Abstract
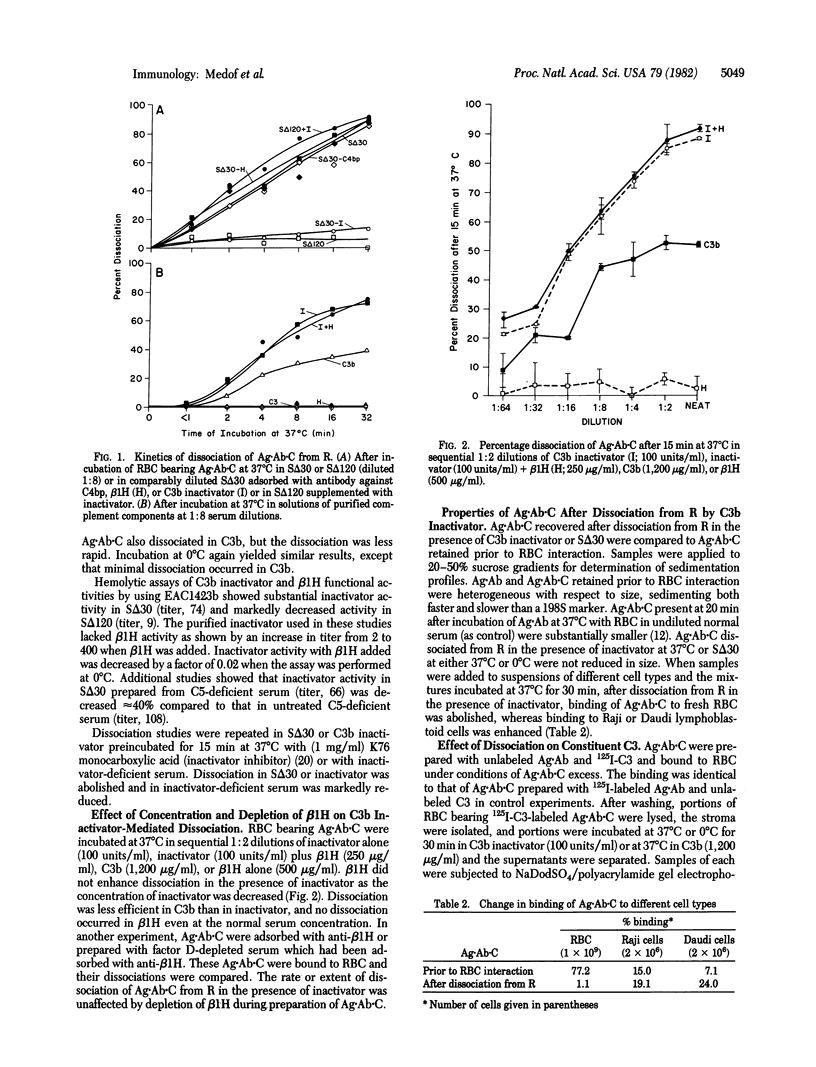
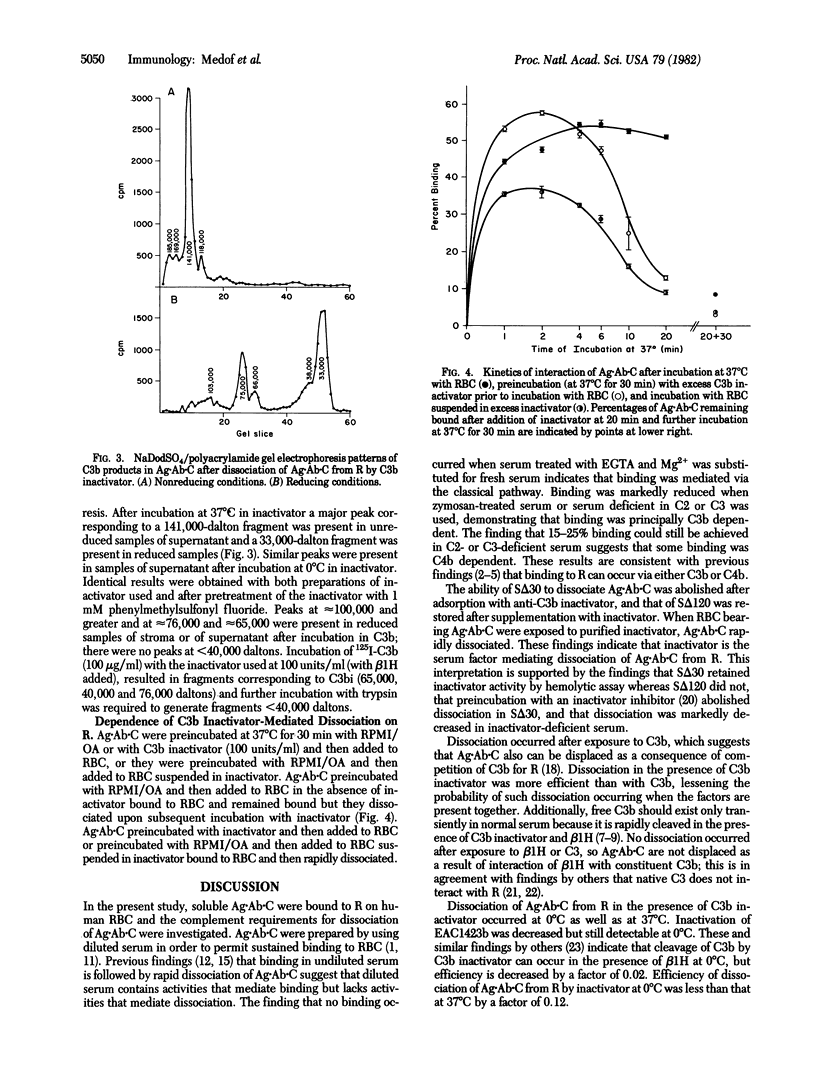
Antigen·antibody complexes (Ag·Ab) prepared from 125I-labeled bovine serum albumin and guinea pig anti-albumin were incubated at 37°C for 30 min with normal human serum diluted optimally for binding (1:16) and then with autologous erythrocytes (RBC). After washing, RBC-bearing antigen·antibody·complement complexes (Ag·Ab·C) were resuspended in serum reagents or solutions of purified complement components, and the kinetics of dissociation were analyzed. Ag·Ab·C dissociated in serum heated at 56°C for 30 min (SΔ30) but not in serum heated for 120 min (SΔ120). Dissociation in SΔ30 markedly decreased after adsorption with anti-C3b inactivator but not anti-β1H or anti-C4 binding protein (C4bp), and dissociation in SΔ120 markedly increased after addition of C3b inactivator. Hemolytic assays revealed that SΔ30 retained inactivator activity whereas SΔ120 lacked significant activity. Ag·Ab·C dissociated in the presence of purified inactivator or C3b but not β1H or C3. Dissociation was more rapid with inactivator than with C3b and occurred at 0°C as well as at 37°C. Treatment with inactivator inhibitor abolished dissociation in SΔ30; dissociation in inactivator deficient serum was markedly reduced. Addition of β1H did not enhance inactivator-mediated dissociation at limiting dilutions of inactivator, and adsorption of Ag·Ab·C with anti-β1H or preparation of Ag·Ab·C with serum adsorbed with anti-β1H did not diminish dissociation. After dissociation with inactivator, Ag·Ab·C were unchanged in size but were no longer able to bind to fresh RBC and gave enhanced binding to Raji and Daudi lymphoblastoid cells. NaDodSO4/polyacrylamide gel electrophoresis of Ag·Ab·C prepared with 125I-labeled C3 revealed that, after binding to RBC, dissociation with inactivator was accompanied by generation of a C3 fragment the size of C3c. Preincubation of Ag·Ab·C with excess inactivator did not prevent subsequent binding of Ag·Ab·C to RBC but, immediately after binding, Ag·Ab·C dissociated rapidly. These findings indicate that C3b inactivator can release immune complexes from immune adherence receptors on human RBC, that release occurs independently of β1H, alters cell binding properties of immune complexes, and involves multiple cleavages of the C3b α′ chain, and that receptors in human RBC membrane are required for this C3b inactivator-mediated breakdown.
