Abstract
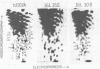
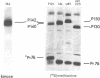
We have isolated a replication-defective rapidly transforming sarcoma virus (designated 16L virus) from a fibro-sarcoma in a chicken infected with td107A, a transformation-defective deletion mutant of subgroup A Schmidt-Ruppin Rous sarcoma virus. 16L virus transforms fibroblasts and causes sarcomas in infected chickens within 2 wk. Its genomic RNA is 6.0 kilobases and contains sequences homologous to the transforming gene (fps) of Fujinami sarcoma virus (FSV). RNase T1 oligonucleotide analysis shows that the 5' and 3' terminal sequences of 16L virus are indistinguishable from (and presumably derived from) td107A RNA. The central part of 16L viral RNA consists of fps-related sequences. These oligonucleotides fall into four classes: (i) oligonucleotides common to the putative transforming regions of FSV and another fps-containing avian sarcoma virus, UR1; (ii) an oligonucleotide also present in FSV but not in UR1; (iii) an oligonucleotide also present in UR1 but not in FSV; and (iv) an oligonucleotide not present in either FSV, UR1, or td107A. Cells infected with 16L virus synthesize a protein of Mr 142,000 that is immunoprecipitated with anti-gag antiserum. This protein has protein kinase activity. These results suggest that 16L virus arose by recombination between td107A and the cellular fps gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduzzi P. C., Notter M. F., Morgan H. R., Shibuya M. Some biological properties of two new avian sarcoma viruses. J Virol. 1981 Oct;40(1):268–275. doi: 10.1128/jvi.40.1.268-275.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Neiman P. E. Two distinct candidate transforming genes of lymphoid leukosis virus-induced neoplasms. Nature. 1981 Aug 27;292(5826):857–858. doi: 10.1038/292857a0. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Neil J. C., Vogt P. K. Cleavage of four avian sarcoma virus polyproteins with virion protease p15 removes gag sequences and yields large fragments that function as tyrosine phosphoacceptors in vitro. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5847–5851. doi: 10.1073/pnas.78.9.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Generation of novel, biologically active Harvey sarcoma viruses via apparent illegitimate recombination. J Virol. 1981 Apr;38(1):136–150. doi: 10.1128/jvi.38.1.136-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Structure of the provirus within NIH 3T3 cells transfected with Harvey sarcoma virus DNA. J Virol. 1981 Apr;38(1):125–135. doi: 10.1128/jvi.38.1.125-135.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY J. J. AN UNIDENTIFIED VIRUS WHICH CAUSES THE RAPID PRODUCTION OF TUMOURS IN MICE. Nature. 1964 Dec 12;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- Halpern C. C., Hayward W. S., Hanafusa H. Characterization of some isolates of newly recovered avian sarcoma virus. J Virol. 1979 Jan;29(1):91–101. doi: 10.1128/jvi.29.1.91-101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Mathey-Prevot B., Feldman R. A., Hanafusa H. Mutants of Fujinami sarcoma virus which are temperature sensitive for cellular transformation and protein kinase activity. J Virol. 1981 Apr;38(1):347–355. doi: 10.1128/jvi.38.1.347-355.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Hayward W. S., Hanafusa H. Cellular information in the genome of recovered avian sarcoma virus directs the synthesis of transforming protein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3154–3158. doi: 10.1073/pnas.76.7.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Duesberg P. H., Hanafusa H. Transformation-defective mutants of Rous sarcoma virus with src gene deletions of varying length. J Virol. 1977 Dec;24(3):910–914. doi: 10.1128/jvi.24.3.910-914.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Hanafusa H., Kawai S. A cellular protein is immunologically crossreactive with and functionally homologous to the Fujinami sarcoma virus transforming protein. Cell. 1982 Apr;28(4):897–906. doi: 10.1016/0092-8674(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Moloney J. B. A virus-induced rhabdomyosarcoma of mice. Natl Cancer Inst Monogr. 1966 Sep;22:139–142. [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Breitman M. L., Vogt P. K. Characterization of a 105,000 molecular weight gag-related phosphoprotein from cells transformed by the defective avian sarcoma virus PRCII. Virology. 1981 Jan 15;108(1):98–110. doi: 10.1016/0042-6822(81)90530-4. [DOI] [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Okazaki W., Vogt P. K., Hanafusa H., Burmester B. R., Crittenden L. B. Oncogenicity of avian leukosis viruses of different subgroups and of mutants of sarcoma viruses. Infect Immun. 1977 Feb;15(2):423–428. doi: 10.1128/iai.15.2.423-428.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Todaro G. J. Generation of oncogenic mouse type C viruses: in vitro selection of carcinoma-inducing variants. Proc Natl Acad Sci U S A. 1980 Jan;77(1):624–628. doi: 10.1073/pnas.77.1.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Huebner R. J. In vitro isolation of stable rat sarcoma viruses. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2972–2976. doi: 10.1073/pnas.75.6.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Oskarsson M., Maizel J., Vande Woude G. Molecular cloning of Snyder-Theilen feline leukemia and sarcoma viruses: comparative studies of feline sarcoma virus with its natural helper virus and with Moloney murine sarcoma virus. J Virol. 1980 Apr;34(1):200–212. doi: 10.1128/jvi.34.1.200-212.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H., Balduzzi P. C. Cellular sequences related to three new onc genes of avian sarcoma virus (fps, yes, and ros) and their expression in normal and transformed cells. J Virol. 1982 Apr;42(1):143–152. doi: 10.1128/jvi.42.1.143-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H., Balduzzi P. C. Cellular sequences related to three new onc genes of avian sarcoma virus (fps, yes, and ros) and their expression in normal and transformed cells. J Virol. 1982 Apr;42(1):143–152. doi: 10.1128/jvi.42.1.143-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa T., Hanafusa H., Stephenson J. R. Homology exists among the transforming sequences of avian and feline sarcoma viruses. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6536–6540. doi: 10.1073/pnas.77.11.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Czernilofsky A. P., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence at the 5' terminus of the avian sarcoma virus genome. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1473–1477. doi: 10.1073/pnas.74.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Stavnezer E., Gerhard D. S., Binari R. C., Balazs I. Generation of transforming viruses in cultures of chicken fibroblasts infected with an avian leukosis virus. J Virol. 1981 Sep;39(3):920–934. doi: 10.1128/jvi.39.3.920-934.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Robins T., Yokota H., Vogt P. K. The terminal oligonucleotides of avian tumor virus RNAs are genetically linked. Virology. 1977 Oct 15;82(2):472–492. doi: 10.1016/0042-6822(77)90020-4. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Feldman R., Shibuya M., Hanafusa H., Notter M. F., Balduzzi P. C. Genetic structure, transforming sequence, and gene product of avian sarcoma virus UR1. J Virol. 1981 Oct;40(1):258–267. doi: 10.1128/jvi.40.1.258-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Halpern C. C., Nadel M., Hanafusa H. Recombination between viral and cellular sequences generates transforming sarcoma virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5812–5816. doi: 10.1073/pnas.75.12.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Hanafusa H., Notter M. F., Balduzzi P. C. Genetic structure and transforming sequence of avian sarcoma virus UR2. J Virol. 1982 Mar;41(3):833–841. doi: 10.1128/jvi.41.3.833-841.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]