Abstract
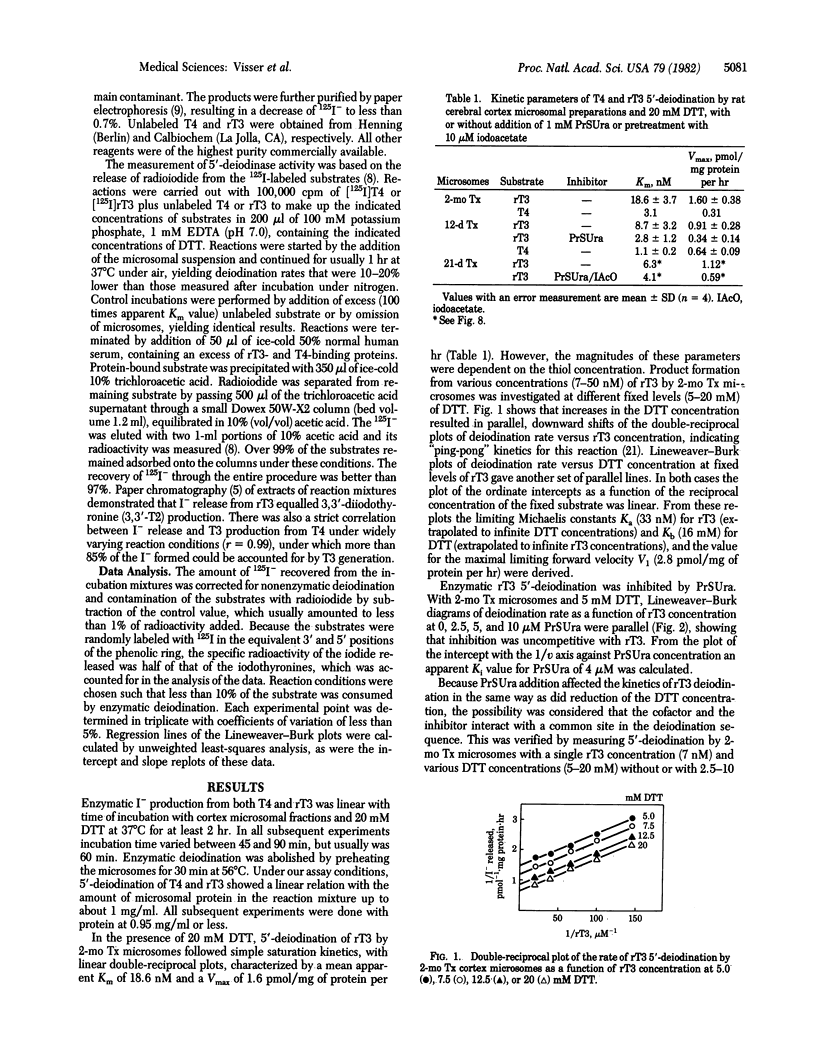
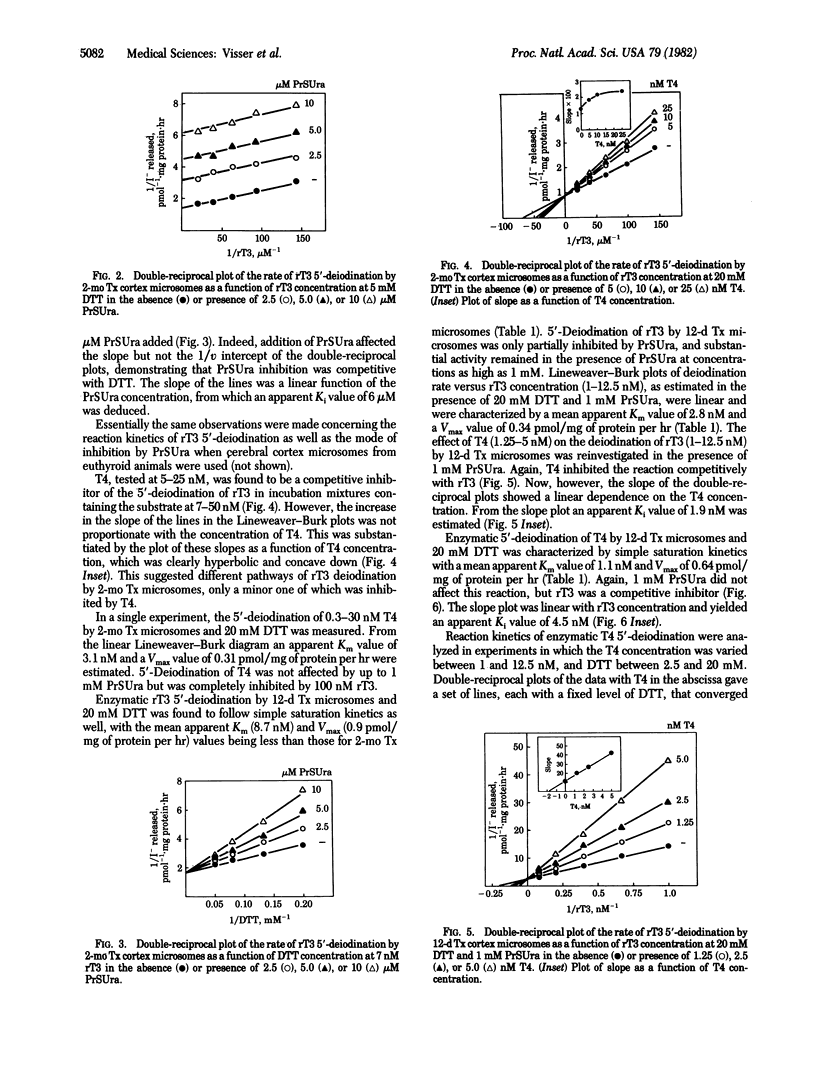
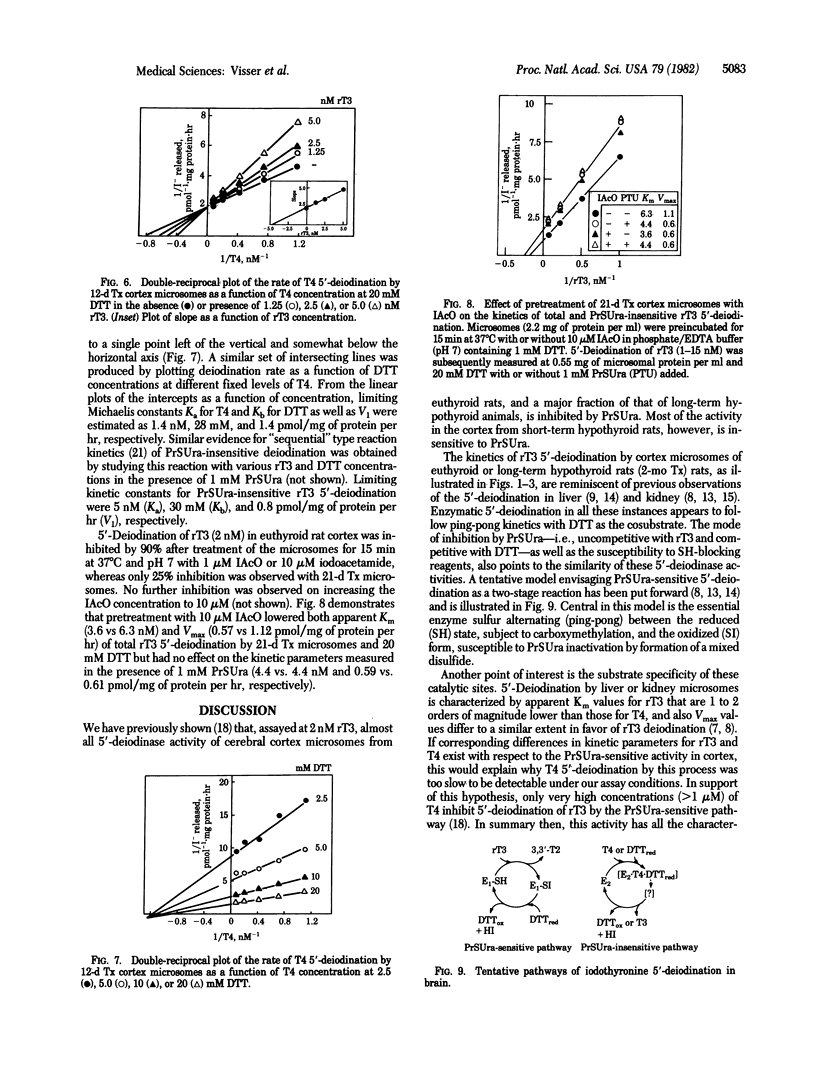
Enzymatic 5'-deiodination of 3,3',5'-triiodothyronine (rT3) and 3,3',5,5'-tetraiodothyronine (thyroxine, T4) was studied in microsomal preparations of rat cerebral cortex. Evidence was obtained for the existence of two thiol-dependent 5'-deiodinase entities. One of these predominates in tissue from euthyroid and long-term hypothyroid rats, is specific for rT3, follows "ping-pong" kinetics with dithiothreitol as the cosubstrate, and is inhibited by propylthiouracil (PrSUra) and iodoacetate. Inhibition by PrSUra is uncompetitive with rT3 and competitive with dithiothreitol. These properties are shared with the 5'-deiodinase activity of liver and kidney. The activity of a second type of 5'-deiodinase is highest in cerebral cortex from short-term hypothyroid rats, prefers T4 to rT3 as the substrate, is insensitive to PrSUra and iodoacetate, and follows "sequential" reaction kinetics. A similar PrSUra-insensitive 5'-deiodinase activity is also found in pituitary but is not detectable in liver and kidney; it seems, therefore, characteristic of tissues in which local T4 to 3,3',5-triiodothyronine (T3) conversion supplies a major portion of the total intracellular T3.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheron R. G., Kaplan M. M., Larsen P. R. Physiological and pharmacological influences on thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat anterior pituitary. J Clin Invest. 1979 Nov;64(5):1402–1414. doi: 10.1172/JCI109598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekkes D., van Overmeeren-Kaptein E., Docter R., Hennemann G., Visser T. J. Location of rat liver iodothyronine deiodinating enzymes in the endoplasmic reticulum. Biochim Biophys Acta. 1979 Sep 20;587(1):12–19. doi: 10.1016/0304-4165(79)90215-0. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Thyroxine 5'-monodeiodination in rat anterior pituitary homogenates. Endocrinology. 1980 Feb;106(2):567–576. doi: 10.1210/endo-106-2-567. