Abstract
Interlocked feedback loops may represent a common feature among the regulatory systems controlling circadian rhythms. The Neurospora circadian feedback loops involve white collar-1 (wc-1), wc-2, and frequency (frq) genes. We show that WC-1 and WC-2 proteins activate the transcription of frq gene, whereas FRQ protein plays dual roles: repressing its own transcription, probably by interacting with the WC-1/WC-2 complex, and activating the expression of both WC proteins. Thus, they form two interlocked feedback loops: one negative and one positive. We establish the physiological significance of the interlocked positive feedback loops by showing that the levels of WC-1 and WC-2 determine the robustness and stability of the clock. Our data demonstrate that with WC-1 being the limiting factor in the WC-1/WC-2 complex, the greater the levels of WC-1 and WC-2, the higher the level of the FRQ oscillation and the more robust the overt rhythms. Our data also show that, despite considerable changes in the levels of WC-1, WC-2, and FRQ, the period of the clock has been limited to a small range, suggesting that the interlocked circadian feedback loops are also important for determining the circadian period length of the clock.
In eukaryotic and certain prokaryotic organisms, circadian clocks are responsible for controlling a wide variety of physiological, behavioral, cellular, and biochemical activities. At the molecular level, a common theme of various circadian oscillators is a network of positive and negative elements that form the core of the oscillators that establish the negative feedback loops generating the basic circadian rhythmicity (1). In a simple view, every oscillator has both positive and negative elements to comprise the feedback loop. The positive elements of the loop activate the expression of the negative elements, whereas the negative elements feedback to block their own activation by the positive elements. The identified positive elements in Neurospora, Drosophila, and mammals are all PAS domain-containing transcription factors (2–7). These factors form heterodimeric complexes and activate the transcription of the negative elements in each system, and the protein products of these negative elements feedback to inhibit their own expression (8–12).
Recently, studies in Neurospora, Drosophila, and mammals have significantly furthered our view of the negative feedback nature of the circadian oscillator with the identification of interlocked feedback loops (13–15). In each system, the negative elements of the oscillator have been found to activate the expression of one of the positive elements. Thus, the negative and the positive elements form another positive feedback loop interlocked with the negative feedback loop. The similarity of such an arrangement in different clock systems suggests that it may be a common aspect in the eukaryotic circadian oscillators. However, evidence to support the physiological significance of the positive feedback loops is still lacking.
In the Neurospora frq-wc based circadian feedback loops, the two PAS domain-containing transcription factors, WHITE COLLAR-1 (WC-1) and WC-2, form heterodimeric complexes and function as the positive components (2, 16), whereas two forms of the FRQ protein are the negative elements (9, 17). WC-1 and WC-2 proteins have two different roles in Neurospora (2, 18, 19). First, they are both essential for the light induction of gene expression of all known light-induced genes, including light induction of frq and light resetting of the clock. Second, in constant darkness, WC-1 and WC-2 are required for the activation of frq and the generation of circadian rhythms. In mutants with either wc-1 or wc-2 lesions, the levels of frq mRNA and FRQ protein are very low, and the clock is not running at normal conditions (2).
The frequency (frq) gene, the first known Neurospora clock gene, plays an essential role in the frq-wc based circadian feedback loop (1, 9, 20, 21). Both forms of FRQ protein (large and small FRQ forms) can negatively feedback to repress their own transcription (9, 17). This repression is probably achieved through protein–protein interactions between FRQ and the WC proteins (22). Recently, Lee et al. (15) revealed that FRQ also positively regulates the level of WC-1, leading to rhythmic expression of WC-1 through a posttranscriptional mechanism, and thereby they form a second positive feedback loop. In this study, we demonstrate that both WC proteins are positively regulated by FRQ and that the levels of WC-1 and WC-2 determine the robustness and stability of the Neurospora clock. Thus, the positive feedback loops formed by FRQ and the WCs are important for maintaining robustness and stability of the clock.
Materials and Methods
Strains and Culture Conditions.
The bd, a (wild-type clock) strain was used as the wild-type strain in this study. 93-4 (his-3, bd, frq10), 87-12 (wild-type clock, his-3, bdA), 161-8 (his-3, bd, wc-1), and 241-23 (his-3, bd, wc-2−) were the host strains for various his-3 targeting constructs used (2, 23, 24). In frq10, qa-FRQ; wc-1−, qa-WC-1; wc-2−, qa-WC-2; wc-1+, qa-WC-1; and wc-2+, qa-WC-2 strains, qa-FRQ and qa-WC constructs were transformed into various host strains at the his-3 locus (9, 25). For each transformation, the transformants were first checked by Western blot analysis, and the positive transformants were examined by race tube assays.
Liquid culture conditions were the same as previously described (21), except a lower glucose concentration was used in the media (1× Vogel's/0.1% glucose/0.17% arginine). Such a concentration of glucose was used because the induction of the qa-2 promoter is significantly inhibited by the normally used 2% glucose in the media (9). For rhythmic experiments, the Neurospora cultures were moved from LL to DD at time 0 and were harvested in constant darkness at the indicated time (hours). Race tube assay media contains 1× Vogel's/0% glucose/0.17% arginine/50 ng/ml biotin/1.5% agar. Densitometric analysis of race tubes and calculations of period length were performed as described (17).
