Abstract
The depigmenting effect of kojic acid esters synthesized by the esterification of kojic acid using Rhizomucor miehei immobilized lipase was investigated in B16F1 melanoma cells. The depigmenting effect of kojic acid and kojic acid esters was evaluated by the inhibitory effect of melanin formation and tyrosinase activity on alpha-stimulating hormone- (α-MSH-) induced melanin synthesis in B16F1 melanoma cells. The cellular tyrosinase inhibitory effect of kojic acid monooleate, kojic acid monolaurate, and kojic acid monopalmitate was found similar to kojic acid at nontoxic doses ranging from 1.95 to 62.5 μg/mL. However, kojic acid monopalmitate gave slightly higher inhibition to melanin formation compared to other inhibitors at doses ranging from 15.63 to 62.5 μg/mL. Kojic acid and kojic acid esters also show antioxidant activity that will enhance the depigmenting effect. The cytotoxicity of kojic acid esters in B16F1 melanoma cells was significantly lower than kojic acid at high doses, ranging from 125 and 500 μg/mL. Since kojic acid esters have lower cytotoxic effect than kojic acid, it is suggested that kojic acid esters can be used as alternatives for a safe skin whitening agent and potential depigmenting agents to treat hyperpigmentation.
1. Introduction
Melanin is synthesized via melanogenesis process to give pigment of skin, brain, eye, and hair [1–3]. Tyrosinase is a key enzyme that is responsible for melanogenesis in melanoma and melanocytes [4, 5]. The inhibition of tyrosinase will greatly affect the melanogenesis process and melanin production. The occurrence of abnormal melanin production is the cause for many hyperpigmentation, postinflammatory pigmentation, melasma, and skin-aging process [6–8]. Kojic acid is a well-known antityrosinase agent, efficiently used for skin lightening cosmetic products and widely used to treat hyperpigmentation, melasma, and wrinkle [5, 9–11]. However, most of the kojic acid and its derivatives are not oil soluble and unstable at high temperature for long term storage, prohibiting them to be directly incorporated in oil base cosmetic and skin-care products. Therefore, a few attempts had been made to improve the physical properties and biological activities of kojic acid (KA) via esterification with fatty acids aimed at better industrial application [12–14].
The physical properties of kojic acid esters (KA esters) are important factor for inhibition of melanin synthesis where it must penetrate into the cell membrane to inhibit cellular tyrosinase and melanin synthesis. Thus, appropriate hydrophobic and hydrophilic balance of the derivatives is important for the inhibition of melanin synthesis [15]. The improvements of the characteristics of depigmenting agents are very important to enhance their applications in cosmetic and skin-health industries. Reports on the antimelanin and tyrosinase inhibitory of KA esters in cell system are not available in the literature.
The objective of this study was to analyze the cytotoxicity and depigmenting activities of KA and KA esters such as KA monooleate (KAMO), KA monolaurate (KAML), and KA monopalmitate (KAMP) in B16F1 melanoma cells. KA was produced by the fermentation employing Aspergillus flavus link and KA esters were produced by the esterification of purified KA with various fatty acids using immobilized lipase.
2. Materials and Methods
2.1. Materials
Immobilized lipase from Rhizomucor miehei (RMIM) was purchased from Novo-Nordisk (Denmark). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) penicillin, and streptomycin were purchased from Invitrogen (Grand Island, NY, USA). Glycerol tributyrate, L-dihydroxyphenylalanine (L-DOPA), L-tyrosine, mushroom tyrosinase, phenylmethanesulfonyl fluoride (PMSF), L-ascorbic acid, and alpha-melanocyte stimulating hormone (α-MSH) were purchased from Sigma-Aldrich (Steheim, UK). All other chemicals and solvents used in this study were the analytical grade.
2.2. Production of Kojic Acid
KA was produced through the fermentation by Aspergillus flavus link 44–1 according to the method as described by Mohamad and Ariff [16]. In this method, the fermentation medium consisted of glucose (100 g/L), yeast extract (5 g/L), potassium dihydrogen phosphate (1 g/L), magnesium sulphate (0.5 g/L), and methanol (10 mL/L). The fungal spores suspension (1 × 105 spores/mL) was inoculated into 100 mL medium in 250 mL shake-flasks. The flasks were incubated at 30°C and agitated at 250 rpm for 5 days. The mycelia were filtered, washed, and transferred into 200 mL medium containing 100 g/L glucose in 500 mL shake flasks. The flasks were incubated in an orbital shaker at 30°C and agitated at 250 rpm for 30 days for the conversion of glucose into KA by the actions of cell-bound enzymes in nongrowing mycelia.
Cell mycelia were separated from the broth by centrifugation at 10 000 g (GRX-250, Tomy Seiko Co., Ltd. Japan). The supernatant containing KA was concentrated to get a final KA concentration of above 80 g/L using rotary evaporator (BUCHI model R-220, Germany). The concentrated KA solution was kept at 30°C for 24 h for crystallization. The KA crystals were separated by centrifugation and redisolved in methanol for the subsequent crystallization process to remove further pigments and impurities. High-purity KA (99.8%) was obtained after recrystallization with methanol for three times.
2.3. Enzymatic Synthesis of Kojic Acid Esters
The esterification of KA to KA esters (KAMO, KAML, and KAMP) was performed according to the method previously described [12, 17]. The reaction mixture for esterification process consisted of fatty acids (oleic acid, lauric acid, and palmitic acid), KA, and immobilized lipase in acetonitrile. The flasks containing the reaction mixture were incubated in a horizontal water bath shaker at 50°C, agitated at 180 rpm for 42 h. The reaction was terminated, by removing the enzyme from the mixture through filtration using filter paper. KA esters were purified using crystallization method similar to that used for KA.
