Abstract
Distal renal tubular acidosis (dRTA) can be caused by mutations in the gene encoding the anion exchanger 1 (AE1) and is characterized by defective urinary acidification, metabolic acidosis, and renal stones. AE1 is expressed at the basolateral membrane of type A intercalated cells in the renal cortical collecting duct (kAE1). Two dRTA mutations result in the carboxyl-terminal truncation of kAE1; in one case, the protein trafficked in a nonpolarized way in epithelial cells. A recent yeast two-hybrid assay showed that the carboxyl-terminal cytosolic domain of AE1 interacts with adaptor protein complex 1 (AP-1A) subunit μ1A (mu-1A; Sawasdee N, Junking M, Ngaojanlar P, Sukomon N, Ungsupravate D, Limjindaporn T, Akkarapatumwong V, Noisakran S, Yenchitsomanus PT. Biochem Biophys Res Commun 401: 85–91, 2010). Here, we show the interaction between kAE1 and mu-1A and B in vitro by reciprocal coimmunoprecipitation in epithelial cells and in vivo by coimmunoprecipitation from mouse kidney extract. When endogenous mu-1A (and to a lesser extent mu-1B) was reduced, kAE1 protein was unable to traffic to the plasma membrane and was rapidly degraded via a lysosomal pathway. Expression of either small interfering RNA-resistant mu-1A or mu-1B stabilized kAE1 in these cells. We also show that newly synthesized kAE1 does not traffic through recycling endosomes to the plasma membrane, suggesting that AP-1B, located in recycling endosomes, is not primarily involved in trafficking of newly synthesized kAE1 when AP-1A is present in the cells. Our data demonstrate that AP-1A regulates processing of the basolateral, polytopic membrane protein kAE1 to the cell surface and that both AP-1A and B adaptor complexes are required for normal kAE1 trafficking.
Keywords: adaptor protein 1, anion exchanger 1, membrane proteins, kidney, distal renal tubular acidosis, trafficking
newly synthesized glycoproteins whose final destination is the plasma membrane traffic through the Golgi to the trans-Golgi network (TGN) from which they can reach the plasma membrane either directly by constitutive secretion or indirectly via early endosomes and even recycling endosomes (RE; Refs. 1, 2). Typical cargoes that traffic directly from the TGN to the plasma membrane include FcII-B2 receptor and a mutant low density lipoprotein receptor (LDLR-Y18A; Ref. 14). In contrast, examples of cargos trafficking through RE before reaching the plasma membrane are vesicular stomatitis virus glycoprotein (VSV-G) or a truncated version of LDLR (LDLR-CT27; Refs. 1, 14). In the TGN, cargoes are loaded into trafficking vesicles via the interaction of canonical motifs (di-leucine based or YXXΦ, where Y is tyrosine, X is any residue, and Φ is a hydrophobic bulky amino acid) in their cytoplasmic domain with adaptor proteins, which in turn bind to clathrin to form clathrin-coated vesicles (24). Four types of adaptor protein (AP) complexes, AP-1 to AP-4, have been identified, each consisting of two large subunits, one medium subunit and one small subunit. AP-1, AP-3, and AP-4 localize to the TGN and endosomes (21). A recently described fifth adaptor protein complex localizes in late endosomes and does not appear to associate with clathrin, a feature shared with AP-4 and possibly AP-3 (20).
AP-1 is responsible for tethering the interaction between clathrin and cargo proteins that exit the TGN en route to endosomes. Two forms of AP-1 have been described: AP-1A is ubiquitously expressed (32), while AP-1B expression is restricted to polarized epithelial cells (15) and is targeted to common REs (13). The classical cargo protein of AP-1A is the cation-dependent mannose 6-phosphate receptor 46 (CD-MPR 46), a type I transmembrane glycoprotein that recycles between the TGN and endosomes (17). Knocking out mu-1A adaptin proved to be embryonic lethal in mice, but in derived mouse embryonic fibroblasts, CD-MPR 46 was mis-localized to endosomes and lysosomal enzymes were missorted to secretions (23).