Plasmids.
To make the his-3 targeting qa-WC constructs, a PCR fragment containing the promoter of qa-2 (9) was inserted into the BglII site of the pDE3dBH. The resulting plasmid pDE3dBH-qa-2 was the parental vector for pqa-WC constructs. pqa-WC vectors were made by inserting the PCR fragment containing the entire WC-1 ORF (from nucleotide −18 to the SnaBI site of the wc-1 locus) or the StuI–SmaI fragment containing the entire wc-2 ORF into the SmaI site of pDE3dBH-qa-2 (18, 19). The resulting pqa-WC constructs were targeted by transformation to the his-3 locus of the host strains as previously described (25).
Protein and RNA Analyses.
Protein extraction, Western blot analysis, and immunoprecipitation assay are as previously described (22, 26). Equal amounts of total protein (40–100 μg) were loaded in each protein lane, and after the blots were developed by chemiluminescence (ECL, Amersham Pharmacia), they were stained by amido black to verify equal loading of protein (17).
RNA extraction and Northern blot analysis were performed as previously described (20). Equal amounts of total RNA (40 μg) were loaded onto agarose gels for electrophoresis, and the gels were blotted and probed with RNA probe specific for frq, wc-1, or wc-2 (18–20).
Results
The Levels of Both WC-1 and WC-2 Proteins Are Positively Regulated by FRQ.
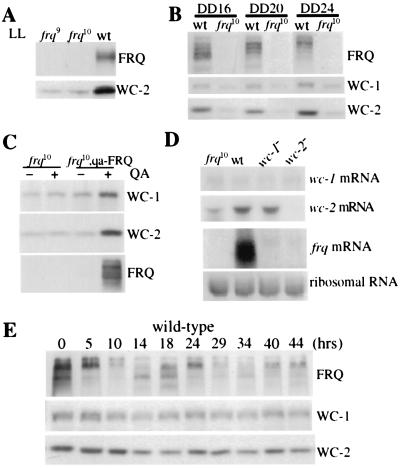
To investigate the role of FRQ in regulating the expression of WC-2, we first examined the level of WC-2 in frq null strains in constant light (LL) and in constant darkness (DD). As shown in Fig. 1 A and B, the level of WC-2 was significantly lower under both conditions in the frq null strains than in the wild-type, suggesting that, like WC-1, the expression of WC-2 is also positively regulated by FRQ. The various FRQ bands seen on Western blots are the results of protein phosphorylation and two alternatively translated FRQ forms (26). To demonstrate the positive role of FRQ on both WC-1 and WC-2, their protein levels were examined in a strain (frq10, qa-FRQ) in which the endogenous frq locus is deleted (23) and the FRQ ORF is under the control of the quinic acid (qa-2)-inducible promoter (9). In the presence of 1 × 10−2 M quinic acid (QA), the levels of both WC-1 and WC-2 were significantly increased in this strain, whereas their amounts were unchanged by the addition of QA in the frq10 strain (Fig. 1C). Together, these results demonstrate that FRQ positively regulates the expression of both WC-1 and WC-2.
Figure 1.
Both WC-1 and WC-2 are positively regulated by FRQ. The WC-2 level is low in the frq null strains in constant light (LL) (A) and constant darkness (DD) (B). Total protein extracts were prepared from the wild-type and the frq null strains (frq9 and frq10) grown in LL or in DD (at different times). Western blot analysis was performed by using WC-1, WC-2, or FRQ antisera (22). Representative results from three independent experiments are shown. (C) Western blot analysis shows that FRQ positively regulated the levels of WC-1 and WC-2 in the dark. Liquid cultures were first grown in LL in media with or without 1 × 10−2 M QA for several hours before being transferred into constant darkness and were harvested at DD24. (D) Northern blot analysis reveals that frq differentially regulates wc-1 and wc-2. Cultures were harvested at DD14. Similar results were obtained for cultures harvested in LL. (E) Western blot analysis shows that the WC-2 level does not fluctuate much in constant darkness in the wild-type strain.
To understand how FRQ regulates wc-1 and wc-2, mRNA levels of wc-1 and wc-2 were examined in frq10, wc-1−(making a truncated WC-1 protein), and wc-2− (wc-2 knock-out) strains (Fig. 1D). Our data indicate that the mechanisms of FRQ controlling wc-1 and wc-2 are different. In agreement with previous reports, the level of frq mRNA is extremely low in the wc mutant strains (2), and the regulation of wc-1 by frq appears to be posttranscriptional because the level of wc-1 mRNA in frq10 is similar to that of the wild-type (15). In addition, wc-1 does not appear to autoregulate its own mRNA level in constant conditions, as indicated by the similar wc-1 level in the wc-1 mutant strain. In contrast, the level of wc-2 mRNA is much lower in frq10, indicating that the positive regulation of FRQ on WC-2 is at least partially achieved by increasing the abundance of wc-2 mRNA.
