Abstract
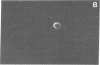
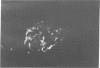
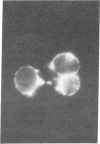
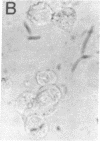
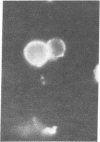
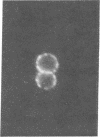
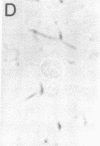
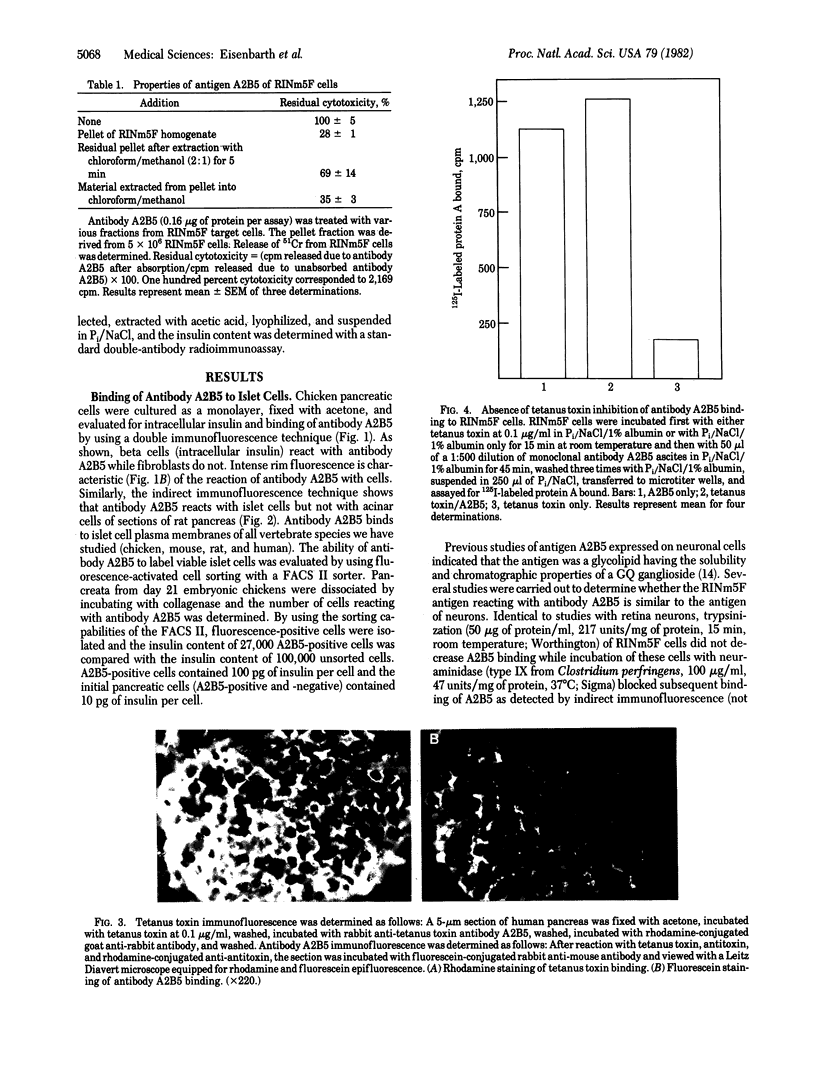
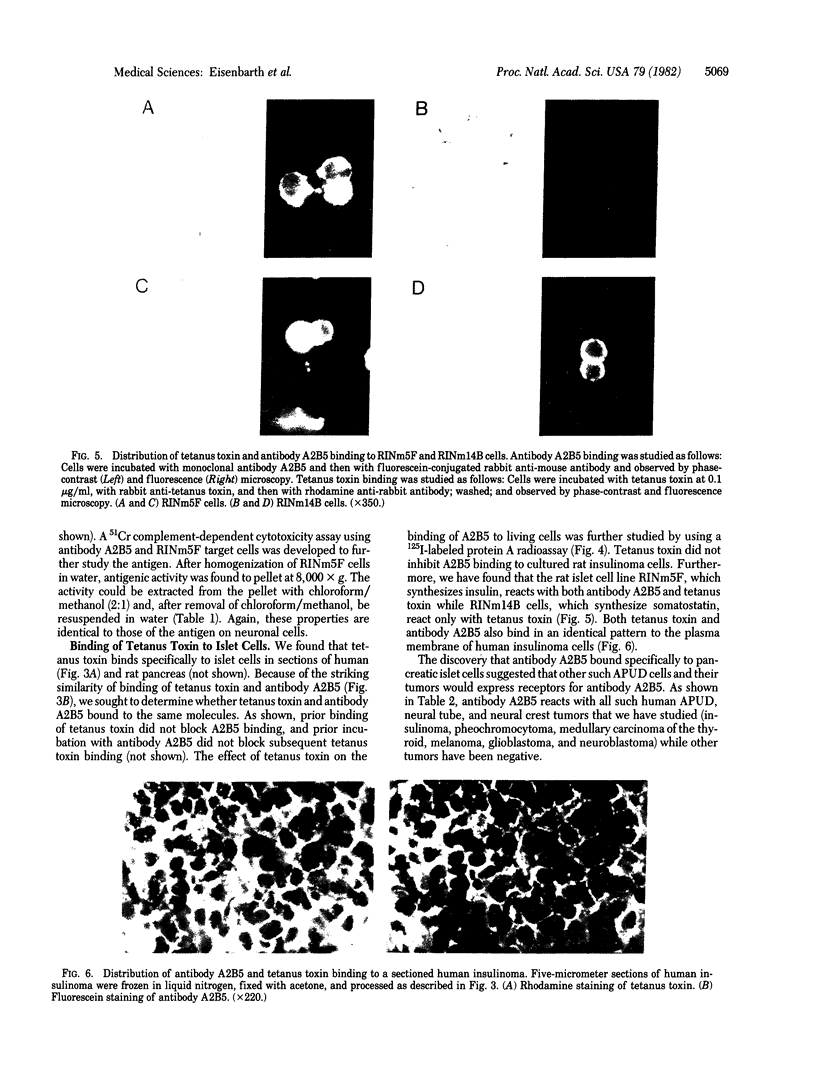
Studies of the reaction of antibody A2B5 and tetanus toxin with pancreatic islet cells, islet cell tumors, and other human amine precursor uptake and decarboxylation (APUD) tumors are described. By indirect immunofluorescence, antibody A2B5 and tetanus toxin were shown to specifically bind to the plasma membrane of human, rat, chicken, and mouse islet cells. The binding of antibody A2B5 to the cell surface of living islet cells has allowed isolation of these cells from a suspension of pancreatic cells by using a fluorescence-activated cell sorter. In studies designed to determine whether tetanus toxin and antibody A2B5 bound to the same surface antigen, A2B5 and tetanus toxin did not compete for binding to normal islet cells, a human islet cell tumor, or a rat islet cell tumor. In addition to binding to islet cell tumors, antibody A2B5 reacts with frozen sections, isolated cells, and cell lines of neural, neural crest, and APUD origin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew A. APUD cells in the endocrine pancreas and the intestine of chick embryos. Gen Comp Endocrinol. 1975 Aug;26(4):485–495. doi: 10.1016/0016-6480(75)90171-9. [DOI] [PubMed] [Google Scholar]
- Andrew A. An experimental investigation into the possible neural crest origin of pancreatic APUD (islet) cells. J Embryol Exp Morphol. 1976 Jun;35(3):577–593. [PubMed] [Google Scholar]
