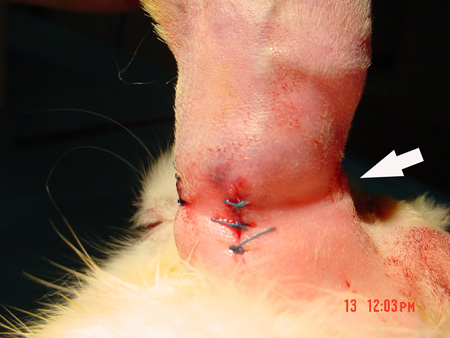 Figure 4. After the skin incisions are closed, the indention over the subcutaneous tunnel is clearly seen.
Figure 4. After the skin incisions are closed, the indention over the subcutaneous tunnel is clearly seen.
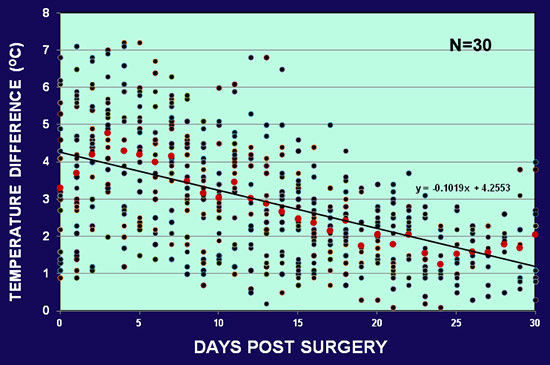 Figure 5. The average skin temperature difference between the normal and ischemic ear in 30 rabbits. The early difference ranges from 7 °C to 10 °C after surgery. The difference decreases gradually but still maintained at the end of one month.
Figure 5. The average skin temperature difference between the normal and ischemic ear in 30 rabbits. The early difference ranges from 7 °C to 10 °C after surgery. The difference decreases gradually but still maintained at the end of one month.
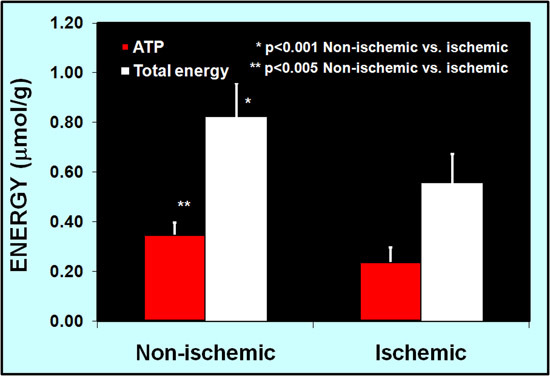 Figure 6. Ear tissue high-energy phosphate contents are reduced in the ischemic ear as compared to non-ischemic ear at 23 days after surgery (p<0.001).
Figure 6. Ear tissue high-energy phosphate contents are reduced in the ischemic ear as compared to non-ischemic ear at 23 days after surgery (p<0.001).
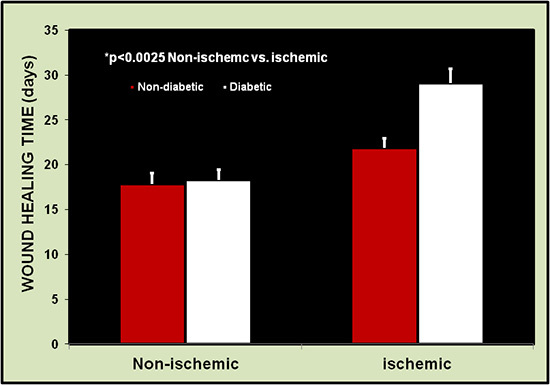 Figure 7. Wound healing time is longer in the ischemic ears than the non-ischemic ears (26 wounds each). Healing is defined as the restoration of integrity to injured tissue, i.e., the complete reepithelialization of wound sites.
Figure 7. Wound healing time is longer in the ischemic ears than the non-ischemic ears (26 wounds each). Healing is defined as the restoration of integrity to injured tissue, i.e., the complete reepithelialization of wound sites.
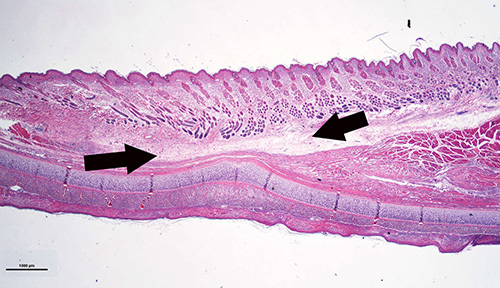 Figure 8. Histology sample showing the ear portion containing subcutaneous tunnel. The healed tissue is thinner than normal ear, the original skeletal muscle is absent and is replaced by fibrous tissues. However, the skin is intact.
