Abstract
Following peripheral axotomy of the facial nerve in mice, T lymphocytes cross the blood-brain-barrier (BBB) into the central nervous system (CNS), where they home to neuronal cell bodies of origin in the facial motor nucleus (FMN) and act in concert with microglial cells to support the injured motor neurons. Several lines of evidence suggested normal aging may alter the injury-related responses of T cells, microglia, and motor neurons in this model. In this study, we therefore sought to test the hypothesis that compared to 8 week old mice (young adult), 52 week old mice (advanced middle age) would exhibit more neuronal damage and increased T cell trafficking into the injured FMN following facial nerve resection. Comparison of 8 and 52 week old mice at 7, 14, 21 and 28 days post-resection of the facial nerve, confirmed our hypothesis that age influences the kinetics of CD3+ T lymphocyte trafficking in the axotomized FMN. The peak T cell response was significantly higher, occurred later, and remained elevated longer in the injured FMN of mice in the 52 week age group. Although the kinetics of motor neuron death (identified by quantifying CD11b+ perineuronal microglial phagocytic clusters engulfing the dead neurons at 7, 14, 21 and 28 days post-rection) differed between the age groups, motor neuron profile counts at day 28 showed that levels of cummulative motor loss did not differ between the age groups. Compared to 8 week old mice, however, there was small reduction in the mean cell size of the surviving motor neurons in the 52 week age group. Since T lymphocyte function decreases with normal aging, it will be important to determine if increased T cell trafficking into the injured CNS is a compensatory response to the decreased function of older T cells, and if these and related neuroimmunological changes are more pronounced in mice in the late stages of the life cycle.
Keywords: age, facial nerve axotomy, resection, T cells, trafficking, perineuronal microglial phagocytic clusters, motor neuron loss
Introduction
Under normal conditions, continuous immune surveillance of the CNS occurs by small numbers of circulating peripheral T lymphocytes (Cose et al., 2006; Hickey et al., 1991). Whereas during pathogenic states such as multiple sclerosis and infection, the presence of T cells in the brain can have detrimental effects, in other contexts T cells act in concert with glial cells to promote neuroprotection and survival (Byram et al., 2004; Martino and Hartung, 1999; Nau and Bruck, 2002; Raivich et al., 1998; Schwartz, 2003). The facial nerve axotomy model has been used to elucidate the role of T cells in preventing initial neuronal death or slowing the rate of neurodegeneration and neuronal loss following facial nerve axotomy (Jones et al., 2005; Serpe et al., 1999; Serpe et al., 2000). Following facial nerve axotomy in mice, T cells cross the blood-brain-barrier (BBB) and home to nerve cell bodies in the facial motor nucleus (FMN) (Raivich et al., 1998). Severe combined immunodeficient (SCID) and recombination activating gene-2 knockout (RAG-2 KO) mice, which both lack functionally mature T and B lymphocytes, exhibit a faster rate of neuronal death than wild-type (WT) mice (Armstrong et al., 2004; Serpe et al., 2000).
Several factors suggest that the kinetics of T trafficking to injured motor neurons may be altered in normal aging. As mice age, increased baseline levels of T cells are found in the CNS of normal mice (Stichel and Luebbert, 2007), and aging mice exhibit higher expression of certain immune response genes following immune challenge with LPS (Terao et al., 2002). Though limited, there is some evidence that normal aging impairs motor neuron survival following axotomy. In a series of two studies, Vaughan showed that compared to rats 3 months of age, injured facial motor neurons of 15 month old rats had decreased recovery rates of enzymatic activity for acetylcholinesterase and cytochrome oxidase, as well as smaller increases in nuclear size associated with functional recovery after nerve crush injury (Vaughan, 1990, 1992). Our research indicates the level of T cell trafficking into the axtomized FMN is related to the the severity of neuronal injury (Ha et al., 2008). Together, these data suggest that if facial motor neurons of aging mice are more vulnerable to damage than those of younger mice, then higher levels of T cells trafficking to the FMN of aging mice may be necessary to maintain comparable levels of neuronal survival to younger mice. Moreover, as aging is associated with decreased T cell function ( Franceschi et al., 2000; Linton and Thoman, 2001; Miller, 1996; Nikolich-Zugich, 2008; Pawelec et al., 1999), older T cells may be less effective at protecting axotomized facial motor neurons. In this study, we therefore sought to test the hypothesis that compared to young adult mice, older mice would exhibit more neuronal damage and higher levels of T cell trafficking to the FMN following facial nerve resection. Specifically, we compared 8 week (young adult) versus 52 week old (advanced middle age) mice at 7, 14, 21 and 28 days post-resection of the facial nerve for differences in numbers of CD3+ T lymphocytes entering the axotomized FMN, and CD11b+ perineuronal microglial phagocytic clusters - a group of microglia that surround a motor neuron that is dead (or dying) and thus serve as a cross-sectional snapshot of the number of dead motor neurons/section at a given point in time (Streit and Kreutzberg, 1988; Raivich et al., 1998; Petitto et al., 2003). Additional assessments of neuronal status were made between the 8 and 52 week old groups at day 28 post-resection to quantify the levels of motor neuron survival and the mean cell size of these axotomized motor neurons.