Recently, it was reported that in human embryonic kidney (HEK) 293 cells, the kidney anion exchanger 1 (kAE1) interacts with the mu-1A adaptin of AP-1A adaptor, via a Y904DEV907 canonical motif within kAE1 cytoplasmic carboxyl-terminal domain (kAE1 Cter) (27). To gain greater insights into this interaction, we have characterized the physiological role of this interaction in renal epithelial cells. The human kidney AE1, encoded by the solute carrier family 4, anion exchanger, member 1 (SLC4A1) gene, is a membrane glycoprotein that exchanges bicarbonate for chloride at the basolateral membrane of renal type A intercalated cells (ICCs; Ref. 6). Naturally occurring mutations in the SLC4A1 gene can lead to distal renal tubular acidosis (dRTA; Ref. 34), which is characterized by metabolic acidosis, metabolic bone disease, failure to thrive, and nephrolithiasis or nephrocalcinosis. SLC4A1 mutations associated with dRTA (dRTA mutations hereon) can be either dominantly or recessively inherited. One dominant dRTA mutation, R901X, truncates the last 11 amino acids of kAE1. When expressed in epithelial Madin-Darby canine kidney (MDCK) cells, this mutant protein was mis-localized either to both basolateral and apical membrane or exclusively at the apical membrane, depending on the degree of polarization of the cells (10, 31). Interestingly, when expressed in porcine LLC-PK1 cells that lack endogenous AP-1B but contain endogenous AP-1A, kAE1 wild-type (WT) protein was still located at the basolateral membrane, demonstrating that AP-1B is not required for basolateral targeting of kAE1 WT protein (10). The machinery regulating the normal processing of kAE1 in epithelial cells is undetermined, and it remains unclear whether failure of this machinery to interact with kAE1 results in dRTA. We hypothesize that in kidney cells interaction with AP-1A via mu-1A adaptin is crucial for proper basolateral membrane targeting of kAE1 WT protein and that mis-sorting of the kAE1 R901X dRTA mutant could be due to the deletion of an YXXΦ motif located within the last 11 residues of the AE1 protein.
In this study, we first confirmed the interaction between kAE1 and mu-1A adaptor protein in MDCK cells using coimmunoprecipitation with endogenous or heterologously expressed mu-1A. In addition, we present evidence that kAE1 also binds to mu-1B and mu-3 proteins from adaptor complex AP-1B and AP-3, respectively. In agreement with these results, we found that kAE1 protein and mu-1A and/or B proteins colocalize in intracellular vesicles in intercalated cells of mouse kidney sections and coimmunoprecipitate from a mouse kidney homogenate. Moreover, in MDCK cells where endogenous mu-1A was the predominant isoform to be knocked down, kAE1 protein was prematurely degraded via a lysosomal pathway and kAE1 was no longer detectable at the plasma membrane. In MDCK cells, reintroducing small interfering (si)RNA-resistant mu-1A allowed proper targeting of newly synthesized kAE1 to the cell surface. Thus these data highlight a novel role for AP-1A in normal processing of kAE1 in epithelial cells. Finally, our results show that newly synthesized kAE1 does not traffic through REs before reaching the cell surface in cells that contain endogenous AP-1A. These findings suggest that 1) AP-1A is the primary adaptor complex required for kAE1 processing to the plasma membrane; and 2) AP-1B can partially compensate for the absence of AP-1A in kAE1 normal trafficking. Together, our results demonstrate that AP-1 adaptor complexes are crucial for normal targeting of newly synthesized kAE1 to the cell surface of renal epithelial cells.
EXPERIMENTAL PROCEDURES
Recombinant plasmid constructs and antibodies.
The pFB-Neo plasmid construct containing human kAE1 WT cDNA with a hemagglutinin (HA) or myc epitope in position 557 (in the third extracellular loop) was previously described (7, 8). The constructs encoding human mu-1A-HA and mu-1B-HA were provided by Dr. Heike Folsch (Northwestern University), and those encoding VSV-G protein were kindly provided by Dr. Paul Melancon (University of Alberta). The kAE1 halotag (HT) in pFN21A plasmid (reference no. FHC11947) was purchased from the Kazusa DNA Research Institute (associated with Promega, Madison WI). This construct contains the HT protein fused to the amino terminus of human kAE1 cDNA that was inserted between the SgfI restriction site (5′) and PmeI restriction site (3′). This construct does not contain an HA epitope.
The rabbit polyclonal antibody that detects both dog mu-1A and mu-1B adaptor proteins was generously provided by Dr. Linton Traub (University of Pittsburgh) and is not suitable for immunofluorescence experiments. The rabbit polyclonal antibody that detects mu-1A from murine kidney homogenates was provided by Dr. Peter Schu (University of Goettingen) and is not suitable for immunoprecipitations or immunofluorescence (personal communication). Mouse antibody against epsilon adaptin (no. 612018) and goat antibody against mu-3 adaptor protein (sc-46771) were purchased from BD Transduction Laboratories and Santa Cruz Biotechnologies, respectively. The mouse antibody against gamma-adaptin subunit was purchased from BD Transduction Laboratories (San Jose, CA). The rabbit polyclonal antibody detecting murine AE1 was provided by Dr. Sebastian Frische (Aarhus University) and is not suitable for Western blots (5). A mouse monoclonal antibody against AE1 developed by Dr. M. Jennings was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biology (Iowa City, IA). Bric 6 antibody that detects an exofacial epitope of kAE1 was purchased from the International Blood Group Reference Laboratory (Bristol, UK). Goat antibody detecting mouse AE1 (C-17) was purchased from Santa Cruz (Santa Cruz Biotechnology, Santa Cruz, CA). Mouse monoclonal anti-HA and mouse anti-myc antibodies were purchased from Covance (Covance, Princeton, NJ); rat anti-HA antibody was purchased from Roche (Roche Diagnostics, Basel, Switzerland); and mouse monoclonal antibody against MPR was purchased from Abcam (Abcam, Cambridge, MA).