Keywords: complement, C3b degradation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper N. R. Immune adherence by the fourth component of complement. Science. 1969 Jul 25;165(3891):396–398. doi: 10.1126/science.165.3891.396. [DOI] [PubMed] [Google Scholar]
- Czop J., Nussenzweig V. Studies on the mechanism of solubilization of immune precipitates by serum. J Exp Med. 1976 Mar 1;143(3):615–630. doi: 10.1084/jem.143.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Fujita T., Nussenzweig V. Modulation of the classical pathway C3 convertase by plasma proteins C4 binding protein and C3b inactivator. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6596–6600. doi: 10.1073/pnas.76.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Kinoshita T., Kitajima H., Inoue K. Inhibitory effect of K-76 monocarboxylic acid, an anticomplementary agent, on the C3b inactivator system. J Immunol. 1981 Jul;127(1):104–108. [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981 May 1;153(5):1138–1150. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Müller-Eberhard H. J. The demonstration in human serum of "conglutinogen-activating factor" and its effect on the third component of complement. J Immunol. 1968 Apr;100(4):691–698. [PubMed] [Google Scholar]
- Lesavre P. H., Müller-Eberhard H. J. Mechanism of action of factor D of the alternative complement pathway. J Exp Med. 1978 Dec 1;148(6):1498–1509. doi: 10.1084/jem.148.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linscott W. D., Ranken R., Triglia R. P. Evidence that bovine conglutinin reacts with an early product of C3b degradation, and an improved conglutination assay. J Immunol. 1978 Aug;121(2):658–664. [PubMed] [Google Scholar]
- May J. E., Kane M. A., Frank M. M. Immune adherence by the alternate complement pathway. Proc Soc Exp Biol Med. 1972 Oct;141(1):287–290. doi: 10.3181/00379727-141-36760. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Oger J. J. Competition for immune complexes by red cells in human blood. J Clin Lab Immunol. 1982 Jan;7(1):7–13. [PubMed] [Google Scholar]
- Medof M. E., Prince G. M., Oger J. J. Kinetics of interaction of immune complexes with complement receptors on human blood cells: modification of complexes during interaction with red cells. Clin Exp Immunol. 1982 Jun;48(3):715–725. [PMC free article] [PubMed] [Google Scholar]
- Miller G. W., Nussenzweig V. A new complement function: solubilization of antigen-antibody aggregates. Proc Natl Acad Sci U S A. 1975 Feb;72(2):418–422. doi: 10.1073/pnas.72.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllerèberhard H. J., Dalmasso A. P., Calcott M. A. The reaction mechanism of beta-1C-globulin (C'3) in immune hemolysis. J Exp Med. 1966 Jan 1;123(1):33–54. doi: 10.1084/jem.123.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON R. A., Jr The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953 Dec 18;118(3077):733–737. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981 Sep 1;154(3):856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Polley M. J. Specificity of human lymphocyte complement receptors. J Exp Med. 1975 May 1;141(5):1163–1180. doi: 10.1084/jem.141.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J Immunol. 1971 Sep;107(3):742–750. [PubMed] [Google Scholar]
- Schreiber R. D., Pangburn M. K., Müller-Eberhard H. J. C3 modified at the thiolester site: acquisition of reactivity with cellular C3b receptors. Biosci Rep. 1981 Nov;1(11):873–880. doi: 10.1007/BF01114821. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J., Bokisch V. A. Binding of soluble immune complexes to human lymphoblastoid cells. I. Characterization of receptors for IgG Fc and complement and description of the binding mechanism. J Exp Med. 1974 Oct 1;140(4):877–894. doi: 10.1084/jem.140.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemasu K., Stroud R. M. Clq: rapid purification method for preparation of monospecific antisera and for biochemical studies. J Immunol. 1971 Feb;106(2):304–313. [PubMed] [Google Scholar]


