Abstract
Background
In humans and in baboons, protracted gastro-esophageal reflux (GER) transforms the squamous-lined esophagus into columnar-lined (that is Barrett's mucosa, BM). Alcian blue stain (AB) is used to evidence sialomucin-producing goblet cells in human BM.
Aim
To assess the frequency and distribution of sialomucin-producing cells in BM in baboons.
Materials and Methods
Sections from 137 consecutive baboon esophagi were alternatively stained with hematoxylin-eosin (H&E) and with AB (pH 2.5), without counterstain.
Results
Out of 137 baboons, 131 (95.6%) had BM. Columnar and intramucosal glandular cells produced sialomucin in all 131 of these animals. Many BM cells were ballooned and filled with sialomucins, despite goblet cells not being found in H&E sections.
Conclusion
In humans, protracted GER is a disease requiring medication that may lead to BM; AB stains mainly goblet cells and occasional columnar cells in BM. In baboons, in contrast, BM is a natural postnatal process of adaptation to GER, triggered by regurgitation and rumination. AB stains all columnar and intra-mucosal glandular cells. Sialomucin-overstuffed cells were more frequent and larger in baboons than in humans. The extra load of sialomucin in BM might be an integrated part of the postnatal life-long process of adaptation to regurgitation and rumination in baboons.
Keywords: Esophagus, baboons, glandular metaplasia, sialomucins
The esophagus in baboons is a tubular, predominantly intrathoracic organ, covered with stratified squamous epithelium having discrete papillae. Near the gastric junction the papillae are more prominent and intramucosal and submucosal accessory glands may be present (1). Under the influence of protracted gastric reflux generated by the chewing of regurgitated food, that is rumination (2), the multilayered squamous cell-lined mucosa becomes columnar-lined, having intramucosal mucus-producing accesory glands (3). The function of this glandulo-metaplastic mucosa is to buffer the low pH of the gastric juices entering the esophagus during regurgitation (4).
In humans, the stratified squamous epithelium of the esophagus also becomes columnar-lined under the influence of protracted gastric reflux (5). This transformation, known as Barrett's mucosa (BM)6 exhibits three main histological phenotypes: i) columnar-lined mucosa with accessory cardio-pyloric glands, ii) columnar-lined mucosa with accessory fundic glands, and iii) columnar-lined mucosa with crypts showing intercalated goblet cells and occasional Paneth cells. The latter epithelium is referred to as intestinal metaplasia (IM) (6).
In clinical praxis, sialomucin-producing goblet cells are evidenced in sections stained with Alcian blue (AB) (pH 2.5), counterstained with 0.5 aqueous neutral red. But when AB (pH 2.5) is used without the interference of other colorants, sialomucin-producing cells are more easily identified in both humans (7–9) and rodents (10, 11). This procedure even allows the quantification of sialomucin-producing cells (7, 9–11).
In all three BM histological phenotypes in humans, columnar cells secrete neutral mucins whereas goblet cells in IM produce sialomucins (12). In clinical biopsies Chen et al. (13) found, however, that even columnar cells in IM may occasionally contain sialomucins. These authors postulated that sialomucin-positive columnar cells might represent an early or incomplete form of IM (13).
The purpose of the present work was to assess the frequency and distribution of sialomucin-producing cells in the columnar-lined esophageal mucosa in baboons.
Materials and Methods
A cohort of 163 consecutive esophagi from adult baboons was investigated. The baboons were members of colonies at the Southwest National Primate Research Center, Southwest Foundation for Biomedical Research. The housing conditions have been reported elsewhere (3). Briefly, the animals were housed in metal and concrete indoor-outdoor cages and fed commercial monkey diets occasionally supplemented with a variety of fruit and vegetables. Water was available ad libitum. Baboons were euthanized with a commercial barbiturate euthanasia agent or died naturally. All procedures were carried out in accordance with the Institutional Animal Care and Use Committee guidelines.
On necropsy, tissue samples from the esophagus and the stomach were fixed in 10% buffered formalin, embedded in paraffin and cut at 5 μm. Sections were alternatively stained with hematoxylin-eosin (HE) and with AB (pH 2.5) without counterstain (9).