Because FRQ levels oscillate daily, one would predict that the level of WC-2 protein, like WC-1, would show a circadian rhythm because of the positive effect of FRQ (15). However, we found no robust rhythm of wc-2 mRNA (data not shown) or WC-2 protein in constant darkness under the conditions examined. Despite the lack of a clear rhythm in WC-2 protein level (see Figs. 3 and 4), in some of our rhythmic experiments, we found that WC-2 level, like that of WC-1, showed a trough around DD14–18 (Fig. 1E). Therefore, it is also possible that WC-2 oscillates with a very low amplitude. Similar to what has been reported before (15), the level of WC-1 was rhythmic with a trough around DD14–18 and a peak around DD 24–29. However, the amplitude of the WC-1 rhythm has been variable in our hands, especially with the second trough of the rhythm (compare WC-1 rhythms in this and other figures in this study).
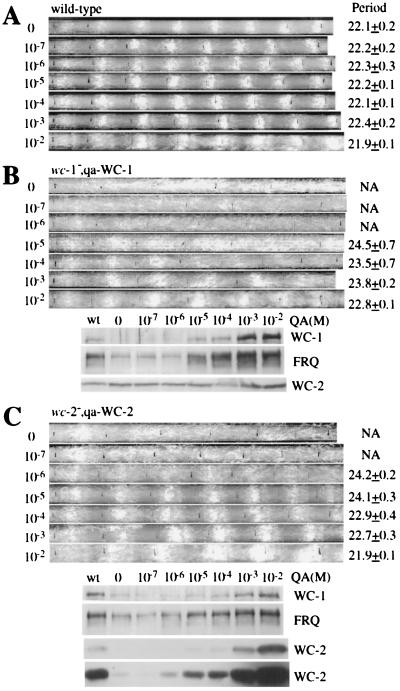
Figure 3.
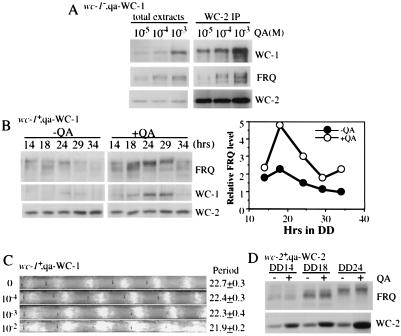
WC-1 and WC-2 are required for circadian rhythmicity, and their levels determine the robustness and stability of the clock. Race tube assays show the conidiation rhythms of the wild-type (A), wc-1−, qa-WC-1 (B, Upper), and wc-2−, qa-WC-2 (C, Upper) strains in the presence of different concentrations of QA (labeled at left). The race tubes shown are representative samples from six replicate tubes. The period lengths of the rhythm (Ave. + SEM) are labeled at right. The lower panels of B and C are Western blot analysis results showing the levels of WC-1, WC-2, and FRQ in the wild-type, wc-1−, qa-WC-1 or wc-2−, qa-WC-2 strain. Liquid cultures were grown in media with different concentrations (indicated above) of QA and were harvested at DD24. No QA was added to the wild-type culture. In C, two different exposures of the WC-2 blot are shown.
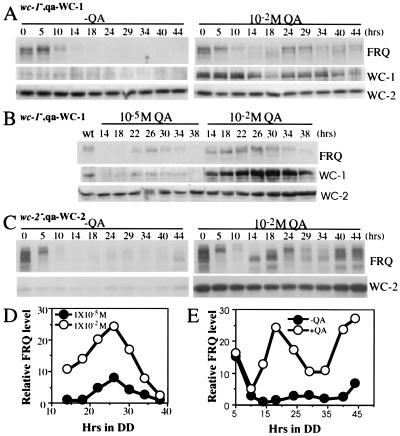
Figure 4.
Western blot analysis results show that a higher level of WC-1 or WC-2 leads to a higher level of FRQ oscillation. (A and B) wc-1−, qa-WC-1 cultures were grown in media with or without QA (1 × 10−5 or 1 × 10−2 M) in DD. The left lane in B shows the protein levels of the wild-type culture grown in LL. (C) A higher level of WC-2 resulted in a higher level of FRQ oscillation. wc-2−, qa-WC-2 cultures were grown in media with or without 1 × 10−2 M QA in DD. (D and E) Densitometric analyses of the Western blots shown in B and C, respectively.
WC-1 and WC-2 Positively Regulate frq.