2. Materials and Methods
2.1. Animals
C57BL/6Tac male mice were obtained from Taconic Farms (Germantown, NY)
Mice were acclimated to our facilities for 2 weeks prior to the beginning of these experiments. Mice were cared for in compliance with the NIH Guide for the Care and Use of Laboratory Animals and housed under specific pathogen free conditions in individual microisolator cages.
2.2. Facial nerve resection
Mice were either 8 weeks or 52 weeks old at the time of surgery. Animals were anesthetized with 4 % isoflurane. The right facial nerve was exposed, and a portion of the main branch resected to prevent nerve reconnection as described previously (McPhail et al., 2004; Ha 2007). The whisker response was assessed after surgery to ensure complete whisker paralysis. Mice in each group were then sacrificed at 7, 14, 21, and 28 days after resection injury to assess levels of CD3+ Tcells and CD11b+ perineuronal microglial phagocytic clusters (a measure of motor neuron death) in the injured FMN. At day 28, neuronal profile counts and neuronal cell size assesment were also made. For euthanasia, mice were anesthetized via intraperitoneal injection of 0.5 mg/mL ketamine cocktail (ketamine/xylazine/acepromazine given in a 3:3:1 ratio), and perfused with 4 % paraformaldehyde (PF). Brains were harvested, post-fixed in 4 % PF for 2 h at room temperature, and cryoprotected in 25 % sucrose at 4 °C overnight. Samples were then snap frozen in isopentane at − 80 °C. Using the ambiguous nucleus and facial nerve root as starting and ending points, respectively, 16 μm coronal sections were cut throughout the FMN. Sections were collected on Superfrost/Plus slides (Fisher Scientific) and stored at − 80 °C.
2.3. Light microscopic immmunohistochemistry
Tissue sections were incubated 1 h at room temperature in 1:30 normal goat serum (Vector) followed by overnight incubation at 4 °C with 1:500 anti-mouse CD3 (17A2; Pharmingen) or anti-mouse CD11b (5C6; Serotec). Sections were then incubated in 1:2000 goat anti-rat secondary antibody (Vector Labs) for 1 h at room temperature followed by 1:500 avidin-peroxidase conjugates (Sigma) for 45 minutes. Incubation with either the primary antibodies or secondary antibody alone produced no signal. Visualization of antiboby conjugates was performed by incubation in 3,3′-diaminobenzidine (DAB)-H2O2 solution (Sigma; 0.07%DAB/0.004%H2O2). Sections were counterstained with cresyl violet, dehydrated with ascending concentration EtOH washes, cleared with xylenes, and coverslipped.
2.4. Quantification and statistical analyses
CD3+ T cells and CD11b+ microglial perineuronal phagocytic clusters in the FMN were quantified under blind conditions at 7, 14, 21 and 28 days. Eight sections per animal (approximately 1/5 of the entire FMN) were used to assess each variable. Facial motor neuron counts and cell size assessment were performed at 28 days post-injury in both injured and uninjured FMN (on opposite side of each section). Nissl stained motor neurons were quantified by counting neuronal profiles that contained a visible nucleolus in eight representative sections throughout the extent of the FMN (Deboy et al 2006; Ha et al., 2007). Neuronal cell size was measured in 3 representative sections of the FMN each spaced 60 μM using ImageJ software (National Institutes of Health) (McPhail et al., 2004; Ha et al., 2007). Analysis of variance (ANOVA) was used to make statistical comparisons between groups, and the area under the curve across the 4 post-resection assessment times (7, 14, 21, and 28 days) was used to compare differences in the kinetics of CD3+ T cell counts and numbers of CD11b+ microglial perineuronal phagocytic clusters.