Cell culture.
MDCK (CCL-34), LLC-PK1 (CL-101), and HEK 293T (CRL-11268) cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA), and MDCK cells expressing kAE1 WT were prepared according to methods previously described (7). Briefly, HEK 293 cells were transfected with p-VPack-GP, p-VPack-VSV-G, and pFB-Neo-kAE1-HA557 WT or mutant plasmids using FuGENE 6 (Roche Applied Science, Indianapolis, IN). Cell culture supernatants containing infectious viral particles were added to dividing MDCK cells complemented with 8 μg/ml of polybrene (Sigma-Aldrich, Oakville, ON, Canada). After a 24-h incubation, an heterogenous population of MDCK cells expressing kAE1 was selected with 1 mg/ml geneticin (Sigma-Aldrich). MDCK or LLC-PK1 cells were also transiently transfected using the Neon transfection system (Invitrogen, Burlington, Canada), following the manufacturer's instructions. For immunofluorescence experiments, 1 × 106 cells were transfected with 2–6 μg of cDNA, and for immunoprecipitation experiments, 3 × 106 cells were transfected with 24 μg of cDNA. Both LLC-PK1 and MDCK cells were transfected using 1,400-V pulse voltage, 20-ms pulse width, and 3 pulses.
Immunoprecipitation.
MDCK cells expressing kAE1-HA557 WT were lysed in PBS containing 1% Triton X-100 and protease inhibitors (1 μg/ml aprotinin, 2 μg/ml leupeptin, 1 μg/ml pepstatin A, and 100 μg/ml PMSF). Aliquots of the cell lysates were saved as total fraction, the remaining cell lysates were incubated with rabbit anti-mu-1A/B or with rat anti-HA antibodies, followed by precipitation with protein G-Sepharose (Thermo Scientific, Rockford, IL). The bound proteins were eluted with Laemmli buffer before detection by Western blot using either a mouse anti-HA antibody followed by an anti-mouse antibody coupled to horseradish peroxidase (HRP), rabbit anti-mu-1A/B anti-rabbit antibody followed by an anti-rabbit antibody coupled to HRP, mouse anti-gamma adaptin antibody followed by an anti-mouse antibody coupled to HRP, or mouse anti-actin antibody followed by an anti-mouse antibody coupled to HRP. Alternatively, proteins in cell lysates were immunoprecipitated with a rabbit anti-myc antibody and detected using the mouse anti-HA antibody.
Kidney from PBS-perfused mouse kidneys were freshly dissected, and extracts were prepared in PBS (pH 7.5), 1 mM EDTA, 1% Triton X100, and protease inhibitors at 4°C. The PBS perfusion allowed the removal of a majority of red blood cells that would contain extensive amounts of erythroid AE1. After insoluble materials were removed with two low speed centrifugations (15,000 g for 10 min), an aliquot of the Triton soluble extract (60 μg of proteins) was saved while the remaining lysate (3 mg of total proteins) was precleared with protein G beads, before immunoprecipitation with 5 μl of goat anti-AE1 antibody (Santa Cruz C-17). The eluted proteins were detected on Western blot using a rabbit anti-mu1A antibody. All experiments were performed in compliance with the University of Alberta, Health Sciences Section, Animal Ethics Board (protocol 576).
Immunocytochemistry.
MDCK cells expressing kAE1-HA557 WT were grown on glass coverslips, fixed with 4% paraformaldehyde, permeabilized with 0.3% Triton X-100, and blocked with 1% BSA. Cells were then incubated with mouse anti-HA antibody and rabbit polyclonal anti-gamma adaptin antibody or rat anti-HA and mouse anti-CD-MPR antibodies. Secondary antibodies were donkey anti-mouse or donkey anti-rat antibodies coupled to Cy3 (Jackson Immunoresearch, West Grove, PA) and goat anti-mouse antibody coupled to Alexa 488 (Molecular Probes, Carlsbad, CA). Nuclei were stained with DAPI (Sigma-Aldrich). Samples were examined using an Olympus IX81 microscope equipped with a Nipkow spinning-disk optimized by Quorum Technologies (Guelph, ON, Canada) and a ×100 lens.