Figure 8. Histology sample showing the ear portion containing subcutaneous tunnel. The healed tissue is thinner than normal ear, the original skeletal muscle is absent and is replaced by fibrous tissues. However, the skin is intact.
Discussion
Diabetes affects 23.6 million people in the US,2,3 and 15-20% of these patients develop foot ulcers in their lifetime,4,5 at an annual treatment cost of more than $25 billion.3,6 However, even the best available treatments achieve only a 50% healing rate and this is often temporary,7 with recurrence rates as high as 66% in diabetics.3,6 When ulcers occur in diabetic patients, major complications such as infection and amputation increase dramatically. Despite almost a century of extensive research and the development of thousands of dressing products, none has yet proven effective. The statement that "Old ulcers in 1830 will be the older ulcers in 1860," made in one of the first papers describing chronic wound treatment, still applies today.8 Finding an appropriate animal model for wound study has been a major challenge for scientists as well as for clinicians. Many animal models have been created or suggested for wound care study, but a wound model comparable to a chronic, non-healing human wound is nonexistent.9,10 This is because all human diabetic wounds have a combination of tissue ischemia, chronic hyperglycemia, oxidative stress, neuropathy, and bacterial colonization. Ischemia may not be the earliest cause of diabetic foot ulcers, but it is the main reason for the persistence of these wounds,11. This is because wounds do not heal in tissue that does not bleed, whereas they almost always heal in tissue that bleeds extensively.12-15 Unfortunately, none of the currently used animal ischemic wound models has yet been used successfully in diabetic animals, and this has created a major obstacle for the testing of wound drugs. Small scale clinical trials of growth factors, such as bFGF, have shown them to be highly effective, but they are ineffective in hypoxic dermal ulcer models.16,17
We published an article in 2007 describing an ischemic rabbit ear wound model that was used successfully in diabetic animals.18. The technique is somewhat difficult to perform because a careful dissection of the cranial neurovascular bundle is needed. Because these vessels are small, their contribution to the postoperative recovery is negligible. Since then, we have simplified the procedure by cutting the entire cranial neurovascular bundle. To compare with the procedure in which the cranial bundle was preserved, there was no difference in wound healing or ear complications. This modified minimally invasive procedure has the following advantages: 1). It is simple, safe, and has a high success rate, which reduces pain and suffering for the animals. No surgical complication has occurred in these animals, including a few rabbits with 24 months of diabetic time; 2). It has a long ischemic time. Our measurements indicated a skin temperature difference at the end of one month. It is possible that the extensive subcutaneous tissue dissection without requiring suturing the tissue back results in delayed collateral formation; 3). It is very flexible. Because the three vascular bundles are all under surgical control, this model can easily be made to mimic various chronic wounds (see below); 4). Unlike the skin in other locations, these wounds have no hair follicles in the wound bed. The healing is totally from the wound edge; 5). This procedure creates 4 ischemic wounds and 4 non-ischemic wounds in the same rabbit for paired comparison, thereby reducing individual variations; 6). Skin contraction is minimal. While the dorsal skin of the rabbit ear has some mobility, the ventral skin is splinted by cartilage, allowing more accurate study of re-epithelialization;19 7). Rabbits are generally timid and nonaggressive. Anesthetics are needed only during wounding surgery. This can reduce any potential confounding factors that may affect wound healing processes; 8). It is also very economical because up to 8 wounds can be made in one rabbit. 9). In contrast to some previous reports, we have not seen any hypertrophic scar formation in either non-diabetic or diabetic rabbits observed for more than 24 months after surgery. The mechanism is still unclear.
Several approaches can be taken to modify the operation to meet different testing requirements. 1). Further extension of ischemic time. Our current results show that the skin temperature difference is maintained at least one month. If some inert material (such as silicone) is used to fill the tunnel, to delay the collateral formation, ischemic time could be extended. We tried this technique in some rabbits and found very little tissue reaction; 2). Total ear denervation. Our technique cuts the nerves in the two vascular pedicles (central and cranial), leaving the caudal branch intact. If total wound denervation is needed, the nerve branch in the caudal pedicle can also be severed as well; 3). If wound bacterial inoculation is needed, it can be applied to the wound. Because the base of the wound is ear cartilage, it has the potential to get infection and perforation. To prevent cartilage complications, bacterial inoculation should be applied after the cartilage is covered by granulation tissue. It should be noted that bacterial contamination is easily made and it can significantly extend the healing time. However, establishment of biofilm in acute wounds is more difficult. Using clinical material and state-of-the-art technology, biofilm has been shown to be established in only 6% of acute wounds, while in chronic wounds the incidence is ten times higher (60%);20 Our experience has shown that when diabetic times reach two years, most of the wounds on the ischemic ear can become infected even with strict aseptic technique. 4). Pressure ulcers often occur in patients with central or peripheral nerve damage due to loss of sensation. Using this model, a pressure ulcer can also be created by various compression methods.21,22
Disclosures
There is no financial conflict of interest involved in this study.