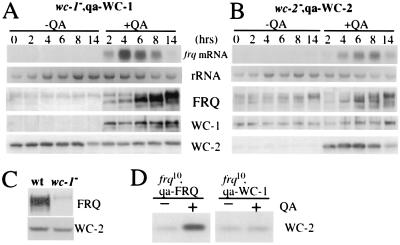
In wc-1 or wc-2 mutants, the levels of frq mRNA and FRQ protein are very low (Fig. 1D), and the clock is not running at normal conditions (2), suggesting that both WC-1 and WC-2 are the positive elements of the frq negative feedback loop. To directly demonstrate the positive role of WC-1 and WC-2 on frq expression, we made strains (wc-1−, qa-WC-1 and wc-2−, qa-WC-2) in which the ORF of either WC-1 or WC-2 is under the control of the quinic acid-inducible promoter (qa-2) at the his-3 locus in either the wc-1− or wc-2− strain (2, 24). In the presence of QA, the qa-2 promoter results in the constitutive induction of the controlled gene (9, 27). At DD14, 1 × 10−2 M QA was added, and protein expressions were monitored afterward. As can be seen from the control lanes (−QA) in Fig. 2 A and B, there is little WC-1 or WC-2 expressed without the inducer (the low-level expression was because of the basal activity of the qa-2 promoter, also see Figs. 3 and 4). The level of FRQ in the wc-1−, qa-WC-1 strain was also very low (only the extensive phosphorylated forms of FRQ can be seen) (28), but its level in the wc-2−, qa-WC-2 strain was significant and may be rhythmic (note the change in FRQ phosphorylation status). These data indicate that even a low level of WC-2 is able to support a significant level of FRQ expression, suggesting that WC-2 is not a limiting factor for frq expression in a wild-type strain. After the addition of QA, the level of WC-1 or WC-2 was increased immediately (peaking after 2 h), suggesting that there was almost no delay between their transcription and translation (Fig. 2 A and B). The induction of either WC-1 or WC-2 quickly leads to the induction of the frq mRNA and FRQ protein. Note that the low molecular weight FRQ forms (the newly synthesized and little phosphorylated two alternatively translated FRQ forms) started to appear in 2–4 h, and FRQ levels reached the peak in 8 h. Interestingly, the level of frq RNA decreased after about 6 h of induction, suggesting that the induction of WC-1 or WC-2 in these strains started running of the clock.
Figure 2.
WC-1 and WC-2 activate the expression of frq. wc-1−, qa-WC-1 (A) and wc-2−, qa-WC-2 (B) strains were used in these experiments. At DD14, 1 × 10−2 M QA was added to half of the cultures. Northern blot and Western blot analyses were performed. The representative results from several independent experiments are shown. The weak and high molecular weight FRQ signals in (A) are the extensively phosphorylated large FRQ forms. (C) Western blot analyses show that there is a low level of FRQ expression in wc-1− strain. (D) Western blot analyses show that the level of WC-1 does not affect the abundance of WC-2 in a frq10, qa-WC-1 strain.
Somewhat to our surprise, we found that the levels of WC-2 were comparable when with or without QA in the wc-1−, qa-WC-1 strain. Two lines of evidence suggest that even the low-level expression of FRQ in wc-1− strain is able to maintain a normal WC-2 level. First, some FRQ is still expressed, albeit at a low level, in wc-1− strain (Fig. 2C). Second, WC-1 does not appear to negatively regulate WC-2 because the induction of WC-1 in a frq− strain did not affect the level of WC-2 (Fig. 2D); therefore, the normal WC-2 level in wc-1− strain is not because of the absence of WC-1. These data also help to explain the fact that the level of WC-2 does not fluctuate much during a circadian cycle.
The Levels of WC-1 and WC-2 Determine the Robustness of the Neurospora Circadian Conidiation Rhythm.
What is the biological significance of the positive role of FRQ on the expression of the WCs? One hypothesis is that the positive regulation of FRQ on WCs would promote the robustness and stability of the clock, as more WC proteins will require more FRQ protein to block their transcriptional activation, thus allowing a more robust oscillation of FRQ. To test this hypothesis, we examined the circadian conidiation rhythms by race tube assays in the wc-1−, qa-WC-1 and wc-2−, qa-WC-2 strains. Because the level of WC-1 or WC-2 can be induced to different levels in these strains by different QA concentrations, the clock phenotypes under these conditions should inform us about the importance of the WC levels on the clock. For the wild-type strain, the presence of different concentrations of QA (−glucose media) had no influence on the conidiation rhythms (Fig. 3A), and it also had no effect on the expression levels of FRQ, WC-1, and WC-2 (Fig. 1C and data not shown). The broader conidiation peaks observed at 1 × 10−2 M QA was because of the carbon source effects of the high concentration of QA. This phenomenon is known to occur when the concentration of the carbon source, such as glucose, sucrose, or QA, is high, as more conidia and aerial hyphae are made, but the clock is not affected by these conditions. As shown in Fig. 3B (Upper), the wc-1−, qa-WC-1 strain was arrhythmic when QA concentration was less than 1 × 10−7 M. At 1 × 10−6 M QA, it showed one conidiation peak in the first day in DD, but the rhythm could not be sustained. When QA was at 1 × 10−5 M, clear circadian conidiation rhythms could be observed, but the very broad conidiation peaks suggest that the amplitude of rhythm was low. As the concentration of QA further increased, the conidiation rhythms became more robust, as indicated by more defined conidiation peaks. These conidiation rhythm data were in agreement with the induction of WC-1 and FRQ in the cell (Fig. 3B, Lower). When QA was less than 1 × 10−5 M, the levels of WC-1 and FRQ were low. At 1 × 10−5 M QA, a significant amount of WC-1 was induced, leading to a higher FRQ expression level. At 1 × 10−4 M QA, the levels of both WC-1 and FRQ were comparable with those in the wild-type strain. At higher QA concentrations, their levels were significantly higher than those of the wild-type. In contrast to FRQ and WC-1, the WC-2 level changed little, suggesting that even low-level expression of FRQ can support normal WC-2 expression.