For immunohistochemistry experiments, formalin-fixed, paraffin-embedded 2-μm thick mouse kidney sections were baked overnight at 60°C, dewaxed in xylene, and hydrated in decreasing concentrations of alcohol in distilled water. Sections were heated to retrieve antigen in TEG buffer at pH 9 (10 mM Tris and 0.5 mM EGTA pH 9.0) for 20 min before blocking then immunostained with mouse anti-gamma adaptin and rabbit anti-AE1 antibodies, followed by anti-mouse antibody coupled to Cy3 and anti-rabbit antibody coupled to Alexa 488. The sections were examined with the Olympus IX81 microscope described above and a ×20 objective.
Knockdown of canine mu-1A adaptin with siRNA duplexes.
Subconfluent MDCK cells (2 × 105 cells per well) expressing kAE1-HA557 WT, grown in six-well plates, were transiently transfected with either 200 nM of canine-specific siRNA duplexes targeting dog mu-1A adaptin (5′-GGTCCGAGGGCATCAAGTA) or 200 nM of control siRNA targeting luciferase (Dharmacon, Lafayette, CO) using 12 μl of OLIGOFectamine (Invitrogen) resuspended in opti-MEM reduced serum medium (Invitrogen; protocol provided by Dr. Ashley Toye, Bristol University). Four hours later, the cell culture medium was replaced by DMEM-F12 containing 10% FBS, 0.5% penicillin/streptomycin, and 1 mg/ml geneticin to maintain expression of kAE1-HA557 WT protein. Transfected cells were grown for 48 or 72 h at 37°C before lysis in PBS containing 1% Triton X-100 and protease inhibitors. Approximately 20% of the cells died after a 72-h incubation with siRNA against canine mu-1A but not with siRNA against luciferase. After cells were lysed, 7 μg of total proteins were loaded per lane on an 8% SDS-PAGE gel. After transfer to a nitrocellulose membrane, the samples were incubated with mouse anti-HA antibody, rabbit anti-mu-1A/B antibody, and mouse anti-actin antibody, followed by anti-mouse and anti-rabbit secondary antibodies coupled to HRP. After visualization by enhanced chemiluminescence, intensities of the bands of interest were compared using densitometric analysis with ImageJ software. Alternatively, after 48- or 72-h incubation posttransfection in six-well plates containing glass coverslips, cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 1% BSA. Cells were then incubated with rat anti-HA antibody and mouse anti-CD-MPR antibody. Secondary antibodies were donkey anti-rat antibodies coupled to Cy3 and donkey anti-mouse antibody coupled to Cy5 (Jackson Immunoresearch). Nuclei were stained with DAPI. Samples were examined using an Olympus IX81 microscope equipped with a Nipkow spinning-disk optimized by Quorum Technologies (Guelph) and a ×100 objective. There is a 3-base mismatch between our mu-1A siRNA and canine mu-1B, 10-base mismatch between our mu-1A siRNA and canine mu-3, and 10-base mismatch between mu-1A siRNA and canine mu-4 from AP-4.
Expression of siRNA-resistant mu-1A in mu-1A/B siRNA-transfected MDCK cells.
MDCK cells (1 × 106 cells) expressing kAE1-HA557 WT were transiently transfected with 5 μg of either pCDNA3 empty vector or mu-1A HA cDNA in the pCDNA3 vector using the NEON system. Twenty-four hours later, 200 nM of either canine-specific siRNA duplexes targeting dog mu-1A protein or control siRNA targeting luciferase were transfected using the protocol described in Knockdown of canine mu-1A adaptin with siRNA duplexes. Forty-eight hours later, the cells were lysed and kAE1 and mu-1A were both detected by Western blot using a mouse anti-HA antibody followed by anti mouse antibody coupled to HRP. Mu-1A HA was detectable by Western blot after up to 72 h posttransfection, and 5 μg of mu-1A HA cDNA were the minimum amount required for maximal expression of mu-1A HA protein for this number of cells (data not shown). To calculate the amount of rescued kAE1, intensities of the respective bands were measured using the ImageJ software. We normalized the intensities of kAE1 to the internal control actin and then compared the percentage of kAE1 band in mu-1A/B siRNA cells to kAE1 in luciferase siRNA cells in each condition (pcDNA3-, mu-1A HA-, or mu-1B HA-transfected cells).
Recycling endosomes inactivation and immunolocalization.