3. Results
3.1 T Lymphocyte and microglia measures
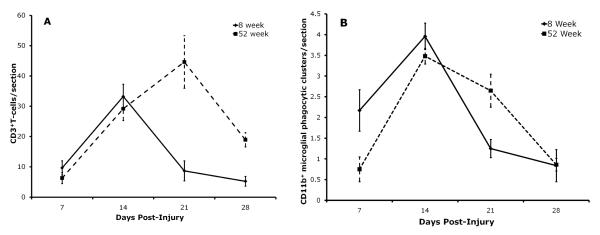
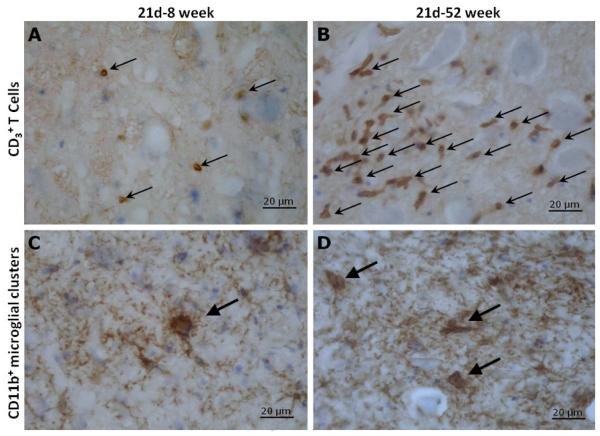
Figure 1A compares T cell numbers in the resected FMN of 8 and 52 week old mice at 7, 14, 21, and 28 days of age. ANOVA to compare the area under the curve of the time course of T cell trafficking into the injured FMN showed that there was a significant difference in the kinetics of this response between 8 and 52 week old mice (F(1,12)=13.04, p<0.005). As shown in Figure 1A, T lymphocytes between the groups were similar between days 7 and 14 post-injury. Whereas T cell levels peaked at day 14 in the 8 week group, in the 52 week group levels continued to rise and peaked at day 21 post-injury and remained higher through day 28 post-injury in the older mice. The photomicrographs, Figure 2A and B, illustrate an example of the differences seen between the 8 and 52 week groups at day 21.
Figure 1.
Comparison between the 8 week old (solid line) and the 52 week old (dashed line) subject groups for the kinetics of 1). CD3+ T lymphocyte trafficking into the injured FMN (A), and; 2) neuronal death represented by CD11b+ perineuronal microglial phagocytic clusters in the injured FMN (B). Each time point represents the mean ± S.E.M. of 8 mice in the 8 week group and 6 mice in the 52 week group.
Figure 2.
Immunohistochemistry for CD3+ T lymphocyte and CD11b+ perineuronal microglial phagocytic clusters at day 21 post-resection of the facial nerve in 8 week versus 52 week old mice. CD3+ T lymphocytes and CD11b+ perineuronal microglial phagocytic clusters were immunostained (brown) and are indicated by arrows in facial motor nuclei of 8 week (A,C) and 52 week (B,D) old mice. Neuronal and glial cell bodies were counterstained with cresyl violet (blue). Incubation with either the primary antibodies or secondary antibody alone produced no signal. Scale bar = 20 μm.
Figure 1B compares CD11b+ microglial perineuronal phagocytic clusters in the resected FMN of 8 and 52 week old mice at 7, 14, 21, and 28 days of age. ANOVA to compare the area under the curve of the time course of CD11b+ microglial perineuronal phagocytic clusters in the injured FMN showed that there was a significant difference in the kinetics of neuronal death between 8 and 52 week old mice (F(1,12)=6.10, p<0.05). As can be seen in Figure1B, dead neurons labeled by perineuronal microglial phagocytic clusters were initially increased in the 8 week group mice, compared to the 52 week group at day 7 a post-resection, and levels in both groups peaked at day 14. Whereas levels of perineuronal microglial phagocytic clusters remained elevated at day 21 post-injury in the 52 week group, they decreased substantially in the 8 week group. At day 28 post-injury, lower levels of perineuronal microglial phagocytic clusters were the similar in both age groups. The differences in dead neurons/section marked by perineuronal microglial phagocytic clusters between the age groups at day 21 can be seen in the photomicrographs, Figures 2C and D.
3.2 Quantification of motor neurons and neuronal cell size
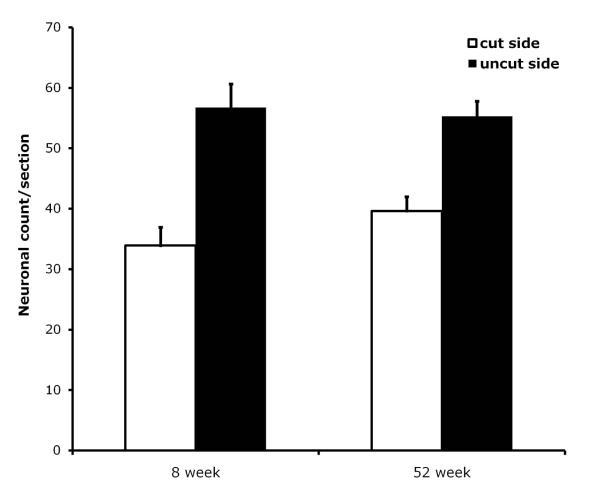
We quantified motor neurons in the cut and uncut sides of FMN sections at day 28 post-resection, and compare their levels in the two age groups as can be seen in Figure 3. Analyses confirmed that there were no statistically significant differences between the 8 and 52 week groups in motor neurons profile counts/section in either the resected or uncut, control sides. ANOVA also confirmed that motor neuron survival (the number of neurons in the injured, ipsilateral FMN expressed as a percentage of the number of motor neurons in the uninjured, contralateral FMN) did not differ between the two age groups (data no shown).
