Abstract
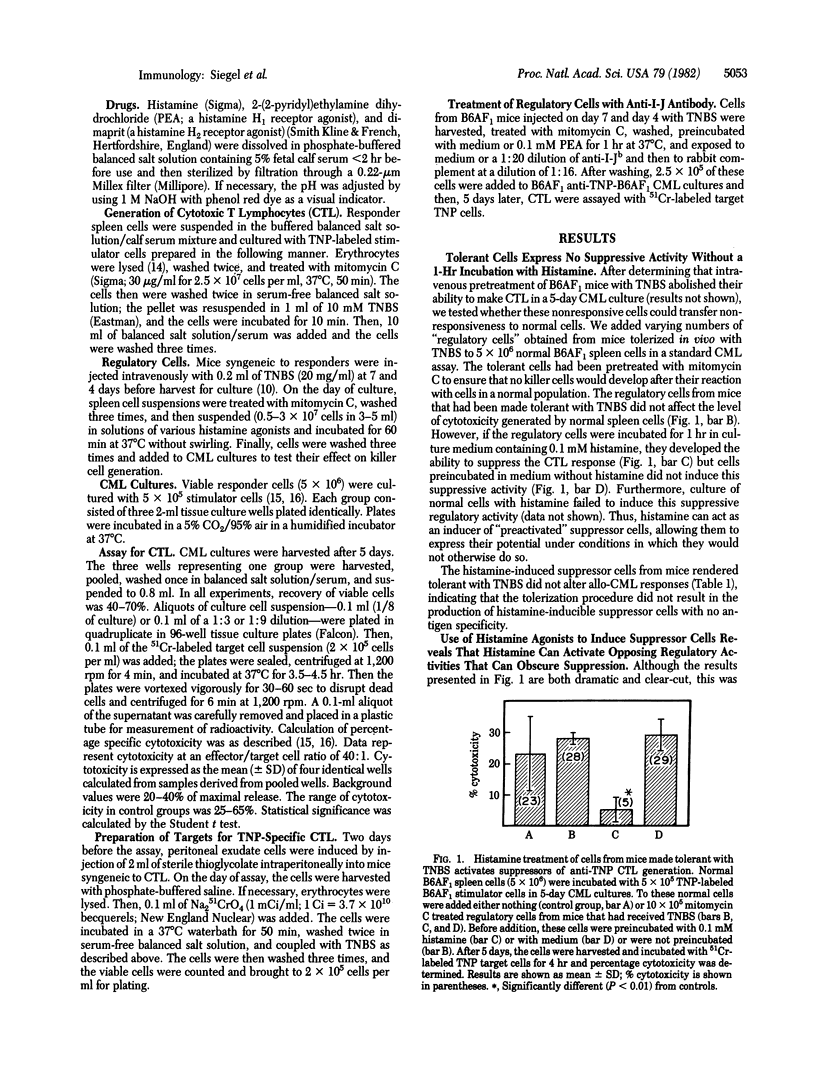
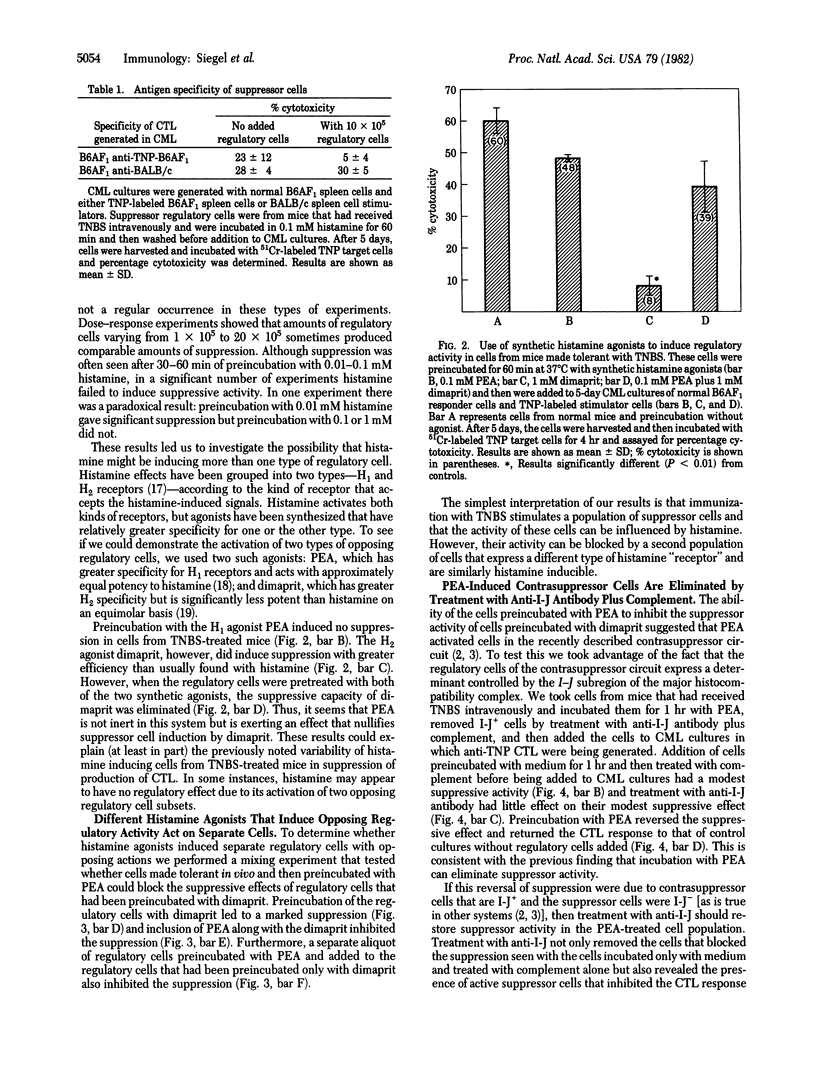
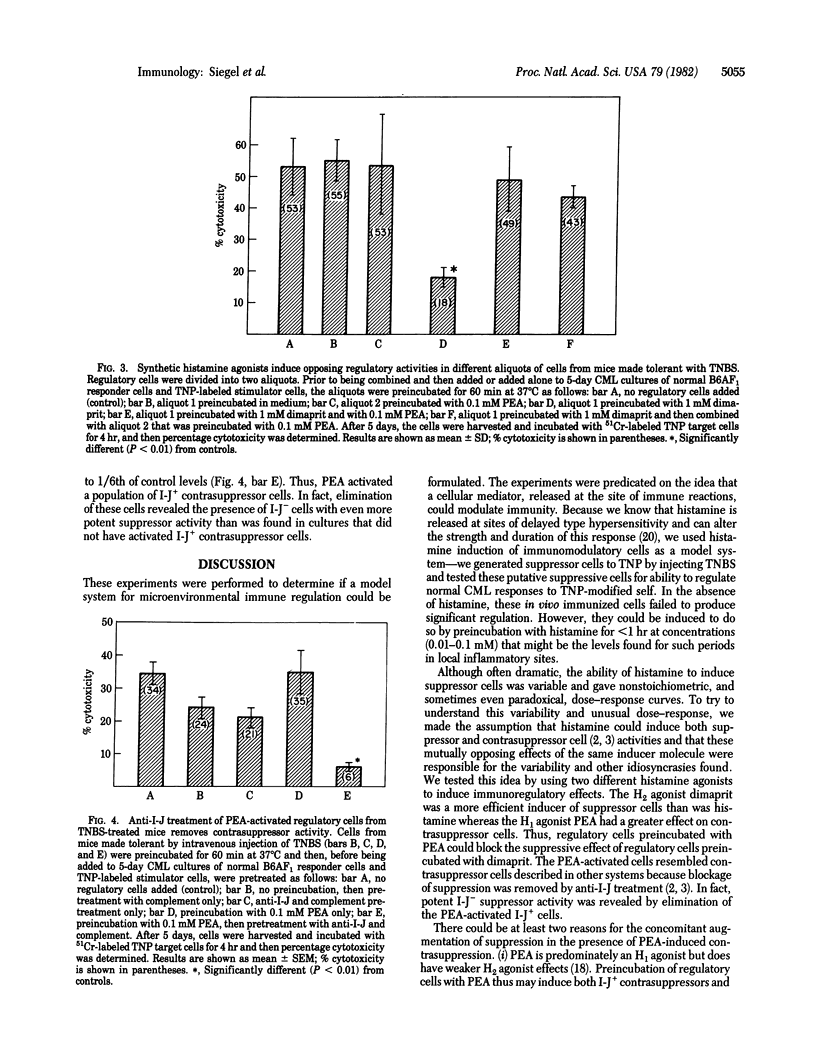
The intensity of Lyl+T helper and delayed type hypersensitivity effector cell activities is governed, in part, by an interplay between two classes of immunoregulatory T cells: suppressor cells and contrasuppressor cells. We asked whether histamine, at concentrations and duration of exposure that we calculated might be achieved at local sites of inflammation, could activate either or both of these classes of regulatory cells in vitro. To answer this question we used spleen cells from mice treated in vivo with the toleragen trinitrobenzenesulfonic acid as regulators of in vitro generation of primary anti-trinitrophenyl self-cytotoxic T lymphocytes. Under the conditions used, these spleen cells had no major regulatory effects. However, if these cells were preincubated with histamine at 0.1 mM for 30-60 min, suppressor activity was induced, but this occurred inconsistently and with nonstoichiometric results. The use of synthetic histamine agonists revealed that histamine may activate both suppressor and contrasuppressor cell subsets. A histamine H1 receptor agonist [2-(2-pyridyl)-ethylamine dihydrochloride] had a propensity to activate contrasuppression, whereas an H2 receptor agonist (dimaprit) tended to activate suppressor cells. Thus, histamine may have opposing actions that obscure suppression. This duality was shown by treatment of pyridylethylamine-induced contrasuppressor cells with complement and anti-I-J antibody that kills contrasuppressor cells. This treatment revealed a high level of suppressor cell activity that was not expressed until the opposing contrasuppressor cells were removed. Because histamine is released at local sites of delayed type hypersensitivity, these results indicate that histamine may serve as an inducer of microenvironmental immunomodulation by activating regulatory T cells at sites where immune responses are taking place.