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in liver and kidney homogenates from hyperthyroid and hypothyroid rats. Endocrinology. 1978 Jul;103(1):156–161. doi: 10.1210/endo-103-1-156. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Phenolic and tyrosyl ring deiodination of iodothyronines in rat brain homogenates. J Clin Invest. 1980 Sep;66(3):551–562. doi: 10.1172/JCI109887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Silva J. E., Kaplan M. M. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev. 1981 Winter;2(1):87–102. doi: 10.1210/edrv-2-1-87. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Kaplan M. M., Visser T. J., Silva J. E., Larsen P. R. Cerebral cortex responds rapidly to thyroid hormones. Science. 1981 Oct 30;214(4520):571–573. doi: 10.1126/science.7291997. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Characterization of essential enzyme sulfhydryl groups of thyroxine 5'-deiodinase from rat kidney. Endocrinology. 1980 Feb;106(2):444–451. doi: 10.1210/endo-106-2-444. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Iodothyronine 5'-deiodinase from rat kidney: substrate specificity and the 5'-deiodination of reverse triiodothyronine. Endocrinology. 1980 Nov;107(5):1376–1383. doi: 10.1210/endo-107-5-1376. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Subcellular distribution of thyroxine 5'-deiodinase in the rat kidney: a plasma membrane location. Endocrinology. 1978 Jul;103(1):274–280. doi: 10.1210/endo-103-1-274. [DOI] [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Thyroxine 5'-deiodinase activity of rat kidney: observations on activation by thiols and inhibition by propylthiouracil. Endocrinology. 1978 Dec;103(6):2137–2144. doi: 10.1210/endo-103-6-2137. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Leonard J. L., Crantz F. R., Larsen P. R. Evidence for two tissue-specific pathways for in vivo thyroxine 5'-deiodination in the rat. J Clin Invest. 1982 May;69(5):1176–1184. doi: 10.1172/JCI110554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe G. A., Duncan M. W., Bradshaw J. E., Cai W. Y. Serotoninergic control of growth hormone secretion: hypothalamic dopamine, norepinephrine, and serotonin levels and metabolism in three hyposomatotropic rat models and in normal rats. Endocrinology. 1982 Feb;110(2):376–383. doi: 10.1210/endo-110-2-376. [DOI] [PubMed] [Google Scholar]