Figure 3.
Motor neuron profile counts in the resected (ipsilateral) and uninjured (contralateral control) facial motor nuclei comparing the 8 week versus 52 week groups at day 28 post-injury. The number of neurons on the resected side (empty bar) and uninjured control side (black bar) are expressed as the mean ± S.E.M. of 6 mice/group.
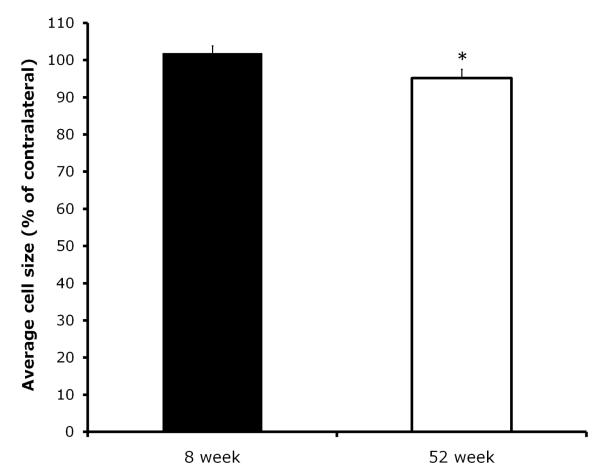
At day 28, we also compared the change in the cross-sectional area (mean cell size) of injured motor neurons (injured, ipsilateral motor neuron size expressed as percentage of uninjured, contralateral motor neuron size) between the two age groups. There was a significant difference between the groups in the change in motor neuron size at 28 days post-resection (F(1,10)=6.82, p<0.05). As seen in Figure 4, whereas the 8 week group had a slight increase in motor neuron size, by contrast, the 52 week group old mice exhibited a small reduction in motoneuron size. We also performed a direct comparison of the raw data, the mean size of injured motoneurons between the groups. Although the analysis showed that the magnitude of the reduction of the group mean of the 52 week vs. the 8 week group was comparable to the small reduction in motoneuron size that was found when the change in injured motoneuron size was calculated relative to each animal’s uninjured control side (injured motoneuron size decreased 8.7% in the 52 week old group compared to the 8 weeks old group; mean neuron size ± S.E.M. of injured motoneurons in the 8 week group was 180.3 ± 8.9 vs. 164.6 ± 9.8 for the 52 week group), it was not statistically significant. This is likely due to the increased variance and loss of statistical power when making such direct comparison of the mean motoneuron size of the injured side between the two groups (as compared to the analysis calculating the change in motoneurons size relative to each animal’s uninjured control side).
Figure 4.
Comparison of mean motor neuron cell size in the injured facial motor nuclei (expressed % of the uninjured, contralateral side) between the 8 and 52 week groups. Each bar represents the mean ± S.E.M. of 6 mice/group.
4. Discussion
These data confirmed our hypothesis that the kinetics of T trafficking to injured motor neurons is altered in normal aging. CD3+ T cell increased to comparable levels in both groups at day 7 through day 14 post-injury, however, whereas levels then decreased by day 21 in the 8 week group, they rose still further in the 52 week group and peaked at day 21 where they remained substantially elevated at day 28. In the processes of examining axotomy-induced glial cell responses related to aging, although they did not quantify T cells, Hurley and Coleman (2003) previously made the qualitative observation that old rats appeared to have substantially more T cells in the injured FMN than young rats. As depicted in Figure 1B, enumeration of dead neurons using CD11b+ perineuronal microglial phagocytic clusters revealed that the kinetics of motor neuron death also differed between the age groups. Compared to the 8 week group, in the 52 week group dead neurons were initially lower at day 7 before the magnitude of the response peaked at day 14 post-resection in both age groups; the response remained near peak levels at day 21 in the older mice, while levels decreased substantially in the younger mice. Thus, although the initial rise of dead motor neurons was slower in the 52 week old mice, the response remained elevated out to 3 weeks before decreasing to levels similar to the younger mice at day 28.