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W., Bursztajn S., Gershon M. D., Gershon R. K. T cell-dependent mast cell degranulation and release of serotonin in murine delayed-type hypersensitivity. J Exp Med. 1980 Nov 1;152(5):1358–1374. doi: 10.1084/jem.152.5.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenase P. W., Schwartz A., Siegel J. N., Gershon R. K. Role of histamine in the regulation of cell-mediated immunity. Int Arch Allergy Appl Immunol. 1981;66 (Suppl 1):225–233. doi: 10.1159/000232907. [DOI] [PubMed] [Google Scholar]
- Beer D. J., Osband M. E., McCaffrey R. P., Soter N. A., Rocklin R. E. Abnormal histamine-induced suppressor-cell function in atopic subjects. N Engl J Med. 1982 Feb 25;306(8):454–458. doi: 10.1056/NEJM198202253060804. [DOI] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Durant C. J., Ganellin C. R., Parsons E. M. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Cantor H., Gershon R. K. Immunological circuits: cellular composition. Fed Proc. 1979 Jun;38(7):2058–2064. [PubMed] [Google Scholar]
- Durant G. J., Ganellin C. R., Parsons M. E. Chemical differentiation of histamine H1- and H2-receptor agonists. J Med Chem. 1975 Sep;18(9):905–909. doi: 10.1021/jm00243a009. [DOI] [PubMed] [Google Scholar]
- Finberg R., Burakoff S. J., Benacerraf B., Greene M. I. The cytolytic T lymphocyte response to trinitrophenyl-modified syngeneic cells. II. Evidence for antigen-specific suppressor T cells. J Immunol. 1979 Sep;123(3):1210–1214. [PubMed] [Google Scholar]
- Finberg R., Greene M. I., Benacerraf B., Burakoff S. J. The cytolytic T lymphocyte response to trinitrophenyl-modified syngeneic cells. I. Evidence for antigen-specific helper T cells. J Immunol. 1979 Sep;123(3):1205–1209. [PubMed] [Google Scholar]