The wc-2−, qa-WC-2 strain showed results very similar to those of the wc-1−, qa-WC-1 strain, except that it became rhythmic at a lower QA concentration (Fig. 3C). When QA concentration was less than 1 × 10−7 M, one conidiation peak could be observed in the first day, but the rhythm could not be sustained in the later days. When QA was above 1 × 10−6 M, the strain exhibited clear circadian rhythms of conidiation, and the rhythms became more robust at higher QA concentrations. At the molecular level, some FRQ and WC-1 were expressed even when there was no QA present, which was because of the low expression level of WC-2 resulting from the basal activity of the qa-2 promoter (see the longer exposure of WC-2 Western blot in Fig. 3C). At 1 × 10−6 M QA, the induction of WC-2 and FRQ started to be seen. As more WC-2 was induced by higher concentrations of QA, the levels of FRQ and WC-1 were higher, but the increase of FRQ expression appeared to be limited. At 1 × 10−3 M QA, although the WC-2 level was higher than that of the wild-type, the levels of FRQ were comparable, suggesting that WC-2 is not the limiting factor for the WC-1/WC-2 complex in a wild-type strain.
Interestingly, although the robustness of the conidiation rhythms changed as the levels of the WCs and FRQ changed in these two strains, the period of the conidiation rhythms varied only a couple of hours. When the levels of WCs and FRQ were much lower than those of the wild-type and the amplitude of the overt rhythms was low, the period length of the overt rhythm was only about 2 h longer than that of the wild-type. When the levels of WCs and FRQ were higher than those of the wild-type due to higher concentration of the inducer, the period of the rhythms stayed very close to that of the wild-type (Fig. 3). The small period changes despite dramatic variations in the levels of the clock components suggest that the parallel changes in WCs and FRQ levels, results of the interlocked nature of the feedback loops, is important for determining the circadian period of the clock.
Together, these data suggest that the main effect of high WC levels is to increase the robustness and stability of the circadian clock. When either WC-1 or WC-2 level is low and rate-limiting in the mutant strains, the clock runs poorly and it is hard to sustain, whereas the clock runs more robust when their levels are higher.
Higher Level of WC-1 or WC-2 Leads to Higher Level of FRQ Oscillation.
Because the levels of WC-1 and WC-2 determine the level of FRQ and the robustness of the conidiation rhythms, we would expect to see higher WC-1 and WC-2 levels leading to more robust FRQ oscillations. To confirm this, the levels of WC-1, WC-2, and FRQ were monitored in constant darkness in the wc-1−, qa-WC-1 and wc-2−, qa-WC-2 strains. In general, these molecular data match the race tube data. When there was no inducer, the levels of WC-1 and FRQ were very low and arrhythmic in the dark in the wc-1−, qa-WC-1 strain (Fig. 4A). At 1 × 10−5 M QA, when clear conidiation rhythms started to be seen on race tubes (Fig. 3B), low-level oscillations of WC-1 and FRQ could be observed (Fig. 4 B and D). When QA was increased to 1 × 10−2 M, there was a robust and high level oscillation of FRQ (Fig. 4 A, B, D). Although this high concentration of QA increased WC-1 significantly, the abundance of WC-1 was still rhythmic, suggesting that the posttranscriptional regulation of FRQ on WC-1 expression is still functioning despite the high and constitutive expression of wc-1.
For the wc-2−, qa-WC-2 strain, although the levels of WC-2 and FRQ were low without the inducer (the low level of WC-2 was due to leaky expression from the qa-2 promoter), the level of FRQ was cyclic with a low amplitude (note the changes in FRQ amount and phosphorylation states) (Fig. 4 C and E). However, this weak FRQ rhythm was only limited to the first day or two (Fig. 4C and data not shown), indicating that this low-level expression of FRQ and WC-2 cannot sustain the rhythmicity for long. These data corresponded well with the 1-day conidiation rhythm we observed on race tubes (Fig. 3C). In the presence of 1 × 10−2 M QA, as in the wc-1−, qa-WC-1 strain, the higher level of WC-2 led to a robust and a higher level of FRQ oscillation (Fig. 4 C and E). Surprisingly, we also found that the FRQ level was comparable in constant light with or without QA in both strains despite the dramatic difference in WC-1 or WC-2 level (compare the level of FRQ at time 0 in Fig. 4 A and C), indicating that the requirement for WC-1 and WC-2 on FRQ expression in LL is different from that in the dark. Together, these data indicate that there are threshold levels of WC-1, WC-2, and FRQ required for the functioning of the clock, and higher levels of WC-1 and WC-2 lead to more robust oscillation of FRQ.
WC-1 Is the Limiting Factor in the WC-1/WC-2 Complex.