The term Barrett's mucosa in baboons was applied when the squamous-cell epithelium of the esophagus was replaced by columnar-lined mucosa (14).
Alcian blue (pH 2.5) staining procedure
Paraffin sections were deparaffinized hydrated to distilled water and treated in 3% acetic acid for 2 minutes. The preparations were drained off and stained in 1% AB (DAKO, Glistrup, Denmark) (pH 2.5) for 30 min. They were subsequently washed in water, dehydrated rapidly in alcohol, cleared and mounted.
Results
Of the 163 esophagi, 26 were rejected from the study because of post-morten autolysis. Hence, out of 163 baboons, 137 had well-preserved esophageal mucosa for histological evaluation. Of these, 131 (95.6%) had columnar-lined mucosa and in the remaining 6 (4.4%), the squamous epithelium merged directly into the fundic mucosa. Of these 6 baboons, five were stillborn and one was 4 days old.
The columnar-lined mucosa displayed subjacent accessory glands of antrum-pyloric phenotype (Figure 1). Results showed that the columnar epithelium and subjacent accessory glands were stained with AB (Figure 2), while the normal squamous cell epithelium of the esophagus remained unstained (Figure 3). Both the surface epithelium and accessory glands had many ballooned cells which stained deep blue with AB (Figure 4), thus resembling goblet cells found in the human esophagus with IM. Notwithstanding, the corresponding H&E stained sections in baboons revealed no goblet cells.
Figure 1.

Low power-view of the esophagus in a baboon showing columnar-lined glandular mucosa and squamous cell epithelium (arrow) (H&E, ×2).
Figure 2.
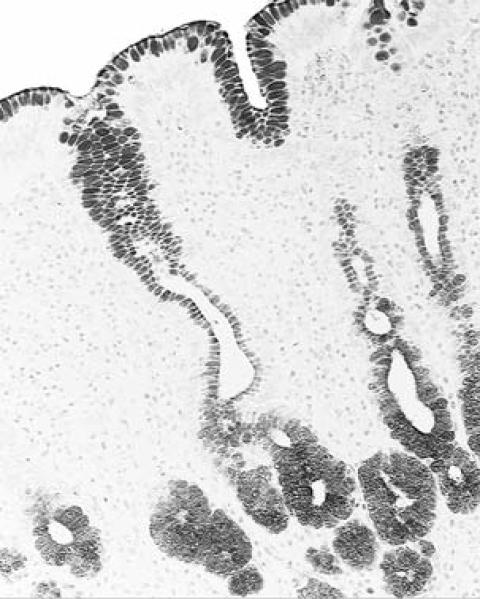
Columnar-lined glandular mucosa of the esophagus in a baboon, showing sialomucin-positive cells, both along the surface epithelium as well as in the epithelium of the glands (Alcian blue, pH 2.5, without counterstain, ×10).
Figure 3.
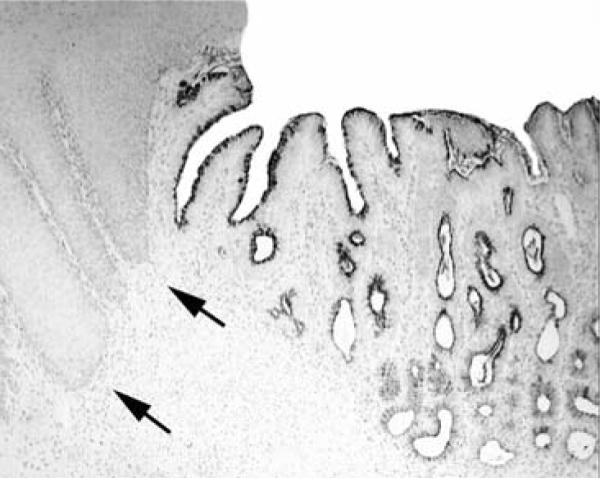
Columnar-lined glandular mucosa of the esophagus in a baboon, showing sialomucin-positive cells, both along the surface epithelium as well as in the epithelium of the glands. In contrast, the squamous epithelium (arrows) remained unstained (Alcian blue, pH 2.5, without counterstain, ×4).