To inactivate REs, we adapted the REs inactivation protocol from (12). MDCK cells (1 × 106 cells per well) were transiently transfected with 2 μg of cDNA encoding kAE1 WT-HA557 and 2 μg of cDNA encoding VSV-G fused to the green fluorescent protein, using the NEON system. The cells were then placed in six-well plates with glass coverslips in CO2-independent culture medium (Invitrogen) supplemented with 10% FBS and incubated for 2 h at 37°C to allow them to reattach to the glass coverslip, before their transfer to 19°C. The following day, the activity of preexisting kAE1 HT proteins was blocked by incubating cells with 500 nM of coumarin-HT substrate (Promega) for 1 h at 19°C in a starving DMEM-F12 medium (containing no serum or antibiotics) to deplete transferrin (Tfn). After three washes with PBS for 5, 5, and 10 min on ice, cells were incubated with 0.01 mg/ml of transferrin-HRP (Tfn-HRP, Accurate Biochemical, US Biological) at 19°C in the starving medium for 2 h. During the last 20 min of incubation, 50 nM of TMR-HT substrate was directly added to the medium to allow labeling of newly synthesized proteins. Cells were then washed three times with PBS and twice with an ice-cold solution containing 150 mM NaCl and 20 mM citric acid and pH 5 to remove the remaining cell surface Tfn-HRP. REs were then inactivated by incubating the cells on ice for 1 h with 0.1 mg/ml DAB and either 0.025% H2O2 or PBS (as a control condition) in the dark. After two washes with PBS containing 1% BSA, the medium was replaced by warm DMEM-F12 medium containing 10% FBS and 10 μg/ml of cycloheximide and transferred to 30°C for 0 (no chase) or 3 h to release proteins from the TGN and allow their trafficking to the cell surface. Cells were then fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked with 1% BSA, and incubated with rabbit anti-GFP antibody followed by anti-rabbit antibody coupled to Alexa488. Samples were examined using an Olympus IX81 microscope equipped with a Nipkow spinning-disk optimized by Quorum Technologies and a ×100 objective. For counting cells, random pictures were taken using a ×20 objective on the same microscope and two individuals independently counted the percentage of cells transfected with kAE1 that had kAE1 cell surface staining as well as the number of cells transfected with VSV-G that displayed VSV-G cell surface staining.
Pulse-chase like experiment.
MDCK cells were transfected with siRNA against either luciferase or fluorescein-labeled siRNA against mu-1A/B as described in Knockdown of canine mu-1A adaptin with siRNA duplexes. Twenty-four hours later, the cells were transfected with kAE1-HT and either mu-1A HA, mu-1B HA, or pcDNA3 vector as a control using the NEON system. Forty-eight hours after this second transfection (72 h after the first transfection), preexisting kAE1 HT proteins were blocked with 500 nM of coumarin-HT substrate, and cells were incubated at 37°C for 30 min to allow synthesis of new kAE1-HT. The newly synthesized kAE1-HT were then either stained with TMR-HT (red) or FAM-HT (green) substrate (50 nM) and either immediately fixed (no chase) or incubated at 37°C for 3 h to allow trafficking of the protein (3 h chase). Cells were then fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked with 1% BSA, and incubated with mouse anti-HA antibody to detect mu-1A HA or mu-1B HA. Slides were examined using an Olympus IX81 microscope equipped with a Nipkow spinning-disk optimized by Quorum Technologies and a ×100 objective.
Statistical analysis.
Experimental results are summarized as means ± SE. All statistical comparisons were made using unpaired Student's t-test. A P < 0.05 was considered significant.
RESULTS
Mu-1A and B subunits bind to kAE1 in epithelial cells.
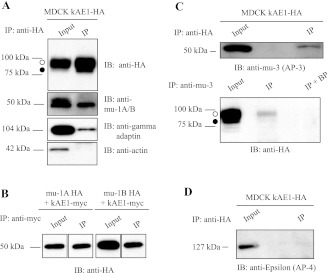
Recently, the carboxyl terminus of the anion exchanger 1 was found to interact with mu-1A subunit from adaptor complex 1A, using a yeast two-hybrid assay (27). We aimed to determine the significance of this interaction in renal epithelial cells. In MDCK cells, we first confirmed that human kAE1 interacted with endogenous canine mu-1A by immunoprecipitation in renal epithelial cells. We immunoprecipitated kAE1-HA557 WT (carrying a HA epitope in position 557, later referred to as kAE1 WT; Ref. 8) expressed in MDCK cells with rat anti-HA antibody and blotted the samples with rabbit anti-mu-1A/B antibody, mouse anti-gamma adaptin, or mouse anti-actin antibodies as a negative control (Fig. 1A). In MDCK epithelial cells, kAE1 protein migrates as two main bands: the top band corresponds to proteins carrying complex oligosaccharide (open circle) and the bottom band corresponds to kAE1 carrying high mannose oligosaccharide (closed circle) as shown on Fig. 1, A and C. In Fig. 1A, we show that kAE1, endogenous mu-1A/B, and gamma adaptin, but not actin, are present in the same protein complex in MDCK cell lysates.