- Sponholtz D. K., Brautigan D. L., Loach P. A., Margoliash E. Preparation of cytochrome c2 from Rhodospirillum rubrum. Anal Biochem. 1976 May 7;72:255–260. doi: 10.1016/0003-2697(76)90528-5. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Does-Tobé I., Docter R., Hennemann G. Subcellular localization of a rat liver enzyme converting thyroxine into tri-iodothyronine and possible involvement of essential thiol groups. Biochem J. 1976 Aug 1;157(2):479–482. doi: 10.1042/bj1570479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Fekkes D., Docter R., Hennemann G. Kinetics of enzymic reductive deiodination of iodothyronines. Effect of pH. Biochem J. 1979 Jun 1;179(3):489–495. doi: 10.1042/bj1790489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Fekkes D., Docter R., Hennemann G. Sequential deiodination of thyroxine in rat liver homogenate. Biochem J. 1978 Jul 15;174(1):221–229. doi: 10.1042/bj1740221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser T. J., Leonard J. L., Kaplan M. M., Larsen P. R. Different pathways of iodothyronine 5'-deiodination in rat cerebral cortex. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1297–1304. doi: 10.1016/0006-291x(81)91588-6. [DOI] [PubMed] [Google Scholar]
- Visser T. J. Mechanism of action of iodothyronine-5'-deiodinase. Biochim Biophys Acta. 1979 Aug 15;569(2):302–308. doi: 10.1016/0005-2744(79)90066-4. [DOI] [PubMed] [Google Scholar]
- Visser T. J., van Overmeeren-Kaptein E. Substrate requirement for inactivation of iodothyronine-5'-deiodinase activity by thiouracil. Biochim Biophys Acta. 1981 Apr 14;658(2):202–208. doi: 10.1016/0005-2744(81)90290-4. [DOI] [PubMed] [Google Scholar]
- Weeke J., Orskov H. Synthesis of 125I monolabelled 3, 5, 3'-triiodothyronine and thyroxine of maximum specific activity for radioimmunoassay. Scand J Clin Lab Invest. 1973 Dec;32(4):357–360. doi: 10.3109/00365517309084359. [DOI] [PubMed] [Google Scholar]