KA esters were examined by thin layer chromatography (TLC) on precoated silica gel plate (60F254) and developed in hexane/ethyl acetate (70 : 30, v/v). The developed bands were visualized using UV light. Then, the products were analyzed on Agilent gas chromatograph after being silylated to TMS derivatives using a nonpolar column ZB-5HT Inferno (15 m × 0.53 mm × 0.15 μm) with nitrogen as carrier gas. The oven temperature was programmed to rise from 100°C to 225°C at 15°C min−1, and to 280°C at 30°C min−1 for 1 min. The injector and flame ionization detectors were set at 340°C, and 350°C respectively. The composition of product was quantified by an integrator with 1,2,3-tributyrylglycerol as internal standard.
2.4. Characterization of KA Esters
The GC-mass spectrometry (GC-MS) analysis of the product isolated using preparative column chromatography was performed on a Perkin Elmer Instrument model Clarus 600 MS spectrometer. The GC was equipped with a non-polar column, ZB-5HT (30 m × 0.32 mm × 0.25 μm). The carrier gas was helium at a flow rate of 1.5 mL min−1. Proton NMR (1H-NMR) and carbon NMR (13C-NMR) spectra were obtained using Varian NMR Unity Inova 500 MHz with Pulsed Field Gradient. The samples were dissolved in deuterated chloroform with tetramethylsilane as internal standard. On the other hand, FTIR spectra were recorded on a Perkin Elmer 100-series FTIR spectrophotometer using Universal Attenuated Total Reflectance (UATR). The IR spectra were used to identify the possible molecular structures for the pure components and also used to determine the chemical changes during the reaction.
2.5. Cell Culture
B16F1 melanoma cells were purchased from American Type Culture Collection (ATCC). The cells were cultured in DMEM with 10% w/v fetal bovine serum and 1% w/v penicillin/streptomycin (100 IU/50 μg/mL) in humidified atmosphere containing 5% CO2 in air at 37°C. B16 cells were cultured in 96-well plates and 24-wells plates for different assays. All the experiments were repeated at least in triplicates.
2.6. Determination of Cell Viability
Cell viability was assessed by the standard MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay with a slight modification [18]. B16F1 melanoma cells (1 × 105 cells/well) were seeded in a 96-well microtiter plate and allowed to adhere completely to the plate overnight [19]. On the next day, the medium was removed and a new medium containing test compounds with doses ranging from 1.9 to 500 μg/mL was added to the plate and then incubated at 37°C in CO2 incubator. After a total of 72 h incubation, medium was removed and 50 μL of MTT solutions (1.0 mg/mL) was added to each well and the incubation was continued for 4 h. Then, formazon was solubilized in dimethyl sulfoxide (DMSO) and the absorbance was measured at 450 nm (reference at 630 nm) using MR-96A microplate reader. DMSO at a toxic concentration of 5% v/v was used as a negative control [20].
2.7. Determination of Melanin Content
The release of extra-cellular melanin was measured according to the method described elsewhere [21]. In brief, B16F1 melanoma cells were seeded into 24-wells tissue culture plates at 1 × 104 cells/mL and incubated for 24 h. Then, alpha-MSH (0.1 μM) was added and cells were treated with increasing doses of KA and KA esters for a total of 72 h incubation. After washing twice with phosphate buffered saline, cells were dissolved in 1 mL of 1 N NaOH. For measurement of melanin content, 100 μL aliquots of solution were then placed in 96-well plates and the absorbance was measured at 450 nm using microplate reader. Antimelanin activity was expressed by the percentage of melanin content in KA and KA esters to that of untreated melanoma cells. L-ascorbic acid, a widely used whitening agent, was used as a reference [22].
2.8. Determination of Cellular Tyrosinase Activity
Cellular tyrosinase activity was determined according to the method described by Shin et al. [23]. In this method, B16F1 melanoma cells were treated with α-MSH alone and α-MSH with the addition of KA and KA esters at various doses for a total of 72 h incubation. The cells were washed with ice-cold PBS then lysed with 100 μL phosphate buffer (pH 6.8) containing 1% Triton X-100 and 0.1 mM phenylmethylsulfonyl fluoride (PMSF). Then, lysate was clarified by centrifugation at 800 rpm for 5 min. The supernatant (100 μL) was added into 50 μL of L-DOPA (1 mM) and 50 uL of L-Tyrosine (2 mM); and the mixtures were placed in a 96-well plate. During the incubation at 37°C, the absorbance was read at 492 nm at every 30 min for 3 h using a microplate reader. L-ascorbic acid was used as a reference.
2.9. Determination of Mushroom Tyrosinase Activity
KA and KA esters at various doses were dissolved in DMSO, 50 μL of L-DOPA (1 mM) and 50 μL of L-tyrosine (2 mM), dissolved in 20 mM phosphate buffer (pH 6.8). DMSO or test sample (50 μL) was added into these mixtures in a 96-well microplate, followed by mixing with 50 μL of mushroom tyrosinase solution (480 units/mL). After incubation at 25°C for 10 min, the amount of dopachrome in the reaction mixture was determined. The inhibitory activity of the sample was expressed as percentage to control based on the absorbance measured at 492 nm [6].
2.10. Determination of Free Radical Scavenging Activity
The effect of KA and KA esters on the free radical scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) activities was estimated on a 96-well plate [18]. Test compounds (100 μL) dissolved in dimethyl sulfoxide solutions were added into 100 μL of 0.2 mM DPPH in ethanol. 100 μL of dimethyl sulfoxide solution alone was added into 100 μL of 0.2 mM DPPH in ethanol as blank. The plates were incubated at 25°C for 30 min and the absorbance was read at 492 nm. The percentage of antioxidative activity was calculated according to (1),
| (1) |
DPPH is a stable free radical with violet colour. It turns to yellow in the presence of antioxidant and scavenging agents.
2.11. Statistical Analysis
Data were collected as mean ± standard error (S.E.M) of at least three determinations. Statistical analysis was performed using Microsoft Excel 2007 (Microsoft, WA, USA). The evaluation of statistical significance was performed by Student t-test (two-tailed). P < 0.05 was considered statistically significant, n ≥ 3, [2, 21, 24–28].