Fig. 1.
Kidney anion exchanger 1 (kAE1) binds to adaptor protein (AP)-1A, AP-1B, and AP-3 adaptor complexes. A: Madin-Darby canine kidney cells (MDCK) expressing kAE1 wild type (WT) were lysed and proteins were either directly resolved on SDS-PAGE (input) or immunoprecipitated (IP) with rat anti-hemagglutinin (HA) antibody before the SDS-PAGE. After proteins were transferred to nitrocellulose membranes, samples were blotted with mouse anti-HA antibody, rabbit anti-subunit μ1A/B (anti-mu-1A/B) antibody, mouse anti-gamma adaptin antibody, or mouse anti-actin antibody followed by anti-mouse horseradish peroxidase (HRP) or anti-rabbit HRP antibodies. Open circle corresponds to kAE1 carrying complex oligosaccharides, and filled circle indicates kAE1 carrying high mannose oligosaccharides. B: MDCK cells expressing kAE1 WT carrying a myc epitope and either mu-1A-HA or mu-1B-HA were lysed, and proteins were either directly loaded on an 8% SDS-PAGE (input) or immunoprecipitated with mouse anti-myc antibody. Mu subunits present in the same protein complex were detected with a rat anti-HA antibody followed by an anti-rat antibody coupled to HRP. C: MDCK cells expressing kAE1 WT were lysed. Twenty micrograms of proteins were directly loaded on an 8% SDS-PAGE. Three aliquots containing each 500 μg of proteins present in the remaining lysate were prepared. Proteins in each aliquot were either immunoprecipitated with a rat anti-HA antibody (Fig. 1 D, top) or with goat anti-mu-3 adaptor protein antibody (bottom) before SDS-PAGE. After proteins were transferred on nitrocellulose membranes, samples were blotted with goat anti-mu-3 adaptor protein or mouse anti-HA antibodies. IB, immunoblot; BP, immunoprecipitating antibody was preincubated with the immunogenic peptide before incubation with the cell lysate. D: MDCK cells expressing kAE1 WT were lysed, and 500 μg of proteins were immunoprecipitated with rat anti-HA antibody followed by SDS-PAGE. After transfer, the proteins were detected with a mouse anti-epsilon adaptor protein antibody. Blots represent 3 independent experiments.
To clarify whether kAE1 can specifically bind to mu-1A and/or B isoform, we transiently coexpressed kAE1 carrying a myc epitope (kAE1 myc; Ref. 7) and either mu-1A HA or mu-1B HA in MDCK cells and examined whether they interact. As seen in Fig. 1B, we observed that both mu-1A and mu-1B coimmunoprecipitated with kAE1 from MDCK cells transiently expressing either human mu-1A or mu-1B carrying a HA epitope (mu-1A HA or mu-1B-HA). This result indicates that kAE1 can be located in the same protein complex as either mu-1A or mu-1B in epithelial cells.
To confirm our previous findings (25), we transiently expressed kAE1 WT in LLC-PK1 cells that are devoid of endogenous mu-1B. In these cells, we were also able to detect coimmunoprecipitation between kAE1 WT and gamma-adaptin (which is only part of the AP-1A complex, since these cells are lacking endogenous mu-1B) as well as with mu-1A adaptin but no coimmunoprecipitation with actin (data not shown). These results confirm that kAE1 WT can interact with AP-1A adaptor protein complex.
kAE1 protein coimmunoprecipitates with AP-3 but not with AP-4 adaptor protein complex.
Since AP-1A recognizes the same YXXΦ motif on cargo proteins as the other clathrin associated adaptors (24), we asked whether other adaptors also interact with kAE1. Specifically, we tested the interaction of kAE1 with AP-4, which is involved in trafficking of basolateral membrane proteins (29). We also determined whether AP-3A, which localizes in the TGN and endosomes, interacts with kAE1 (24). Figure 1, C and D, shows that in MDCK cells kAE1 coimmunoprecipitates with AP-3 adaptor complex but not with AP-4. Since AP-3A is involved in trafficking of cargo proteins to the lysosome, we hypothesize that kAE1 interacts with this adaptor complex on its way to degradation.
kAE1 protein colocalizes with AP-1A and/or B in mouse kidney sections and coimmunoprecipitates with mu-1A from mouse kidney homogenates.