Because both WC proteins are required for the activation of FRQ and they interact with each other to form heterodimeric complexes (16), we wondered which protein is the limiting factor for the complex in a wild-type strain. As described before, the results in Figs. 3 and 4 suggest that WC-1 is the limiting factor for frq expression in the WC-1/WC-2 complex. To directly compare the levels of WC-1 and WC-2, we generated strains in which WC-1 or WC-2 is tagged with the identical five c-Myc epitope tags (22). Western blot analysis by using c-Myc monoclonal antibody revealed that the relative molar concentration of WC-1 to WC-2 is about 1 to 6 in LL (data not shown), similar to what was recently reported (29). Although this ratio can inform us about the relative amount of the two proteins, it does not reflect the ratio of the functional proteins in vivo because the functional levels of the two proteins can be affected by regulations such as nuclear import or homo-multimer formation (16, 30). Therefore, we decided to examine the limiting role of WC-1 by demonstrating its limiting effects on the formation of the WC-1/WC-2 complex and on the activation of FRQ expression.
To show the limiting effect of WC-1 on the WC-1/WC-2 complex formation, we used the same protein extracts of the wc-1−, qa-WC-1 strain shown in Fig. 3B (Lower) and performed immunoprecipitation using WC-2 antiserum. Protein extracts from three different QA concentrations were used (from 1 × 10−5 to 1 × 10−3 M) because at 1 × 10−5 and 1 × 10−4 M of QA, the levels of WC-1 and FRQ were comparable with those in the wild-type strain, whereas their levels were much higher at 1 × 10−3 M QA than those in the wild-type (Fig. 3B). In contrast, there was almost no change in the WC-2 level at different QA concentrations (Figs. 3B and 5A). If WC-1 is the limiting factor in the WC-1/WC-2 complex, the increase of WC-1 level at 1 × 10−3M QA should result in a similar amount of increase of the WC-1/WC-2 complex. As predicted, immunoprecipitation using WC-2 antiserum showed that significantly more WC-1 was coprecipitated with WC-2 at 1 × 10−3 M QA, whereas the amount of WC-2 precipitated was about the same at different QA concentrations (Fig. 5A, Right). The amount of increase of WC-1 found in the complex was similar to the amount of its increase in the total protein extracts. Importantly, FRQ was also found in the complex, and the amount of FRQ coprecipitated increased as the amount of WC-1/WC-2 complex increased.
Figure 5.
WC-1 is the rate-limiting factor in the WC-1/WC-2 complex. (A) Immunoprecipitation analysis shows that WC-1 is the rate-limiting factor in the WC-1/WC-2 complex. The same protein extracts (1 × 10−5, 1 × 10−4, and 1 × 10−5 M QA samples) shown in Fig. 3B were used, and they were immunoprecipitated with the WC-2 antiserum. Both the total extracts and the pellets of immunoprecipitation were subjected to Western blot analysis. (B) Western blot analysis shows that a higher level of WC-1 leads to a higher level of FRQ oscillation in a wild-type strain. wc-1+, qa-WC-1 liquid cultures were grown in media with or without 1 × 10−2 M QA in DD. (Right) The densitometric analysis of the Western blot shown left. (C) Race tube assay results show the conidiation rhythms of the wc-1+, qa-WC-1 strain at different concentrations of QA (labeled at left). (D) Western blot analysis shows that the increase of WC-2 level in a wild-type strain (wc-2+, qa-WC-2) did not result in the increase of FRQ expression.
To examine the limiting role of WC-1 in elevating FRQ levels in a wild-type situation, we introduced the qa-WC-1 construct into a wild-type strain and examined the circadian conidiation rhythms and protein rhythms of the transformants. As we expected, the higher level of WC-1 rhythm led FRQ to oscillate at a higher level, whereas there was almost no change in the level of WC-2 (Fig. 5B). Similar to the results shown in Fig. 3, there was only a small period shortening effect despite higher than wild-type levels of WC-1 and FRQ (the broader conidiation peaks at 1 × 10−2 M QA are a carbon source effect). Consistent with these data, a higher level of WC-2 expression did not result in a higher level of FRQ in a wild-type, qa-WC-2 strain (Fig. 5D). Together, these data demonstrate that WC-1 is the limiting factor for the WC-1/WC-2 complex in the wild-type strain, and a higher level of WC-1/WC-2 complex requires a higher level of FRQ to interact with to block its transcriptional activation of frq and close the circadian negative feedback loop.
Discussion
In this study, we show that WC-1 and WC-2 are required for the activation of frq transcription, with WC-1 being the limiting factor in the WC-1/WC-2 complex. The levels of WC-1 and WC-2 are positively regulated by FRQ, thereby they form positive feedback loops. Interestingly, the mechanisms whereby frq regulates wc-1 and wc-2 are different; frq regulates wc-1 posttranscriptionally, and it does not influence the abundance of wc-1 transcripts, whereas its effect on wc-2 is at least partially achieved by increasing the steady-state level of wc-2 mRNA. When the level of either WC-1 or WC-2 is below a certain threshold level, the level of FRQ is low and arrhythmic, and the circadian rhythmicity cannot be sustained under normal conditions. If the levels of WC-1 and WC-2 are above certain levels, they will increase FRQ level, resulting in its rhythm and the running of the clock. The higher the levels of WC-1 and WC-2, the higher the level of FRQ oscillation and the more robust and stable the overt rhythmicity (Fig. 6). Although our experiments did not completely dissociate the interlocked feedback loops, which will require the elimination of the regulations of FRQ on WCs while keeping frq intact, the positive regulations of FRQ on the levels of both WC-1 and WC-2 suggest that these interlocked positive feedback loops should be important for promoting the robustness and stability of the clock, which is an integral part of an optimally functioning circadian system.