3. Results
3.1. Production of Kojic Acid Esters
The maximum yield of KA esters synthesized by enzymatic esterification using immobilized lipase is shown in Table 1. The structure of KAMO has been identified and characterized in our previous study [12, 17]. The yield for KAMO, KAML, and KAMP was 32.86%, 34.89%, and 29.30%, respectively. The time taken to reach a maximum KAMO, KAML, and KAMP concentration was 42 h, 15 h, and 12 h, respectively. Meanwhile, KAML and KAMP were identified using GC-MS where the purified samples were fragmented to simple ions. The MS data for the esterification of KA and palmitic acid showed that ions at m/z 141 and 365 arise from path a and b cleavage. The m/z 363 is the expected rearrangement ion resulting path c cleavage with loss of OH (m/z 480–17). The fragmentation at m/z 255 and 125 confirm path d and e cleavage. The product of the esterification, KAMP, is shown at m/z 380. Meanwhile, the MS data for the esterification of KA and lauric acid showed that ions at m/z 141 and 309 arise from path a and b cleavage. The m/z 307 is the expected rearrangement of ion resulting to path c cleavage with loss of OH (m/z 480–17). The fragmentation at m/z 199 and 125 confirm path d and e cleavage. The fragmentation at m/z 197 is the expected ion from path f cleavage. The data for esterification reaction of KA also showed that ions at m/z 142. The esterification product, KAML, is shown at m/z 324 (Figure 1).
Table 1.
The yield of KA esters synthesized by enzymatic esterification using immobilized lipase from Rhizomucor miehei at optimal reaction condition.
| Compound | Variables | Yield (%) | |||
|---|---|---|---|---|---|
| ∗Time (h) | Temperature (°C) | Enzyme (g) | Substrate molar ratio (mmol) | ||
| KAMO | 42 | 52.5 | 0.33 | 0.5 | 32.86 |
| KAML | 15 | 50.0 | 0.20 | 5 | 34.89 |
| KAMP | 12 | 50.0 | 0.20 | 5 | 29.30 |
*A time of enzymatic esterification to achieve maximum concentration of KA esters.
Percentage yield was calculated using following equation:
Yield (%) = (Ccomp/mole of KA) × dilution factor × 100
C comp = (Acomp/AIS) × (CIS/DRF),
where, Acomp: area for each component; AIS: area for internal standard; CIS: concentration for internal standard; DRF: DRF standard/DRF internal standard; Ccomp: concentration for each component.
Figure 1.
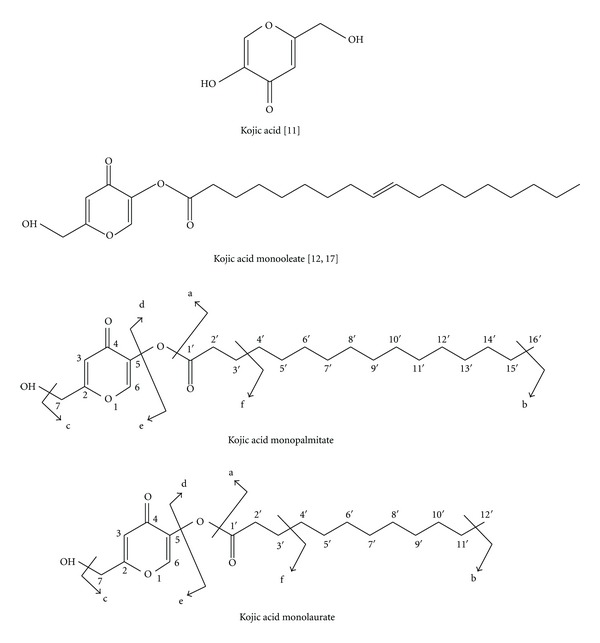
Chemical structure of kojic acid (KA), kojic acid monoeolate (KAMO), kojic acid monopalmitate (KAMP) and kojic acid monolaurate (KAML).
The 1H-NMR spectrum of the KAMP and KAML gave 3-hydrogen triplet at δ 0.88, indicating a terminal methyl group. Two hydrogen methylene signals are observed at H3′–H15′ and H3′–H11′ of KAMP and KAML, respectively. The downfield methylene signal at δ 2.40 was due to the present of CH2 group, next to the ester linkage. The fatty acid chain was thus established to be present in the product. The KA portion of the molecule was confirmed by the presence of two singlet signals at δ 6.49 and δ 7.85 which were assigned to H-3 and H-6. H-7 gave a singlet signal at δ 4.93. The 13C-NMR spectrum gave a total carbon count for KAMP and KAML of 22 and 18, respectively. The C-1′ (ester group) peak appeared at 163. Very low field signals were observed at δ 172 and δ 173 which were due to C-2 and C-4 of the pyrone ring. The other carbon assignments are also shown in Table 2.
Table 2.
1H-NMR and 13C-NMR data for KAMP and KAML.
| KAMP | KAML | |||
|---|---|---|---|---|
| Carbon no. | δ 1H | δ 13C | δ 1H | δ 13C |
| C-2 | — | 172.69 | — | 172.69 |
| C-3 | 6.49 (s) | 110.99 | 6.49 (s) | 110.94 |
| C-4 | — | 173.85 | — | 173.82 |
| C-5 | — | 137.75 | — | 137.67 |
| C-6 | 7.85 (s) | 145.80 | 7.85 (s) | 145.77 |
| C-7 | 4.93 (s) | 61.13 | 4.93 (s) | 61.13 |
| C-1′ester group | — | 163.11 | — | 163.13 |
| C-2′ | 2.40 (t) | 33.88 | 2.40 (t) | 33.88 |
| C-3′ | 1.66 (m) | 24.77 | 1.64 (m) | 24.77 |
| C-4′ | 1.29 | 29.18 | 1.28 | 29.18 |
| C-5′ | 1.29 | 29.34 | 1.28 | 29.23 |
| C-6′ | 1.29 | 29.41 | 1.28 | 29.30 |
| C-7′ | 1.29 | 29.56 | 1.28 | 29.41 |
| C-8′ | 1.29 | 29.64 | 1.28 | 29.56 |
| C-9′ | 1.29 | 29.65 | 1.28 | 29.06 |
| C-10′ | 1.29 | 29.67 | 1.28 | 31.89 |
| C-11′ | 1.29 | 29.67 | 1.30 | 22.66 |
| C-12′ | 1.29 | 29.64 | 0.88 (t) | 14.09 |
| C-13′ | 1.29 | 29.06 | ||
| C-14′ | 1.29 | 31.91 | ||
| C-15′ | 1.30 | 22.67 | ||
| C-16′ | 0.88 (t) | 14.09 | ||
The product formation and reactant disappearance were monitored by IR spectroscopy. The infrared spectrum of the KAMP showed the stretching of CH2 and CH3 which gave absorption peaks at 2848 cm−1 and 2914 cm−1, respectively. The C=O stretching for the expected ester carbonyl gave an absorption peak at 1695 cm−1. The present of aromatic ring gave absorption at 1619 cm−1. The CH2 and CH3 bends showed absorptions at 1458 cm−1 and 1290 cm−1. Meanwhile, O=C–O stretching absorbed at 1109 cm−1. On the other hand, the infrared spectrum of the KAML showed the stretching of CH2 and CH3 which gave absorption peaks at 2849 cm−1 and 2915 cm−1, respectively. The C=O stretching for the expected ester carbonyl gave an absorption peak at 1693 cm−1. The present of aromatic ring gave absorption at 1616 cm−1. The CH2 and CH3 bends showed absorptions at 1427 cm−1 and 1292 cm−1, respectively. Meanwhile, O=C–O stretching absorbed at 1138–11073 cm−1.