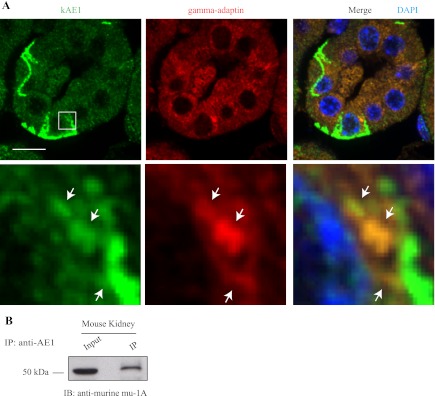
If the interaction between kAE1 protein and mu-1A occurs in vivo, we expect that both proteins colocalize to some extent in the same cells. We thus tested this in ICC of murine kidney. Since AP-1A is ubiquitous and AP-1B adaptor complex is present in cortical collecting ducts (28), it is likely that both kAE1 and AP-1 A and B adaptor complexes are expressed in the same cells. In the absence of an antibody specifically detecting mu-1A subunit that would work in immunofluorescence, we opted to detect the gamma-adaptin subunit present in AP-1A and AP-1B complexes. Mouse kidney sections were incubated with a rabbit polyclonal antibody that specifically recognizes mouse kAE1 protein (stained in green) and with a mouse anti-gamma-adaptin antibody (stained in red; Fig. 2A). In contrast with sections incubated with secondary antibodies only (data not shown), the antibody against kAE1 detected the protein in type-A intercalated cells (the other cells in the tubular section being type-B intercalated cells, non-A and non-B intercalated cells, and principal cells that do not express kAE1) and showed a clear predominant basolateral staining as well as a discrete vesicular intracellular staining. The anti-gamma-adaptin antibody displayed a perinuclear staining, consistent with the previously described localization of AP-1 complexes in the TGN (23). Figure 2A, bottom insets, shows a higher magnification of intracellular red and green staining shows colocalization of both mouse endogenous kAE1 protein with endogenous gamma-1 subunit of the AP-1 complex in a vesicular-like pattern, supporting that kAE1 and AP-1A and/or B complexes can colocalize in the same cell compartment in mouse kidney sections.
Fig. 2.
kAE1 WT immunoprecipitates and colocalizes with the AP-1 adaptor complex. A: paraffin-embedded 2-μm thick mouse kidney sections were submitted to heat-induced antigen retrieval before blocking and incubation with rabbit anti-kAE1 antibody and mouse anti-gamma adaptor subunit. Slides were then incubated with anti-rabbit antibody coupled to Alexa 488 (green) and anti-mouse antibody coupled to Cy3 (red), followed by nuclear staining with DAPI (blue). Samples were examined using an Olympus spinning disk confocal microscope and a ×100 objective. Bottom: enlargement of the region contained in the white square in the above picture. Note that only few cells, that correspond to type-A intercalated cells, show basolateral membrane staining of kAE1. Nonstained cells are likely type-B, non-A, and non-B intercalated cells, and principal cells. White arrows indicate the location of overlapping red and green staining. Bars = 10 μm. B: kidney homogenate was prepared from freshly dissected mouse kidneys and either directly loaded on an 8% gel or immunoprecipitated with a goat anti-AE1 antibody. Coimmunoprecipitated endogenous mu-1A proteins were detected with a rabbit anti-murine mu-1A antibody.
We next determined whether endogenous kAE1 could physically interact with endogenous mu-1A in mouse kidney homogenates. As seen in Fig. 2B, immunoprecipitated kAE1 pulled down a protein with the same molecular weight as endogenous mu-1A strongly suggesting that the two proteins interact in vivo. We were unable to perform the reciprocal immunoprecipitation, as the rabbit anti-mu-1A antibody used in this experiment is not suitable for immunoprecipitations or immunofluorescence. Nevertheless, this immunoprecipitation performed with kidney homogenates confirms that kAE1 and mu-1A physically interact in vivo.
kAE1 is degraded in mu-1A/B knocked-down MDCK cells.