Contrary to our hypothesis, the survival of injured motor neurons between the young and aging mice did not differ. The fate of neurons, measured by the cumulative motor neuron loss at day 28 was not different between the groups (Figure 3), despite the fact that the kinetics motor neuron death identified by perineuronal microglial phagocytic clusters between 7 and 28 days post-resection differed between the age groups (Figure 1B). Therefore, the higher levels of T cell trafficking into the injured FMN of 52 week mice is not attributable to higher levels of injury-induced neuronal damage in the older mice as we had expected (Ha et al., 2008). Surprisingly, the effects of aging on motor neuron survival following facial nerve axotomy had not been reported in the literature. As noted earlier, Vaughan (1990, 1992) found that 15 month old rats had decreased recovery rates of enzymatic activity for acetylcholinesterase and cytochrome oxidase, and smaller increases in nuclear size associated with functional recovery after nerve crush injury than 3 month old rats. Neuronal survival was not assessed in those studies (Vaughan 1990, 1992), most likely because facial nerve crush does not induce notable neuronal loss as is seen following nerve resection (Ha et al., 2008). Tracking the numbers of dead motor neurons by quantifying CD11b+ perineuronal microglial phagocytic clusters at days 7, 14, 21 and 28 provides 4 cross-sectional snapshots in time, where each time point represents the rate of neuronal death at that specific time point post-injury. Together they generate the time course of the rate of neuronal death between the groups. (Theoretically to quantify dead neurons using microglial phagocytic clusters to estimate the cumulative loss of neurons over time to coincide with profile counts of surviving injured motor neurons, the sampling rate - the number of cross-sectional time points at which these phagocytic clusters are quantified over time - would have to be increased to substantially more than 4 time points over the 28 day period used in this study). It is possible, that independent of neuronal death, the differences between 8 and 52 week groups in the time course of perineuronal microglial phagocytic clusters could be influenced by the aging processes in microglial cell function (Miller and Streit, 2007; Streit et al., 2008). Older microglia, like peripheral phagocytic cells, may have reduced phagocytic function and thus have a longer half-life in vivo than phagocytic microglia of young mice (Cambier, 2005). This could account for the elevated levels of perineuronal microglial phagocytic seen between days 14 – 21 post-resection in the 52 week group. It is noteworthy that in both young and aging animals, the present data are consistent with previous studies from our lab and others showing that whether facial motor neurons undergo injury by crush, transaction or resection, the rate of dead motor neurons identified by perineuronal mircroglial phagocytic clusters appear to consistently peak at day 14 post-injury (Moller et al., 1996; Raivich et al., 1998; Ha et al., 2008).
Since we used only the pan T cell marker, CD3, to identify all T cells, we do not know the breakdown of various T cells subtypes in the injured FMN, their different states of activation, or other characteristics (e.g., naïve vs. memory) which could provide insight into their neuroimmunological function in this injury paradigm. Studies using adoptive transfer and T cell subset deficient mice have found that the initial phase of FMN survival after facial nerve axotomy (i.e., the rate of motoneuron loss) is dependent on CD4+ Th2 helper cells through interactions with peripheral antigen presenting cells as well as microglia in the CNS (Serpe et al., 2003; Byram et al., 2004; Armstrong et al., 2006; DeBoy et al., 2006; Xin et al., 2008). Though these elegant adoptive transfer studies have provided important data about the T cell subtypes associated with motoneuron survival, they have not yet identified the presence those T cell subtypes in the injured FMN in vivo. (We have recently found that significant numbers of CD3+, CD4+ and CD8+ T cells traffick to the axotomized FMN of immunodeficient RAG-2 knockout mice adoptively transferred with splenocytes form wild-type mice; Huang et al., manuscript in preparation). The possibility that T cell activity in the periphery also contributes to motoneuron survival in the facial nerve axotomy model is suggested by recent data from Wolf et al. (2009). They found that adoptively transferred CD4+ T cells increased hippocampal neurogenesis and appeared to exert their predominant effect from the periphery without trafficking into the brain, thus illustrating further the complexity of T cell function on neurons in the CNS. Using immunologically intact/normal mice, it has been shown that both CD4+ and CD8+ T cells are present in