Acknowledgments
This study was supported in part by NIH grants 1RO1DK74566 and 1RO1AM52984. The authors would like to thank Ms. Jacqueline McCarty in RRC for her help during surgery, Drs. Jianpu Wang and Harshini Sarojini for their help in surgery and postoperative care of some rabbits, Dr. Rong Wan for her histology work, and Ms. Ming Li for her HPLC measurements of high-energy phosphates.
References
- Ninomiya H. The vascular bed in the rabbit ear: microangiography and scanning electron microscopy of vascular corrosion casts. Anat. Histol. Embryol. 2000;29:301–305. doi: 10.1046/j.1439-0264.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association: Diabetes statistics. American Diabetes Association; 2011. Available from: http://www.diabetes.org/diabetes-basics/diabetes-statistics. [Google Scholar]
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst. Rev. 2010;1:1–40. doi: 10.1002/14651858.CD003556.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdin F, Tennenhaus M, Schaller HE, Rennekampff HO. Evidence-based Management Strategies for Treatment of Chronic Wounds. Eplasty. 2009;9:169–179. [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- Bennett JP, Matthews R, Faulk WP. Treatment of chronic ulceration of the legs with human amnion. Lancet. 1980;1:1153–1156. doi: 10.1016/s0140-6736(80)91616-5. [DOI] [PubMed] [Google Scholar]
- Gottrup F, Agren MS, Karlsmark T. Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair. 2000;8:83–96. doi: 10.1046/j.1524-475x.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- Lindblad WJ. Animal models in wound healing research: do we need more. Wound Repair. 2000;8:81–82. doi: 10.1046/j.1524-475x.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- LoGerfo FW, Gibbons GW. Vascular disease of the lower extremities in diabetes mellitus. Endocrinol. Metab. Clin. North Am. 1996;25:439–445. doi: 10.1016/s0889-8529(05)70332-7. [DOI] [PubMed] [Google Scholar]
- Gottrup F. Oxygen, wound healing and the development of infection. Present status. Eur. J. Surg. 2002;168:260–263. doi: 10.1002/ejs.43. [DOI] [PubMed] [Google Scholar]
- Niinikoski J. The effect of blood and oxygen supply on the biochemistry of repair. 2002. pp. 56–71.
- Schaffer M, Witte M, Becker HD. Models to study ischemia in chronic wounds. Int. J. Low Extrem. Wounds. 2002;1:104–111. doi: 10.1177/1534734602001002005. [DOI] [PubMed] [Google Scholar]
- Niinikoski J, Gottrup F, Hunt TK. The role of oxygen in wound repair. 1991. pp. 165–174.
- Harding KG, Morris HL, Patel GK. Science, medicine and the future: healing chronic wounds. BMJ. 2002;324:160–163. doi: 10.1136/bmj.324.7330.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad WJ. Editorial: How should one study wound healing. Wound Repair. 2007;14:515–515. doi: 10.1111/j.1743-6109.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- Chien S. Ischemic rabbit ear model created by minimally invasive surgery. Wound Repair Regen. 2007;15:928–935. doi: 10.1111/j.1524-475X.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- Joseph J, Townsend FJ. The healing of defects in immobile skin in rabbits. Br. J. Surg. 1961;48:557–564. doi: 10.1002/bjs.18004821121. [DOI] [PubMed] [Google Scholar]
- James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Niitsuma J, Yano H, Togawa T. Experimental study of decubitus ulcer formation in the rabbit ear lobe. J. Rehabil. Res. Dev. 2003;40 doi: 10.1682/jrrd.2003.01.0067. [DOI] [PubMed] [Google Scholar]
- Salcido R, Popescu A, Ahn C. Animal models in pressure ulcer research. J. Spinal Cord Med. 2007;30:107–116. doi: 10.1080/10790268.2007.11753921. [DOI] [PMC free article] [PubMed] [Google Scholar]