Figure 4.
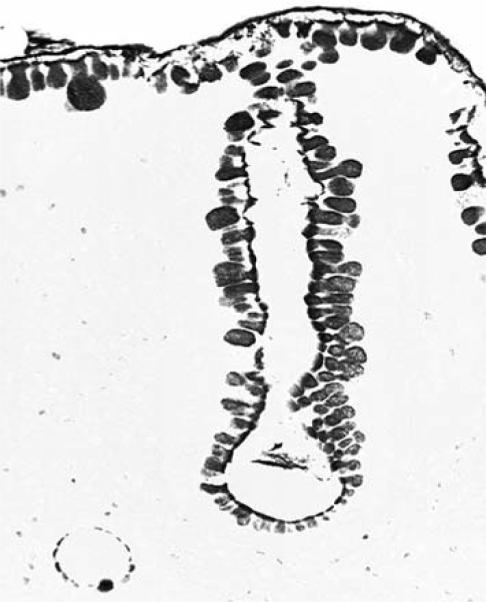
Columnar-lined glandular mucosa of the esophagus in a baboon, demonstrating the variation in size of sialomucin-positive cells. Some cells show a ballooned cytoplasm, but the corresponding H&E-stained sections revealed no classical goblet cells (Alcian blue, pH 2.5, without counterstain, ×40).
In sections from 54 of the 131 animals, gastric fundic mucosa was also present. AB-positive cells regular in size were found along the surface gastric epithelium and in the upper part of foveolar pits. No ballooned, deep blue-stained cells were recorded in the fundic mucosa. Parietal and chief cells were AB negative. The cells in the surface gastric epithelium and in the upper part of the foveolar pits were sialomucin-positive. In the antro-pyloric mucosa, no apparent difference in size was recorded in AB-positive cells.
Discussion
BM occurred in 95.6% of the consecutive esophagi from baboons investigated. This high rate strongly suggests that BM is a habitual event in the distal esophagus, generated by the normal physiological process of food digestion in these animals. This process includes regurgitation and rumination (15) a phenomenon valid not only in baboons but also in other primates such as chimpanzees (16) and gorillas (17). This process transforms the non-secreting multi-layered squamous cell esophagus into sialomucin-producing BM.
It should be understood that during and between meals, baboons adopt, by nature, an oblique position, while humans assume an upright (orthostatic) position. The oblique position in baboons during meals might increase the reflux of the gastric contents into the esophagus during the gastric phase of digestion. Despite the pH of the gastric acid being similar in baboons and in humans at the time of pH testing (18, 19), the daily reflux of this acid into the esophagus seems to be more continuous in baboons than in humans. It should be understood that in baboons, the reflux is life-long and thus do not receive medication, as in humans.
It is known that high fat intake relaxes the lower esophageal sphincter in humans (20, 21), thus encouraging gastro-esophageal reflux (GER). The limiting daily dietary fat recommended by The American Heart Association in humans is 30% (22). In baboons, the regular daily diet is only 4% fat1. Hence, the quantity of daily fat intake does not seem to be a factor responsible for the high frequency of BM in baboons.
Although no goblet cells were found in H&E-stained sections from BM in baboons, the cytoplasm in many BM cells appeared ballooned and deep blue-stained (that is filled with sialomucin). These overstuffed cells were more frequent and their size larger than in human BM showing goblet cells (23, 24). It would appear that this extra load of sialomucin in baboons might, together with the overall sialomucin production in columnar and other glandular cells, contribute to contra rest repeated waves of refluxed gastric acid, particularly following larger meals (2, 15). This notion seems to be substantiated by the fact that similar ballooned, deep blue-stained cells were not present in the fundic or antro-pyloric gastric mucosa in these animals.
In human BM, sialomucins are present in goblet cells and occasionally in columnar cells. The bulk of these secretions appear, however, insufficient to buffer the acidic fluid entering the esophagus, as clinical BM is a disease that requires long-lasting anti-acid medication (25).