3.2. Cytotoxicity Effect of KA and KA Esters
The results of cell viability assay using MTT in B16F1 melanoma cells are shown in Figure 2. There was no significant reduction of cell viability after incubation of pigmented B16F1 melanoma cells with KAMO, KAML, KAMP and KA at doses ranging from 7.81 μg/mL to 31.25 μg/mL. However, the number of viable cells was significantly reduced to below 60% at KA concentration of 125 μg/mL and 500 μg/mL. On the other hand, even at very high dosages of KAMO and KAMP ranging from 125 to 500 μg/mL, more than 90% of B16F1 melanoma cells were still viable. Meanwhile, it was also noted that the number of viable cells was significantly reduced at 5% of DMSO which was used as a negative control.
Figure 2.
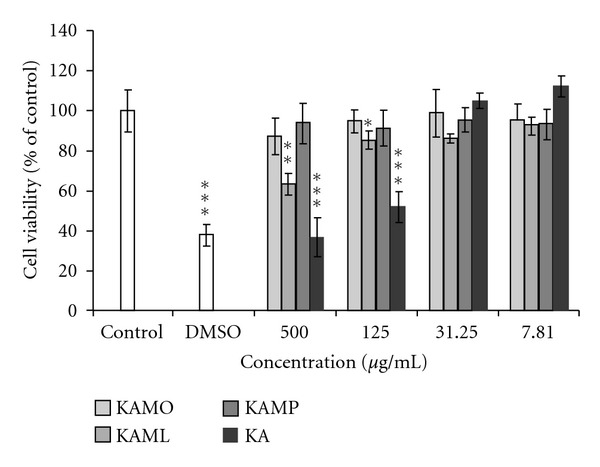
The effects of KAMO, KAML, KAMP, and KA on the viability of B16F1 melanoma cells. DMSO (5%) was used as a negative control. Cells were treated with various doses of KAMO, KAML, KAMP, and KA (7.81–500 μg/mL) for a total of 72 h incubation and were examined by MTT assay. Denotes *P < 0.05, **P < 0.01, ***P < 0.001 compared to untreated control. Data are presented as means ± S.E.M and expressed as % of control, n ≥ 3.
3.3. Inhibitory Effect of KA and KA Esters on Melanin Content
The inhibitory effect of KA and KA esters on melanin formation in B16F1 melanoma cells treated with α-MSH is summarized in Figure 3. The inhibitory effect of KAMO, KAML, KAMP, and KA was evaluated at nontoxic doses ranging from 1.95 to 62.5 μg/mL. The melanin content was significantly reduced at KA and KA esters concentration ranging from 31.3 to 62.5 μg/mL. KA and KA esters showed similar melanin inhibitory effect at the lowest dose tested in this study (1.95 μg/mL). Even at the highest dose tested, KAML was found to have similar melanin inhibitory effect to KA. However, KAMP have slightly higher inhibitory effect than other compounds tested at doses ranging from 15.63 μg/mL to 62.5 μg/mL.
Figure 3.
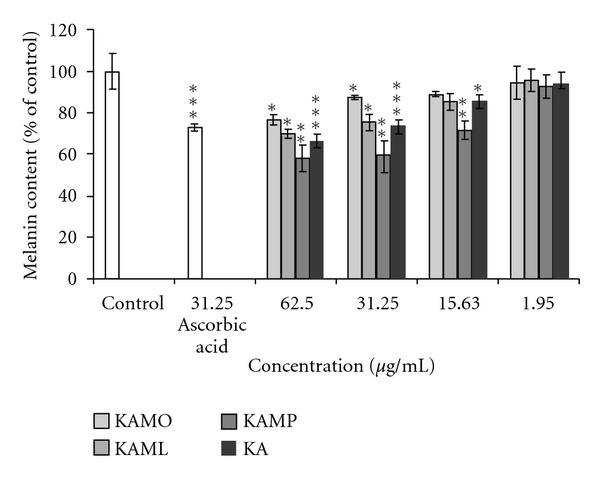
The effects of KAMO, KAML, KAMP, and KA on melanin content in B16F1 melanoma cells. Denotes *P < 0.05, **P < 0.01, ***P < 0.001 compared to α-MSH treated control. Data are presented as means ± S.E.M, and expressed as % of control. 31.25 μg/mL ascorbic acid was used as reference. n ≥ 3.