- Fujiwara H., Levy R. B., Shearer G. M., Terry W. D. Studies on in vivo priming of the TNP-reactive cytotoxic effector cell system. I. Comparison of the effects of intravenous inoculation with TNP-conjugated cells on the development of contact sensitivity and cell-mediated lympholysis. J Immunol. 1979 Jul;123(1):423–425. [PubMed] [Google Scholar]
- Gershon R. K., Eardley D. D., Durum S., Green D. R., Shen F. W., Yamauchi K., Cantor H., Murphy D. B. Contrasuppression. A novel immunoregulatory activity. J Exp Med. 1981 Jun 1;153(6):1533–1546. doi: 10.1084/jem.153.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Eardley D. D., Kimura A., Murphy D. B., Yamauchi K., Gershon R. K. Immunoregulatory circuits which modulate responsiveness to suppressor cell signals: characterization of an effector cell in the contrasuppressor circuit. Eur J Immunol. 1981 Dec;11(12):973–980. doi: 10.1002/eji.1830111205. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Rocklin R. E., Rosenkranz R. P. Autacoids as modulators of the inflammatory and immune response. Am J Med. 1981 Jul;71(1):100–106. doi: 10.1016/0002-9343(81)90264-3. [DOI] [PubMed] [Google Scholar]
- Parsons M. E., Owen D. A., Ganellin C. R., Durant G. J. Dimaprit -(S-[3-(N,N-dimethylamino)prophyl]isothiourea) - a highly specific histamine H2 -receptor agonist. Part 1. Pharmacology. Agents Actions. 1977 Mar;7(1):31–37. doi: 10.1007/BF01964878. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Greineder D. K., Melmon K. L. Histamine-induced suppressor factor (HSF): further studies on the nature of the stimulus and the cell which produces it. Cell Immunol. 1979 May;44(2):404–415. doi: 10.1016/0008-8749(79)90015-7. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Greineder D., Littman B. H., Melmon K. L. Modulation of cellular immune function in vitro by histamine receptor-bearing lymphocytes: mechanism of action. Cell Immunol. 