In light of these considerations, it is not totally inconceivable that GER, a condition demonstrated in patients at all ages (26, 27), might reflect an evolutionary throwback (that is atavism), currently occurring as a natural phenomenon in non-human primates. It should be understood that atavism occurs because genes for previously existing phenotypical features are often preserved in DNA.
In conclusion, this study strongly suggests that sialomucin-producing columnar and intramucosal glandular cells in BM in baboons might be an integrated part of the natural phenomenon of mucosal adaptation to daily regurgitation of gastric acid into the distal esophagus in these animals.
Acknowledgements
Thanks are due to the staff of the Histology Laboratory and to Priscilla Williams, Data Management, Biostatistics and Scientific Computing, at the Southwest Foundation for Biomedical Research, San Antonio, Texas, for their invaluable help. This study was supported by a grant from the Karolinska Institute, Stockholm, Sweden.
References
- 1.Rubio CA, Dick EJ, Jr, Hubbard GB. The columnar-lined mucosa in the distal esophagus. A preliminary study in baboons. In Vivo. 2009;23:273–275. [PMC free article] [PubMed] [Google Scholar]
- 2.Glover EJ, Leland MM, Dick EJ, Jr, Hubbard GB. Gastroesophageal reflux disease in baboons (Papio sp.): a new animal model. J Med Primatol. 2008;37:18–24. doi: 10.1111/j.1600-0684.2007.00217.x. [DOI] [PubMed] [Google Scholar]
- 3.Rubio CA, Dick EJ, Schlabritz-Loutsevitch NE, Orrego A, Hubbard GB. The columnar-lined mucosa at the gastroesophageal junction in nonhuman primates. Int J Clin Exp Pathol. 2009;2:481–488. [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio CA, Dick EJ, Jr, Orrego A, Hubbard GB. Further studies on the frequency and length of the glandulo-metaplastic esophageal mucosa in baboons. In Vivo. 2009;23:955–958. [PMC free article] [PubMed] [Google Scholar]
- 5.Spechler S, Goyal RK. Barretts esophagus. N Engl J Med. 1986;315:362–367. doi: 10.1056/NEJM198608073150605. [DOI] [PubMed] [Google Scholar]
- 6.Playford RJ. New British Society of Gastroenterology (BSG) guidelines for the diagnosis and management of Barrett's oesophagus. Gut. 2006;55:442–444. doi: 10.1136/gut.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio CA, Matthies M, Itabashi M, Hirota T. Image quantitation of intestinal metaplasia in entire gastrectomy specimens from Swedish and Japanese patients. Jpn J Cancer Res. 1996;87:711–717. doi: 10.1111/j.1349-7006.1996.tb00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera F, Rubio CA. Quantitative studies of the extension of gastric intestinal metaplasia in gastrectomy specimens from Swedish patients. Europ J Gastroent Hepatol. 1993;5:521–525. [Google Scholar]
- 9.Rubio CA, Slezak P, Rodensjö M. Differences in the distribution of acidic mucins between flat tubular adenomas and flat serrated adenomas of the colorectal mucosa. In Vivo. 1996;10:383–388. [PubMed] [Google Scholar]
- 10.Rubio CA, Rivera F. Quantification of acid mucins in the descending colon of rats having simultaneously growing colonic tumors. APMIS. 1991;99:993–996. doi: 10.1111/j.1699-0463.1991.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 11.Rubio CA, Huang CB. Quantification of the sulphomucin-producing cell population of the colonic mucosa during protracted stress in rats. In Vivo. 1992;6:81–84. [PubMed] [Google Scholar]
- 12.Sheahan D, Jervis HR. Comparative histochemistry of gastrointestinal mucosubstances. Am J Anat. 1976;146:103–131. doi: 10.1002/aja.1001460202. [DOI] [PubMed] [Google Scholar]