3.4. Inhibitory Effect of KA and KA Esters on Cellular and Mushroom Tyrosinase Activity
The inhibitory effect of KA and KA esters in B16F1 melanoma cells is summarized in Figure 4. The inhibitory effect of KA and KA esters was evaluated at nontoxic doses, ranging from 1.95 to 62.5 μg/mL. Incubation of pigmented melanoma B16F1 melanoma cells with KA and KA esters at doses ranging from 31.25 μg/mL to 62.5 μg/mL showed significant reduction in cellular tyrosinase activity. At very low doses of KA and KA esters, ranging from 1.95 to 15.25 μg/mL, only a slight reduction in cellular tyrosinase activity was observed. The inhibitory effect of KA and KA esters on cellular tyrosinase activity at doses ranging from 31.25 to 62.5 μg/mL was not significantly different. At the same dose (15.63 μg/mL), KAML and KAMP reduced cellular tyrosinase activity at a greater extent than KAMO.
Figure 4.
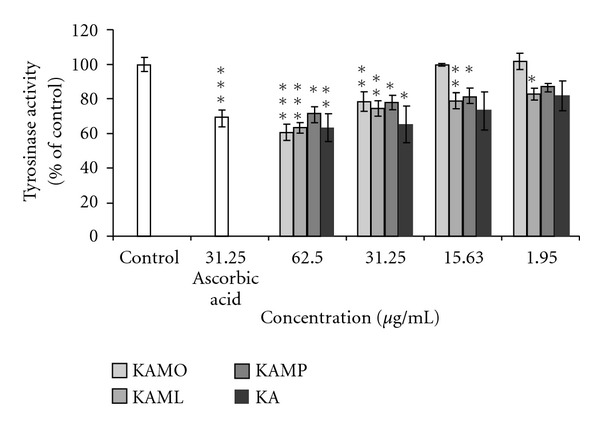
The results of the inhibition of tyrosinase activity by KAMO, KAML, KAMP, and KA in B16F1 melanoma cells. Denotes *P < 0.05, **P < 0.01, ***P < 0.001 compared to α-MSH treated control. Data are presented as means ± S.E.M, and expressed as % of control. 31.25 μg/mL ascorbic acid was used as reference. n ≥ 3.
Inhibitory effect of KA and KA esters on mushroom tyrosinase activity is illustrated in Figure 5. The inhibitory effect of KA and KA esters was evaluated at doses ranging from 3.91 to 250 μg/mL. In this study, mushroom tyrosinase inhibitory was found to be in dose-dependent manner. Among KA esters, KAMO significantly inhibited mushroom tyrosinase superior than KAML and KAMP. The inhibitory effect of mushroom tyrosinase activity of KAMO was not significantly different to KA at doses ranging from 62.5 to 250 μg/mL.
Figure 5.
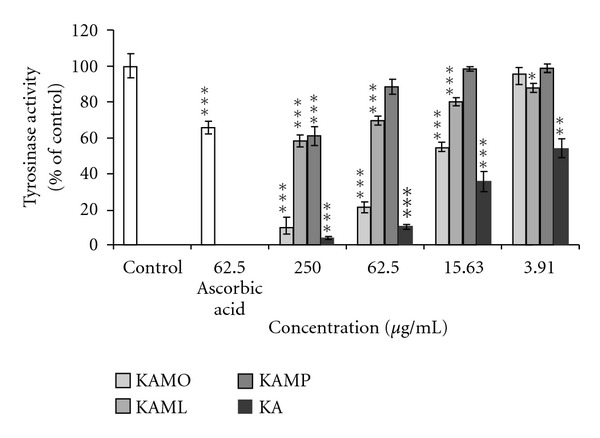
The results of the inhibition of mushroom tyrosinase activity by KAMO, KAML, KAMP, and KA. Denotes *P < 0.05, **P < 0.01, ***P < 0.001 compared to α-MSH treated control. Data are presented as means ± S.E.M, and expressed as % of control. 62.5 μg/mL ascorbic acid was used as reference. n ≥ 3.
3.5. The Antioxidant Activity of KA and KA Esters
The correlation between antimelanogenic activity with oxidative properties of KA and KA esters was also investigated. KA and KA esters showed mild free radical scavenging activity at concentrations ranging from 1.95 to 1000 μg/mL (Table 3). 2,2-diphenyl-1-picrylhydrazyl (DPPH) generation was inhibited by KA and KA esters in dose dependent manner. Among them, KA and KAMO showed slightly better antioxidant activity at higher doses (1000 μg/mL) as compared to KAML and KAMP. L-ascorbic acid, a well-known antimelanogenic vitamin and antioxidant, exerted more scavenging activity than KA and KA esters at 62.5 μg/mL.
Table 3.
Antioxidant effects of KA, KAMP, KAML and KAMO using DPPH. Denotes *P < 0.05, **P < 0.01, ***P < 0.001 compared to untreated control. Data are presented as means ± S.E.M, and expressed as % of control, n ≥ 3.
| Sample | Concentration (μg/mL) | Scavenging activity (% of control) S.E.M |
|---|---|---|
| Control | 0 | 0.06 ± 0.06 |
|
| ||
| KA | 1.95 | 5.86 ± 0.28∗∗∗ |
| 62.5 | 29.24 ± 4.18∗ | |
| 250 | 46.73 ± 5.21∗ | |
| 1000 | 57.07 ± 1.64∗∗∗ | |
|
| ||
| KAMP | 1.95 | 3.00 ± 0.40∗ |
| 62.5 | 4.69 ± 0.34∗∗ | |
| 250 | 9.41 ± 3.43 | |
| 1000 | 53.05 ± 13.47∗ | |
|
| ||
| KAML | 1.95 | 3.28 ± 3.14 |
| 62.5 | 11.96 ± 9.61 | |
| 250 | 16.91 ± 5.06 | |
| 1000 | 44.70 ± 6.66∗ | |
|
| ||
| KAMO | 1.95 | 17.44 ± 3.24∗ |
| 62.5 | 24.54 ± 2.89∗∗ | |
| 250 | 35.20 ± 1.41∗∗ | |
| 1000 | 59.01 ± 2.89∗∗ | |
|
| ||
| L-ascorbic | 62.5 | 48.73 ± 3.84∗∗∗ |
4. Discussion
B16F1 melanoma cells is a widely used model to evaluate depigmentation activity with high level of tyrosinase and melanin content as compared to B16F10, due to high level of msg1 (melanocyte-specific gene) [22, 24, 28–33]. In melanogenesis, tyrosinase-related protein-2 and tyrosinase-related protein-1 catalyzes conversion of DOPachrome to DHICA and oxidation of DHICA, respectively, to form melanin [34]. In the presence of alpha-melanocyte stimulating hormone (α-MSH), and isobutylmethylxanthine (IBMX), B16 melanoma cells expressed great amount of tyrosinase and melanin synthesis [10, 31]. α-MSH binds to melanocortin receptor (MC1R), resulting in the activation of stimulatory GTP-binding protein (Gs), which in turn, stimulates adenylate cyclase to generate cAMP. cAMP increases melanin synthesis via activation of cAMP-dependent protein kinase (PKA) and microphthalmia-associated transcription factor (MITF), a melanocyte-specific transcription factor, leading to induction of tyrosinase expression [35–38]. Hyperpigmentation and melasma are the result from the accumulation of tyrosinase and melanin in cells. Therefore, the ability of KAMO, KAML, and KAMP to inhibit tyrosinase activity and melanin content in alpha-MSH induced B16F1 melanoma cells showed their potential as depigmenting agent and to treat hyperpigmentation in vitro.