- Andrew A. Further evidence that enterochromaffin cells are not derived from the neural crest. J Embryol Exp Morphol. 1974 Jun;31(3):589–598. [PubMed] [Google Scholar]
- Baylin S. B., Mendelsohn G. Ectopic (inappropriate) hormone production by tumors: mechanisms involved and the biological and clinical implications. Endocr Rev. 1980 Winter;1(1):45–77. doi: 10.1210/edrv-1-1-45. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Jackson R. A. Application of monoclonal antibody techniques to endocrinology. Endocr Rev. 1982 Winter;3(1):26–39. doi: 10.1210/edrv-3-1-26. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine J., Le Douarin N. M. Analysis of endoderm formation in the avian blastoderm by the use of quail-chick chimaeras. The problem of the neurectodermal origin of the cells of the APUD series. J Embryol Exp Morphol. 1977 Oct;41:209–222. [PubMed] [Google Scholar]
- Gazdar A. F., Chick W. L., Oie H. K., Sims H. L., King D. L., Weir G. C., Lauris V. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3519–3523. doi: 10.1073/pnas.77.6.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Teillet M. A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973 Aug;30(1):31–48. [PubMed] [Google Scholar]
- Pearse A. G. The APUD concept and hormone production. Clin Endocrinol Metab. 1980 Jul;9(2):211–222. doi: 10.1016/s0300-595x(80)80030-2. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Pearse A. G., Le Lièvre C., Fontaine J., Le Douarin N. M. Immunocytochemical confirmation of the neural crest origin of avian calcitonin-producing cells. Histochemistry. 1974;40(3):209–214. doi: 10.1007/BF00501955. [DOI] [PubMed] [Google Scholar]
- Schmechel D., Marangos P. J., Brightman M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature. 1978 Dec 21;276(5690):834–836. doi: 10.1038/276834a0. [DOI] [PubMed] [Google Scholar]