- 13.Chen YY, Wang HH, Antonioli DA, Spechler SJ, Zeroogian JM, Goyal R, Shahsafaei A, Odze RD. Significance of acid-mucin-positive nongoblet columnar cells in the distal esophagus and gastroesophageal junction. Hum Pathol. 1999;30:1488–1495. doi: 10.1016/s0046-8177(99)90172-7. [DOI] [PubMed] [Google Scholar]
- 14.Rubio CA, Dick EJ, Jr, Orrego A, Hubbard GB. The frequency of glassy cells in Barrett's mucosa: a study in baboons. In Vivo. 2009;23:925–927. [PMC free article] [PubMed] [Google Scholar]
- 15.Glover E, Leland M, Hubbard G. An association between gastric regurgitation and disease in nonhuman primates. Am J Primatol. 2005;66:174–179. [Google Scholar]
- 16.Morgan L, Howell S, Fritz J. Regurgitation and reingestion in a captive chimpanzee (Pan troglodytes) Lab Anim. 2003;22:42–45. [Google Scholar]
- 17.Gould E, Bres M. Regurgitation in gorillas: possible model for human eating disorders (rumination/bulimia) J Dev Behav Pediatr. 1986;7:314–319. doi: 10.1097/00004703-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Lakhoo K, Parekh D, Lawson HH, Rogers G, Van der Walt LA, Hunter S. Gastric acid secretion and gastrin release in the baboon. Dig Dis Sci. 1992;37:1313–1318. doi: 10.1007/BF01295997. [DOI] [PubMed] [Google Scholar]
- 19.Wenner J, Johnsson F, Johansson J, Oberg S. Acid reflux immediately above the squamocolumnar junction and in the distal esophagus: simultaneous pH monitoring using the wireless capsule pH system. Am Gastroenterol. 2006;101:1734–1741. doi: 10.1111/j.1572-0241.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 20.Pehl C, Waizenhoefer A, Wendl B, Schmidt T, Schepp W, Pfeiffer A. Effect of low and high fat meals on lower esophageal sphincter motility and gastroesophageal reflux in healthy subjects. Am J Gastroenterol. 1999;94:1192–1196. doi: 10.1111/j.1572-0241.1999.01064.x. [DOI] [PubMed] [Google Scholar]
- 21.Ledeboer M, Masclee AA, Biemond I, Lamers CB. Effect of medium- and long-chain triglycerides on lower esophageal sphincter pressure: role of CCK. Am J Physiol. 1998;274:1160–1165. doi: 10.1152/ajpgi.1998.274.6.G1160. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AJ, Wong H, Wish K, Carrow J, Bell D, Bindeman J, Watkins T, Lehmann T, Bhattarai S, O'Malley PG. Validation of the MEDFICTS dietary questionnaire: a clinical tool to assess adherence to American Heart Association dietary fat intake guidelines. Nutr J. 2003;13:2–4. doi: 10.1186/1475-2891-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothery GA, Patterson JE, Stoddard CJ, Day DW. Histological and histochemical changes in the columnar-lined (Barrett's) oesophagus. Gut. 1986;27:1062–1068. doi: 10.1136/gut.27.9.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spechler S. Diseases of the esophageal mucosa. In: Shearman DJ, Finlayson N, Camillieri M, Carter D, editors. Diseases of the Gastrointestinal Tract and Liver. Churchill Livingstone; New York: 1977. pp. 191–203. [Google Scholar]
- 25.Smith E, Kelly JJ, Ruskiewicz AR, Sullivan T, Jamieson GG, Drew PA. The effect of long-term control of reflux by fundoplication on aberrant deoxyribonucleic acid methylation in patients with Barrett esophagus. Ann Surg. 2010;252:63–69. doi: 10.1097/SLA.0b013e3181e4181c. [DOI] [PubMed] [Google Scholar]
- 26.Van Howe1 R, Storms M. Gastroesophageal reflux symptoms in infants in a rural population: longitudinal data over the first six months. BMC Pediatrics. 2010;10:7–12. doi: 10.1186/1471-2431-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolia V, Vandenplas Y. Systematic review: the extra-oesophageal symptoms of gastro-oesophageal reflux disease in children. Aliment Pharmacol Ther. 2009;29:258–272. doi: 10.1111/j.1365-2036.2008.03879.x. [DOI] [PubMed] [Google Scholar]