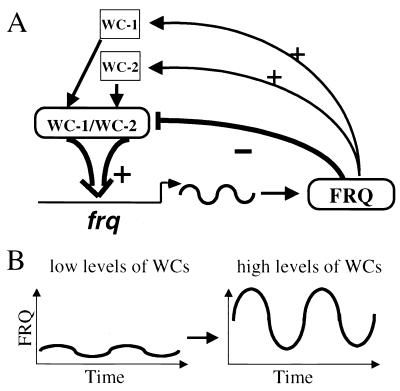
Figure 6.
(A) Model for gene regulation within the Neurospora circadian oscillator. WC-1 and WC-2 form heterodimers to activate the transcription of frq. FRQ proteins interact with the WC-1/WC-2 complex to inhibit their transcriptional activation, forming the negative feedback loop. FRQ also positively regulates the levels of both WC-1 and WC-2, forming the positive feedback loops. (B) The levels of WC proteins determine the robustness of FRQ oscillation. When the levels of WCs are low, there is either no FRQ oscillation or it oscillates at a low level, whereas high levels of WCs lead to a robust and a high-level oscillation of FRQ. The parallel changes in WCs and FRQ limit the period of clock to a small range.
Another important implication of this study is that, because of the interlocked nature of the circadian feedback loops, the levels of the clock components are not the major determining factors in setting period length of the clock. As shown in Figs. 3–5, the parallel changes of WC-1 and FRQ levels had limited the period length to a small range (about 2 h), even though their levels varied considerably from one condition to another (from significantly lower to higher than normal). Therefore, the interlocked feedback loops may be important for setting the period length of the clock to be within the circadian range, and they have also made the Neurospora clock into a highly dynamic but stable process, a fact that may also explain phenomena such as temperature compensation of the clock (21). In this view, we expect that changes in activity or stability of the clock components should play important roles in determining the period length of the clock (24, 28, 31, 32).
Although the expression of both WCs is positively regulated by FRQ, the positive effect of FRQ on WC-1 plays a more important role in the Neurospora clock. Because WC-1 is the limiting factor in the WC-1/WC-2 complex, its rhythmic expression would ensure robust rhythmicity by allowing proper amounts of the transcription activation complexes to be formed at different times of the day to start or close a cycle. The long delay between the expression of FRQ and the increase of WC-1 is important (15) because it allows the negative repression of frq to occur at a time (subjective morning) when FRQ level is high while the amount of the WC-1/WC-2 complex is relatively low. On the other hand, the positive effect of FRQ on the expression of WC-2 seems to have only a limited role in the clock of a wild-type strain. First, WC-2 is not the rate-limiting factor of the WC-1/WC-2 complex, and its level does not fluctuate much in the constant darkness (15). Second, unlike the level of WC-1, WC-2 level does not change significantly with the increase of FRQ level under constant conditions, and even a low level of FRQ is able to maintain normal expression level of WC-2 (Figs. 2–5). Therefore, the positive role of FRQ on WC-2 appears only to maintain the level of WC-2 in excess.
In both Drosophila and mammals, the negative elements of the circadian feedback loops were also found to positively regulate the expression of one of the positive elements and lead to their rhythmic expressions. In flies, PERIOD and TIMELESS elevate the level of dclock, whereas mCRYs and mPER2 increase the expression of Bmal1 mRNA in mammals (13, 14). Unlike the posttranscriptional role of FRQ on WC-1, these regulations appear to be achieved at least partially at the transcript level. In all three systems, the demonstrated or the predicted rate-limiting factors (WC-1 in Neurospora, dCLOCK in Drosophila, and BMAL1 in mammals) of the positive elements in the heterodimeric transcription activation complexes are all expressed rhythmically, whereas the levels of the other partners of the complexes (WC-2 in Neurospora, CYCLE in Drosophila, and CLOCK in mammals) remain pretty much unchanged throughout the day (11, 14, 33, 34). Based on our results in Neurospora, we predict that the interlocked feedback loops in Drosophila and mammals are also important for sustaining robust rhythmicity in those systems.
Acknowledgments
We thank Drs. Michael Collett, Jay Dunlap, and Jennifer Loros for providing the wc-2 knock-out strain before its publication. We thank Drs. Jim Stull, Steven McKnight, Helen Yin, and Stephen Hammes for critical reading of the manuscript. This work was supported by National Institutes of Health Grant GM 62591 (to Y.L.). Y.L. is a Louise W. Kahn endowed scholar in Biomedical Research at the University of Texas Southwestern Medical Center.