significant numbers in the axotomized FMN of mice (Raivich et al., 2003; Bohatschek et al., 2004; Ha et al., 2007). For the present study, we considered using a different immunohistocytochemistry method that we used previously which can identify several T cell subsets in the axotomized FMN in vivo (CD3+, CD4+, and CD8+, and double negative T cells), where tissues are perfused with PBS instead of 4% paraformaldehyde and subsequently fixed with a Zinc immunohistocytochemistry fixative (Ha et al., 2007). Using that method (and modifications of the method), however, we have found that Nissl staining for neuron profile counting and staining of CD11b+ microglia are less intense and more difficult to quantify. Because we had never used that method on brain tissue of mice as old as 52 weeks and we were examining these dependent variables, to ensure the best result we used 4% paraformaldehyde perfusion for the present study thus precluding our ability to also quantify CD4+ , CD8+ , and double negative T cells in the axotomized FMN. We are not aware of studies that have been able to more finely subtype T cells or successfully assess their activation state in vivo by immunohistocytochemistry in brain tissue or by tissue extraction methods (an approach which may itself alter the activation status of T cells) in mice with uncompromised blood-brain-barrier function such as in the facial axotomy paradigm (Neeley et al., 1987; Muhallab et al., 2001; Raivich et al., 1998; Raivich et al., 2003). Although we did not assess whether CD3+ levels in the blood or other peripheral compartments (e.g., spleen, lymph nodes, bone marrow) differ between the 52 and 8 weeks groups, the literature indicates that aging mice have increased CD3+ counts (with a decreasing CD4+ /CD8+ ratios) in peripheral blood, and decreased or unchanged CD3+ levels in the spleen and lymph nodes (Callahan et al., 1993; Miller, et al., 1997; Flurkey et al., 2007). Though it is possible that peripheral changes in T cell distribution in aging could influence T cell trafficking into the injured FMN with aging, we found conversely, that mice with high and low levels of T cells in the axotomized FMN in this model did not differ in splenic T cell density (Ha et al., 2006). Along these lines, discordant peripheral versus central immunological responses have been identified in aging mice (Dilger and Johnson, 2008; Terao et al., 2002). Terao and colleagues (2002) showed that older mice had reduced splenic T mitogen-induced proliferative, but also simultaneously exhibited higher expression of certain immune response genes in the CNS following immune challenge.
One hypothesis that is consistent with the findings of this study is that the increased numbers of T cells in injured FMN of the 52 week group may be because older T cells are less effective at protecting damaged motoneurons (Miller, 1996). The older mice used in this study where late middle aged mice (52 weeks), where clear evidence of senescence has been documented in a number of biological processes including aspects of T lymphocyte function (Flurkey et al., 2007). It is likely therefore that old mice at the end of the life cycle (e.g., 18 - 24 months), may have even more pronounced changes in these T cell and microglia responses than the 52 week old group we used in this study. Reduced T cell function including the balance of naïve to memory cell responses occur with aging in animals and humans (Linton and Thoman, 2001; Linton and Dorshkind, 2004; Miller, 1996; Nikolich-Zugich, 2008; Pawelec et al., 1999). In light of the aforementioned proregenerative actions of CD4+ Th2 helper cells in the facial nerve axotomy model (Serpe et al., 2003; Byram et al., 2004; Armstrong et al., 2006; DeBoy et al., 2006; Xin et al., 2008) and age-related reductions in different CD4+ T subsets and function in the peripheral immune system (Callahan et al., 1993; Miller, et al., 1997; Flurkey et al., 2007), CD4+ Th2 helper cells may be an important population of T cells to examine in future studies of age and immunity for facial motoneuron and other forms of CNS injury. Whether increased T cell trafficking may also be secondary to alterations in intrinsic microglial cell function or growth factor production associated with aging in the FMN (Serpe et al., 2005; Saylor et al., 2006; Njie et al., 2010; Shokouhi et al., 2010), or represent autoimmune-like age-related changes in CNS integrity and function (Chan-Ling et al., 2007; Schindler et al., 2008; Stichel and Luebbert, 2007; Terao et al., 2002) are question of interest going forward.
More CD3+ T cell in the injured FMN in 52 week old mice than 8 week old mice
Differences in CD11b+ clusters between 8 and 52 week old mice in the injured FMN
Cumulative neuronal loss did not differ between 8 and 52 week old mice
There was a small reduction in the size of the surviving motor neurons in older mice.