Melanin synthesis can also be induced by the presence of free radicals and reactive species. Excessive explore of ultraviolet radiation, metal ions, free radicals, and reactive species have significantly stimulate transcription of tyrosinase gene and contribute to hyperpigmentation [4, 39]. The presence of metal ions, free radical, and reactive species caused oxidation in melanogenesis pathway that will result in high melanin synthesis [8]. Antioxidant such as vitamin C and multivitamin were known to scavenge free radicals and inhibit tyrosinase activity [40]. The stable DPPH free radicals are a commonly use model and technique to evaluate antioxidant activities. The effect of antioxidants on DPPH free radicals was due to their hydrogen donating ability. DPPH free radicals accept electron or hydrogen radicals to become stable diamagnetic molecules. The decrease in absorbance of DPPH radical caused by antioxidants, because of the reaction between antioxidant molecules and radical progresses, which results in the scavenging of the radical by hydrogen donation [41]. Therefore, the potency of hydroxyl group (OH) at C-7 of KA esters to stabilize free radicals and chelate metal ions [9] may help to reduce melanogenesis process and downregulate hyperpigmentation. In this study, KA esters synthesized using RMIM have slightly greater scavenging activity than other KA esters that were previously reported in the literature [14]. Tyrosinase is known as copper-containing enzyme, thus the capability of KA and KA esters to chelate metal ions may chelate cooper in tyrosinase, changing its three-dimensional conformation to inhibit its enzymatic activity [11].
The cytotoxicity of KA and KA esters was investigated in this study. It was previously suggested that inhibition of upregulated tyrosinase enzyme in melanoma cells might inhibit cell proliferation of melanoma cells [42, 43]. This is due to the correlation of microphthalmia-associated transcription factor (MITF) and extracellular signal-regulated kinase (ERK) in the pigmentation, proliferation, and survival of melanocytes and melanoma [23, 26, 35]. Due to this reason, it was expected that the inhibition of MITF expression may also inhibit melanoma cells proliferation. However, KA and KA esters were only known to inhibit melanogenesis by direct inhibition to tyrosinase and do not inhibit the expression of the transcription factor [5]. Therefore, the melanoma cells can still proliferate but the tyrosinase produced are not functional due to inhibition of KA and KA esters. In another study, α-melanocyte-stimulating hormone (MSH) decreased a critical mediator in the tumorigenesis (syndecan-2 expression), melanoma cell migration, and invasion in a melanin synthesis-independent manner [44]. Other depigmenting compound like hydroquinone, is a strong tyrosinase inhibitor with bleaching effect and exerted very high cytotoxicity at high concentrations [45]. Besides that, vitamin C and multivitamin showed satisfactory inhibitory effect in melanin content and tyrosinase activity at low concentrations, though it could be toxic at high concentrations [40]. KA was claimed to be nontoxic at doses below 100 μg/mL [18, 27, 46]. KA esters derived in this study have very low cytotoxicity, even at very high doses (up to 500 μg/L). In summary, results from this study indicated that KA and KA esters are potential depigmenting agents with low cytotoxicity for application in cosmetic and skin-care products.
5. Conclusion
KA esters derived from esterification of KA and palm oil based fatty acid have been demonstrated as a safe and nontoxic depigmenting agents with a satisfactory inhibitory effect on melanin formation and tyrosinase activity as determined on α-MSH induced B16F1 melanoma cells. Thus, it can be suggested that these depigmenting compounds have potential to be used in cosmetic formulations and to treat hyperpigmentation.
Acknowledgment
This paper was financially supported by CRDF-MTDC Grant from Malaysian Technology Development Corporation. A. F. B. Lajis is a postgraduate student funded by Graduate Research Fund (GRF) of Universiti Putra Malaysia and Mybrain15 from Ministry of Higher Education of Malaysia.