Abbreviation
- QA
quinic acid
References
- 1.Dunlap J C. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 2.Crosthwaite S K, Dunlap J C, Loros J J. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- 3.Allada R, White N E, So W V, Hall J C, Rosbash M. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 4.Rutila J E, Suri V, Le M, So W V, Rosbash M, Hall J C. Cell. 1998;93:805–813. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 5.King D, Zhao Y, Sangoram A, Wilsbacher L, Tanaka M, Antoch M, Steeves T, Vitaterna M, Kornhauser J, Lowrey P, et al. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gekakis N, Stankis D, Nguyen H B, Davis F C, Wilsbacher L D, King D P, Takahashi J S, Weitz C J. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 7.Kay S A. Science. 1997;276:1093. doi: 10.1126/science.276.5315.1093. [DOI] [PubMed] [Google Scholar]
- 8.Young M W. Recent Prog Horm Res. 1999;54:87–94. [PubMed] [Google Scholar]
- 9.Aronson B, Johnson K, Loros J J, Dunlap J C. Science. 1994;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- 10.Darlington T K, Wager-Smith K, Ceriani M F, Stankis D, Gekakis N, Steeves T, Weitz C J, Takahashi J, Kay S A. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 11.Kume K, Zylka M J, Sriram S, Shearman L P, Weaver D R, Jin X, Maywood E S, Hastings M H, Reppert S M. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 12.Lee C, Bae K, Edery I. Mol Cell Biol. 1999;19:5316–5325. doi: 10.1128/mcb.19.8.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glossop N R, Lyons L C, Hardin P E. Science. 1999;286:766–768. doi: 10.1126/science.286.5440.766. [DOI] [PubMed] [Google Scholar]
- 14.Shearman L P, Sriram S, Weaver D R, Maywood E S, Chaves I, Zheng B, Kume K, Lee C C, van der Horst G T, Hastings M H, Reppert S M. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 15.Lee K, Loros J J, Dunlap J C. Science. 2000;289:107–110. doi: 10.1126/science.289.5476.107. [DOI] [PubMed] [Google Scholar]
- 16.Talora C, Franchi L, Linden H, Ballario P, Macino G. EMBO J. 1999;18:4961–4968. doi: 10.1093/emboj/18.18.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Garceau N, Loros J J, Dunlap J C. Cell. 1997;89:477–486. doi: 10.1016/s0092-8674(00)80228-7. [DOI] [PubMed] [Google Scholar]
- 18.Linden H, Macino G. EMBO J. 1997;16:98–109. doi: 10.1093/emboj/16.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballario P, Vittorioso P, Magrelli A, Talora C, Cabibbo A, Macino G. EMBO J. 1996;15:1650–1657. [PMC free article] [PubMed] [Google Scholar]
- 20.Crosthwaite S K, Loros J J, Dunlap J C. Cell. 1995;81:1003–1012. doi: 10.1016/s0092-8674(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Merrow M M, Loros J J, Dunlap J C. Science. 1998;281:825–829. doi: 10.1126/science.281.5378.825. [DOI] [PubMed] [Google Scholar]
- 22.Cheng P, Yang Y, Heintzen C, Liu Y. EMBO J. 2001;20:101–108. doi: 10.1093/emboj/20.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aronson B D, Johnson K A, Dunlap J C. Proc Natl Acad Sci USA. 1994;91:7683–7687. doi: 10.1073/pnas.91.16.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collett M A, Dunlap J C, Loros J J. Mol Cell Biol. 2001;21:2619–2628. doi: 10.1128/MCB.21.8.2619-2628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell-Pedersen D, Dunlap J C, Loros J J. Mol Cell Biol. 1996;16:513–521. doi: 10.1128/mcb.16.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garceau N, Liu Y, Loros J J, Dunlap J C. Cell. 1997;89:469–476. doi: 10.1016/s0092-8674(00)80227-5. [DOI] [PubMed] [Google Scholar]
- 27.Merrow M, Garceau N, Dunlap J C. Proc Natl Acad Sci USA. 1997;94:3877–3882. doi: 10.1073/pnas.94.8.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Loros J, Dunlap J C. Proc Natl Acad Sci USA. 2000;97:234–239. doi: 10.1073/pnas.97.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denault D L, Loros J J, Dunlap J C. EMBO J. 2001;20:109–117. doi: 10.1093/emboj/20.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballario P, Talora C, Galli D, Linden H, Macino G. Mol Microbiol. 1998;29:719–729. doi: 10.1046/j.1365-2958.1998.00955.x. [DOI] [PubMed] [Google Scholar]
- 31.Price J L, Blau J, Rothenfluh A, Adodeely M, Kloss B, Young M W. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 32.Lowrey P L, Shimomura K, Antoch M P, Yamazaki S, Zemenides P D, Ralph M R, Menaker M, Takahashi J S. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee C, Bae K, Edery I. Neuron. 1998;21:857–867. doi: 10.1016/s0896-6273(00)80601-7. [DOI] [PubMed] [Google Scholar]
- 34.King D P, Takahashi J S. Annu Rev Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]