1978 Apr;37(1):162–173. doi: 10.1016/0008-8749(78)90184-3. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E. Modulation of cellular-immune responses in vivo and in vitro by histamine receptor-bearing lymphocytes. J Clin Invest. 1976 Apr;57(4):1051–1058. doi: 10.1172/JCI108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Askenase P. W., Gershon R. K. Histamine inhibition of the in vitro induction of cytotoxic T-cell responses. Immunopharmacology. 1980 Jun;2(3):179–190. doi: 10.1016/0162-3109(80)90048-x. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Askenase P. W., Gershon R. K. The effect of locally injected vasoactive amines on the elicitation of delayed-type hypersensitivity. J Immunol. 1977 Jan;118(1):159–165. [PubMed] [Google Scholar]
- Schwartz A., Sutton S. L., Askenase P. W., Gershon R. K. Histamine inhibition of concanavalin A-induced suppressor T-cell activation. Cell Immunol. 1981 May 15;60(2):426–439. doi: 10.1016/0008-8749(81)90284-7. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Sutton S. L., Gershon R. K. Regulation of in vitro cytotoxic T lymphocyte generation. I. Evidence that killer cell precursors differentiate to effector cells in two steps. J Exp Med. 1982 Mar 1;155(3):783–796. doi: 10.1084/jem.155.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K., Green D. R., Eardley D. D., Murphy D. B., Gershon R. K. Immunoregulatory circuits that modulate responsiveness to suppressor cell signal. Failure of B10 mice to respond to suppressor factors can be overcome by quenching the contrasuppressor circuit. J Exp Med. 1981 Jun 1;153(6):1547–1561. doi: 10.1084/jem.153.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L. Depression of the T cell phenomenon of contact sensitivity by T cells from unresponsive mice. Nature. 1973 Jul 27;244(5413):227–228. doi: 10.1038/244227a0. [DOI] [PubMed] [Google Scholar]