References
- 1.Hurst EA, Harbour JW, Cornelius LA. Ocular melanoma: a review and the relationship to cutaneous melanoma. Archives of Dermatology. 2003;139(8):1067–1073. doi: 10.1001/archderm.139.8.1067. [DOI] [PubMed] [Google Scholar]
- 2.Huang HP, Shih YW, Chang YC, Hung CN, Wang CJ. Chemoinhibitory effect of mulberry anthocyanins on melanoma metastasis involved in the Ras/PI3K pathway. Journal of Agricultural and Food Chemistry. 2008;56(19):9286–9293. doi: 10.1021/jf8013102. [DOI] [PubMed] [Google Scholar]
- 3.Hearing VJ. The expanding role and presence of neuromelanins in the human brain—why gray matter is gray. Pigment Cell and Melanoma Research. 2009;22(1):10–11. doi: 10.1111/j.1755-148X.2008.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortonne JP, Bissett DL. Latest insights into skin hyperpigmentation. Journal of Investigative Dermatology Symposium Proceedings. 2008;13(1):10–14. doi: 10.1038/jidsymp.2008.7. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhofer G, Tian H, Holmes C, Matsunaga J, Roffler-Tarlov S, Hearing VJ. Tyrosinase: a developmentally specific major determinant of peripheral dopamine. The FASEB Journal. 2003;17(10):1248–1255. doi: 10.1096/fj.02-0736com. [DOI] [PubMed] [Google Scholar]
- 6.Heo SJ, Ko SC, Kang SM, et al. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food and Chemical Toxicology. 2010;48(5):1355–1361. doi: 10.1016/j.fct.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Panich U, Kongtaphan K, Onkoksoong T, et al. Modulation of antioxidant defense by Alpinia galanga and Curcuma aromatica extracts correlates with their inhibition of UVA-induced melanogenesis. Cell Biology and Toxicology. 2010;26(2):103–116. doi: 10.1007/s10565-009-9121-2. [DOI] [PubMed] [Google Scholar]
- 8.Novellino L, Napolitano A, Prota G. 5,6-Dihydroxyindoles in the fenton reaction: a model study of the role of melanin precursors in oxidative stress and hyperpigmentary processes. Chemical Research in Toxicology. 1999;12(10):985–992. doi: 10.1021/tx990020i. [DOI] [PubMed] [Google Scholar]
- 9.Niwa Y, Akamatsu H. Kojic acid scavenges free radicals while potentiating leukocyte functions including free radical generation. Inflammation. 1991;15(4):303–315. doi: 10.1007/BF00917315. [DOI] [PubMed] [Google Scholar]
- 10.Springer M, Engelhart K, Biesalski HK. Effects of 3-isobutyl-1-methylxanthine and kojic acid on cocultures and skin equivalents composed of HaCat cells and human melanocytes. Archives of Dermatological Research. 2003;295(2):88–91. doi: 10.1007/s00403-003-0401-z. [DOI] [PubMed] [Google Scholar]
- 11.Mohamad R, Mohamad MS, Suhaili N, Salleh MM, Ariff AB. Kojic acid: applications and development of fermention process for production. Biological and Molecular Biology Reviews. 2010;5(2):24–37. [Google Scholar]
- 12.Ashari SE, Mohamad R, Ariff A, Basri M, Salleh AB. Optimization of enzymatic synthesis of palm-based kojic acid ester using response surface methodology. Journal of Oleo Science. 2009;58(10):501–510. doi: 10.5650/jos.58.503. [DOI] [PubMed] [Google Scholar]
- 13.Liu KJ, Shaw JF. Lipase-catalyzed synthesis of kojic acid esters in organic solvents. Journal of the American Oil Chemists’ Society. 1998;75(11):1507–1511. [Google Scholar]
- 14.Raku T, Tokiwa Y. Regioselective synthesis of kojic acid esters by Bacillus subtilis protease. Biotechnology Letters. 2003;25(12):969–974. doi: 10.1023/a:1024088303960. [DOI] [PubMed] [Google Scholar]
- 15.Rho HS, Baek HS, Ann SM, Kim DH, Chang IS. Synthesis of new anti-melanogenic compounds containing two molecules of kojic acid. Bulletin of the Korean Chemical Society. 2008;29(8):1569–1571. [Google Scholar]
- 16.Mohamad R, Ariff AB. Biotransformation of various carbon sources to kojic acid by cell-bound enzyme system of A. flavus Link 44-1. Biochemical Engineering Journal. 2007;35(2):203–209. [Google Scholar]
- 17.Khamaruddin NH, Basri M, Lian GEC, et al. Enzymatic synthesis and characterization of palm-based kojic acid Ester. Journal of Oil Palm Research. 2009;20:461–469. [Google Scholar]
- 18.Momtaz S, Mapunya BM, Houghton PJ, et al. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. Journal of Ethnopharmacology. 2008;119(3):507–512. doi: 10.1016/j.jep.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Shibata S, Okano S, Yonemitsu Y, et al. Induction of efficient antitumor immunity using dendritic cells activated by recombinant Sendai virus and its modulation by exogenous IFN-β gene. Journal of Immunology. 2006;177(6):3564–3576. doi: 10.4049/jimmunol.177.6.3564. [DOI] [PubMed] [Google Scholar]
- 20.Cao XG, Li XX, Bao YZ, Xing NZ, Chen Y. Responses of human lens epithelial cells to quercetin and DMSO. Investigative Ophthalmology and Visual Science. 2007;48(8):3714–3718. doi: 10.1167/iovs.06-1304. [DOI] [PubMed] [Google Scholar]
- 21.Makpol S, Arifin NNM, Ismail Z, Chua KH, Yusof YAM, Ngah WZW. Modulation of melanin synthesis and its gene expression in skin melanocytes by palm tocotrienol rich fraction. African Journal of Biochemistry Research. 2009;3(12):385–392. [Google Scholar]
- 22.Choi SW, Lee SK, Kim EO, et al. Antioxidant and antimelanogenic activities of polyamine conjugates from corn bran and related hydroxycinnamic acids. Journal of Agricultural and Food Chemistry. 2007;55(10):3920–3925. doi: 10.1021/jf0635154. [DOI] [PubMed] [Google Scholar]
- 23.Shin YJ, Han CS, Lee CS, et al. Zeolite 4A, a synthetic silicate, suppresses melanogenesis through the degradation of microphthalmia-associated transcription factor by extracellular signal-regulated kinase activation in B16F10 melanoma cells. Biological and Pharmaceutical Bulletin. 2010;33(1):72–76. doi: 10.1248/bpb.33.72. [DOI] [PubMed] [Google Scholar]