Acknowledgments
Supported by NIH Grants R01NS055018 and R01NS048472.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Coute AC, Ngo DH, Waschek JA. Impairment of axotomy-induced pituitary adenylyl cyclase-activating peptide gene expression in T helper 2 lymphocyte-deficient mice. Neuroreport. 2006;27:309–312. doi: 10.1097/01.wnr.0000199465.54907.74. [DOI] [PubMed] [Google Scholar]
- Armstrong B, Abad C, Chhith S, Rodriguez W, Cheung-Lau G, Trinh V, Waschek J. Restoration of axotomy-induced PACAP gene induction in SCID mice with CD4+ T-lymphocytes. Neuroreport. 2004;15:2647–2650. doi: 10.1097/00001756-200412030-00018. [DOI] [PubMed] [Google Scholar]
- Bohatschek M, Kloss CU, Pfeffer K, Hristiova M, Raivich G. Microglial major histocompatibility complex glycoprotein-1 in the axotomized facial motor nucleus: regulation and role of tumor necrosis factor receptors 1 and 2. J Comp Neurol. 2004;470:382–399. doi: 10.1002/cne.20017. [DOI] [PubMed] [Google Scholar]
- Callahan JE, Kappler JW, Marrack P. Unexpected expansion of CD8-bearing cells in old mice. J Immunology. 1993;151:6657–6669. [PubMed] [Google Scholar]
- Byram S, Carson M, DeBoy C, Serpe C, Sanders V, Jones K. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. Immunosenescence: a problem of lymphopoiesis, homeostasis, microenvironment, and signaling. Immunol Rev. 2005;205:5–6. doi: 10.1111/j.0105-2896.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Hughes S, Baxter L, Rosinova E, McGregor I, Morcos Y, van Nieuwenhuyzen P, Hu P. Inflammation and breakdown of the blood-retinal barrier during “physiological aging” in the rat retina: a model for CNS aging. Microcirculation. 2007;14:63–76. doi: 10.1080/10739680601073451. [DOI] [PubMed] [Google Scholar]
- Cose S, Brammer C, Khanna KM, Masopust D, Lefrancois L. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur J Immunol. 2006;36:1423–1433. doi: 10.1002/eji.200535539. [DOI] [PubMed] [Google Scholar]
- Deboy CA, Xin J, Byram SC, Serpe CJ, Sanders VM, Jones KJ. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4(+) T cells. Exp Neurol. 2006;201:212–224. doi: 10.1016/j.expneurol.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison DE. The Mouse in Aging Research. In: Fox JG, et al., editors. The Mouse in Biomedical Research. 2nd Edition American College Laboratory Animal Medicine (Elsevier); Burlington, MA: 2007. pp. 637–672. [Google Scholar]
- Franceschi C, Bonafè M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–1720. doi: 10.1016/s0264-410x(99)00513-7. [DOI] [PubMed] [Google Scholar]
- Ha GK, Huang Z, Parikh R, Pastrana M, Petitto JM. Immunodeficiency impairs reinjury induced reversal of neuronal atrophy: relation to T cell subsets and microglia. Exp Neurol. 2007;208:92–99. doi: 10.1016/j.expneurol.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha GK, Parikh S, Huang Z, Petitto JM. Influence of injury severity on the rate and magnitude of the T lymphocyte and neuronal response to facial nerve axotomy. J Neuroimmunol. 2008;199:18–23. doi: 10.1016/j.jneuroim.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha GK, Huang Z, Streit WJ, Petitto JM. Endogenous T lymphocytes and microglial reactivity in the axotomized facial motor nucleus of mice: effect of genetic background and the RAG2 gene. J Neuroimmunol. 2006;172:1–8. doi: 10.1016/j.jneuroim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hurley SD, Coleman PD. Facial nerve axotomy in aged and young adult rats: analysis of the glial response. Neurobiol Aging. 2003;24:511–518. doi: 10.1016/s0197-4580(02)00097-0. [DOI] [PubMed] [Google Scholar]
- Jones K, Serpe C, Byram S, Deboy C, Sanders V. Role of the immune system in the maintenance of mouse facial motoneuron viability after nerve injury. Brain Behav Immun. 2005;19:12–19. doi: 10.1016/j.bbi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Linton P, Thoman ML. T cell senescence. Front Biosci. 2001;6:D248–261. doi: 10.2741/linton. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Martino G, Hartung H. Immunopathogenesis of multiple sclerosis: the role of T cells. Curr Opin Neurol. 1999;12:309–321. doi: 10.1097/00019052-199906000-00010. [DOI] [PubMed] [Google Scholar]
- McPhail LT, Fernandes KJ, Chan CC, Vanderluit JL, Tetzlaff W. Axonal reinjury reveals the survival and re-expression of regeneration-associated genes in chronically axotomized adult mouse motoneurons. Exp Neurol. 2004;188:331–340. doi: 10.1016/j.expneurol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Miller K, Streit W. The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol. 2007;3:245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Miller RA. Age-related changes in T cell surface markers: a longitudinal analysis in genetically heterogenous mice. Mech Ageing Dev. 1997;96:181–196. doi: 10.1016/s0047-6374(97)01893-9. [DOI] [PubMed] [Google Scholar]