- 24.Aoki Y, Tanigawa T, Abe H, Fujiwara Y. Melanogenesis inhibition by an oolong tea extract in B16 mouse melanoma cells and UV-induced skin pigmentation in brownish guinea pigs. Bioscience, Biotechnology and Biochemistry. 2007;71(8):1879–1885. doi: 10.1271/bbb.70099. [DOI] [PubMed] [Google Scholar]
- 25.Yu JS, Kim AK. Effect of combination of taurine and azelaic acid on antimelanogenesis in murine melanoma cells. Journal of Biomedical Science. 2010;17(supplement 1, article S45) doi: 10.1186/1423-0127-17-S1-S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DS, Park SH, Kwon SB, Li K, Youn SW, Park KC. (-)-Epigallocatechin-3-gallate and hinokitiol reduce melanin synthesis via decreased MITF production. Archives of Pharmacal Research. 2004;27(3):334–339. doi: 10.1007/BF02980069. [DOI] [PubMed] [Google Scholar]
- 27.Ha SK, Koketsu M, Lee K, et al. Inhibition of tyrosinase activity by N,N-unsubstituted selenourea derivatives. Biological and Pharmaceutical Bulletin. 2005;28(5):838–840. doi: 10.1248/bpb.28.838. [DOI] [PubMed] [Google Scholar]
- 28.Kim YJ. Antimelanogenic and antioxidant properties of gallic acid. Biological and Pharmaceutical Bulletin. 2007;30(6):1052–1055. doi: 10.1248/bpb.30.1052. [DOI] [PubMed] [Google Scholar]
- 29.Kim KD, Song MH, Yum EK, Jeon OS, Ju YW, Chang MS. Melanogenesis inhibition by mono-hydroxycinnamic ester derivatives in B16 melanoma cells. Bulletin of the Korean Chemical Society. 2010;31(1):181–184. [Google Scholar]
- 30.Kinoshita M, Hori N, Aida K, Sugawara T, Ohnishi M. Prevention of melanin formation by yeast cerebroside in B16 mouse melanoma cells. Journal of Oleo Science. 2007;56(12):645–648. doi: 10.5650/jos.56.645. [DOI] [PubMed] [Google Scholar]
- 31.Sato K, Takahashi H, Iraha R, Toriyama M. Down-regulation of tyrosinase expression by acetylsalicylic acid in murine B16 melanoma. Biological and Pharmaceutical Bulletin. 2008;31(1):33–37. doi: 10.1248/bpb.31.33. [DOI] [PubMed] [Google Scholar]
- 32.Burchill SA, Bennett DC, Holmes A, Thody AJ. Tyrosinase expression and melanogenesis in melanotic and amelanotic B16 mouse melanoma cells. Pathobiology. 1991;59(5):335–339. doi: 10.1159/000163673. [DOI] [PubMed] [Google Scholar]
- 33.Shioda T, Fenner MH, Isselbacher KJ. msg1, a novel melanocyte-specific gene, encodes a nuclear protein and is associated with pigmentation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(22):12298–12303. doi: 10.1073/pnas.93.22.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato K, Toriyama M. Depigmenting effect of catechins. Molecules. 2009;14(11):4425–4432. doi: 10.3390/molecules14114425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertolotto C, Bille K, Ortonne JP, Ballotti R. Regulation of tyrosinase gene expression by cAMP in B16 melanoma cells involves two CATGTG motifs surrounding the TATA box: implication of the microphthalmia gene product. Journal of Cell Biology. 1996;134(3):747–755. doi: 10.1083/jcb.134.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusciano D, Lorenzoni P, Burger MM. Regulation of c-met expression in B16 murine melanoma cells by melanocyte stimulating hormone. Journal of Cell Science. 1999;112(5):623–630. doi: 10.1242/jcs.112.5.623. [DOI] [PubMed] [Google Scholar]
- 37.Ohguchi K, Banno Y, Akao Y, Nozawa Y. Involvement of phospholipase D1 in melanogenesis of mouse B16 melanoma cells. Journal of Biological Chemistry. 2004;279(5):3408–3412. doi: 10.1074/jbc.M308054200. [DOI] [PubMed] [Google Scholar]
- 38.Hill SE, Buffey J, Thody AJ, Oliver I, Bleehen SS, MacNeil S. Investigation of the regulation of pigmentation in alpha-melanocyte-stimulating hormone responsive and unresponsive cultured B16 melanoma cells. Pigment Cell Research. 1989;2(3):161–166. doi: 10.1111/j.1600-0749.1989.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 39.Lam UDP, Hoang DN, Lee HB, et al. Depigmenting effect of Sterculia lynchnophera on B16F10 melanoma and C57BL/6 melan-a cells. Korean Journal of Chemical Engineering. 2011;28(4):1074–1077. [Google Scholar]
- 40.Choi YK, Rho YK, Yoo KH, et al. Effects of vitamin C vs. multivitamin on melanogenesis: comparative study in vitro and in vivo. International Journal of Dermatology. 2010;49(2):218–226. doi: 10.1111/j.1365-4632.2009.04336.x. [DOI] [PubMed] [Google Scholar]
- 41.Gülçin I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. International Journal of Food Sciences and Nutrition. 2005;56(7):491–499. doi: 10.1080/09637480500450248. [DOI] [PubMed] [Google Scholar]
- 42.Vad NM, Kandala PK, Srivastava SK, Moridani MY. Structure-toxicity relationship of phenolic analogs as anti-melanoma agents: an enzyme directed prodrug approach. Chemico-Biological Interactions. 2010;183(3):462–471. doi: 10.1016/j.cbi.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma HL, Whitters MJ, Konz RF, et al. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-γ. Journal of Immunology. 2003;171(2):608–615. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, Park H, Chung H, et al. Syndecan-2 regulates the migratory potential of melanoma cells. Journal of Biological Chemistry. 2009;284(40):27167–27175. doi: 10.1074/jbc.M109.034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu ZM, Zhou Q, Lei TC, Ding SF, Xu SZ. Effects of hydroquinone and its glucoside derivatives on melanogenesis and antioxidation: biosafety as skin whitening agents. Journal of Dermatological Science. 2009;55(3):179–184. doi: 10.1016/j.jdermsci.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Lee SH, Choi SY, Kim H, et al. Mulberroside F isolated from the leaves of Morus alba inhibits melanin biosynthesis. Biological and Pharmaceutical Bulletin. 2002;25(8):1045–1048. doi: 10.1248/bpb.25.1045. [DOI] [PubMed] [Google Scholar]