- Muhallab S, Lidman O, Weissert R, Olsson T, Svenningsson A. Intra-CNS activation by antigen-specific T lymphocytes in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;113:202–211. doi: 10.1016/s0165-5728(00)00438-0. [DOI] [PubMed] [Google Scholar]
- Moller JC, Klein MA, Haas S, Jones LL, Kreutzberg GW, Raivich G. Regulation of thrombospondin in the regenerating mouse facial motor nucleus. Glia. 1996;17:121–132. doi: 10.1002/(SICI)1098-1136(199606)17:2<121::AID-GLIA4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Nau R, Brück W. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 2002;25:38–45. doi: 10.1016/s0166-2236(00)02024-5. [DOI] [PubMed] [Google Scholar]
- Neeley SP, Conley FK. Extraction and immunocytochemical characterization of viable mononuclear inflammatory cells from brains of mice with chronic Toxoplasma gondii infections. J Neuroimmunol. 1987;15:159–172. doi: 10.1016/0165-5728(87)90090-7. [DOI] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2010 Jun 25; doi: 10.1016/j.neurobiolaging.2010.05.008. 2010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Wagner W, Adibzadeh M, Engel A. T cell immunosenescence in vitro and in vivo. Exp Gerontol. 1999;34:419–429. doi: 10.1016/s0531-5565(99)00002-9. [DOI] [PubMed] [Google Scholar]
- Petitto JM, Haung Z, Lo J, Streit WJ. IL-2 gene knockout affects T lymphocyte trafficking and the microglial response to regenerating facial motor neurons. J Neuroimmunol. 2003;134:95–103. doi: 10.1016/s0165-5728(02)00422-8. [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Werner A, Jones LL, Galiano M, Kloss CU, Zhu XZ, Pfeffer K, Liu ZQ. Lymphocyte infiltration in the injured brain: role of proinflammatory cytokines. J Neurosci Res. 2003;72:726–733. doi: 10.1002/jnr.10621. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor AJ, Meridith GE, Vercillo MS, zahm DS, McGinty JF. BDNF heterozygous mice demonstrate age-related changes in striatal and nigral gene expression. Exp Neurol. 2006;199:362–372. doi: 10.1016/j.expneurol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Schindler MK, Forbes ME, Robbins ME, Riddle DR. Aging-dependent changes in the radiation response of the adult rat brain. Int J Radiat Oncol Biol Phys. 2008;70:826–834. doi: 10.1016/j.ijrobp.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Macrophages and microglia in central nervous system injury: are they helpful or harmful? J Cereb Blood Flow Metab. 2003;23:385–394. doi: 10.1097/01.WCB.0000061881.75234.5E. [DOI] [PubMed] [Google Scholar]
- Serpe C, Kohm A, Huppenbauer C, Sanders V, Jones K. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe C, Sanders V, Jones K. Kinetics of facial motoneuron loss following facial nerve transection in severe combined immunodeficient mice. J Neurosci Res. 2000;62:273–278. doi: 10.1002/1097-4547(20001015)62:2<273::AID-JNR11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Byram SC, Sander VM, Jones KJ. Brain-derived neurotrophic factor supports facial motoneuron survival after facial nerve transection in immunodeficient mice. Brain Behav Immun. 2005;19:173–180. doi: 10.1016/j.bbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Shokouhi BN, Wong B.Z.Y, Siddiqui, S., Lieberman AR, Campbell G, Tohyama K, Anderson PN. Microglial responses around intrinsic CNS neurons are correlated with axonal regeneration. BMC Neurosci. 2010;11:13. doi: 10.1186/1471-2202-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2007;28:1507–1521. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Kreutzberg GW. Response of endogenous glial cells to motor neuron degeneration induced by toxic ricin. J Comp Neurol. 1988;268:248–263. doi: 10.1002/cne.902680209. [DOI] [PubMed] [Google Scholar]
- Streit W, Miller K, Lopes K, Njie E. Microglial degeneration in the aging brain--bad news for neurons? Front Biosci. 2008;13:3423–3438. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- Terao A, Apte-Deshpande A, Dousman L, Morairty S, Eynon BP, Kilduff TS, Freund YR. Immune response gene expression increases in the aging murine hippocampus. J Neuroimmunol. 2002;132:99–112. doi: 10.1016/s0165-5728(02)00317-x. [DOI] [PubMed] [Google Scholar]
- Vaughan DW. The effects of age on enzyme activities in the rat facial nucleus following axotomy: acetylcholinesterase and cytochrome oxidase. Exp Neurol. 1990;109:224–236. doi: 10.1016/0014-4886(90)90077-6. [DOI] [PubMed] [Google Scholar]
- Vaughan DW. Effects of advancing age on peripheral nerve regeneration. J Comp Neurol. 1992;323:219–237. doi: 10.1002/cne.903230207. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kemperman G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Xin J, Wainwright DA, Serpe CJ, Sanders VM, Jones KJ. Phenotype of CD4+ T cell subsets that develop following mouse facial nerve axotomy. Brain Behav Immun. 2008;22:528–537. doi: 10.1016/j.bbi.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]