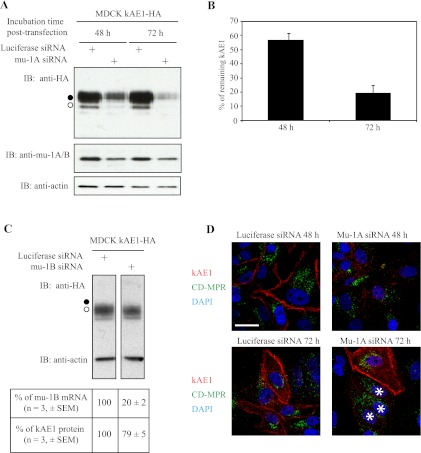
To determine whether mu-1A protein is important for kAE1 sorting, we designed dog-specific siRNA duplexes, transiently transfected subconfluent MDCK cells expressing kAE1 WT protein with either mu-1A-specific siRNA or control siRNA against luciferase (Fig. 3A) and detected endogenous mu-1A/B, kAE1 and actin proteins by immunoblot. Densitometric comparison of the immunoblot bands indicated that there was no change in the amount of endogenous mu-1A/B proteins in cells transfected with siRNA against luciferase after 48- or 72 h-incubation. In contrast, we observed a 40 ± 4% (n = 3; ±SE) reduction of endogenous mu-1A/B proteins after a 48-h incubation posttransfection with siRNA against mu-1A and a 50 ± 13% (n = 3; ±SE) reduction after a 72-h incubation. Mu-1A is 79% identical to mu-1B (25, 26); thus although the siRNA we designed presents 3 bases out of 19 mismatching with mu-1B mRNA, we performed a quantitative RT-PCR to determine the respective amount of endogenous mu-1A or B mRNAs remaining in cells knocked down with siRNAs against mu-1A, relative to cells treated with siRNAs against luciferase. Quantitative RT-PCR indicated that 48 h posttransfection there was 63.2 ± 2.8% (n = 7; ±SE) endogenous mu-1B and only 37.9 ± 4.4% (n = 7; ±SE) endogenous mu-1A mRNAs remaining in cells transfected with siRNAs against mu-1A. Concomitant with this significant decrease of endogenous mu-1A/B mRNA and proteins, our immunoblot showed an abrupt decrease (43 ± 5%; n = 3; ± SE) in the amount of kAE1 WT protein after a 48-h incubation and an 81 ± 5% (n = 3; ± SE) decrease of kAE1 protein amount after 72 h (Fig. 3, A and B). No change of endogenous amount of housekeeping protein actin was observed in any of these conditions. The quick disappearance of kAE1 in MDCK cells where mu-1A and to a lesser extent mu-1B were knocked down suggests mis-sorting and premature degradation of kAE1 in these cells.
Fig. 3.
Knocking down mu-1A affects the stability of kAE1 as well as trafficking of cation-dependent mannose 6-phosphate receptor (CD-MPR) and kAE1 in MDCK cells. A: MDCK cells expressing kAE1 WT were transiently transfected with either 200 nM of canine-specific small interfering (si)RNA duplexes targeting mu-1A or 200 nM of control siRNA targeting luciferase and grown for 24, 48, or 72 h. Cell lysates (7 μg of proteins) were loaded on SDS-PAGE gel and proteins were examined by Western blotting using mouse anti-HA antibody, rabbit anti-mu-1A/B antibody, and mouse anti-actin antibody, followed by anti-mouse and anti-rabbit secondary antibodies coupled to HRP. Blot represents 3 independent experiments. B: histogram representing the percentage of kAE1 remaining in cells transfected with siRNA against mu-1A, 48 or 72 h after transfection, after normalization to amount of actin. Amounts were calculated by densitometric analysis of 3 independent experiments, including the 1 presented on A. Intensity of the bands was measured using the ImageJ software. Error bars correspond to means ± SE. C: MDCK cells expressing kAE1 WT were transfected three times at 72-h intervals with 200 nM of siRNA against mu-1B using the NEON transfection system according to a previously published protocol (19). Seventy-two hours after the third transfection, 15 μg of proteins in the cell lysates were immunoblotted with anti-mu1A/B, anti-actin or anti-HA antibodies. Table indicates the percentage of endogenous mu-1B mRNA quantified by RT-PCR (n = 3), and the percentage of remaining kAE1 protein in these cells (n = 3). D: 48 or 72 h posttransfection, cells grown on glass coverslips were fixed, permeabilized and blocked before incubation with rat anti-HA antibody and mouse anti-CD-MPR antibody. Secondary antibodies were donkey anti-rat antibodies coupled to Cy3 (red) and donkey anti-mouse antibody coupled to Cy5. Nuclei were stained with DAPI (blue). For the purpose of this figure, Cy5 staining (corresponding to mu-1A) is here shown in green. White stars indicate the location of 3 cells transfected with the siRNA against mu-1A. Images represent 3 separate experiments.
To determine the respective role of mu-1A or mu-1B knockdown on kAE1 stability, we specifically knocked down endogenous mu-1B using a previously published siRNA sequence (19) (Fig. 3C). Interestingly, this siRNA was reported to knockdown 90% of endogenous mu-1B protein in MDCK cells but increased mu-1A protein levels by a 1.5 to 2 factor without altering mu-1A mRNA levels. Accordingly, we were unable to detect a significant decrease of mu1-A and B proteins using the nondiscriminating rabbit antibody against mu-1A/B. However, although RT-PCR results indicated that endogenous mu-1B mRNA was knocked down by 80 ± 2% (n = 3; ±SE), consistently with data previously published, there was only a 21 ± 5% (n = 3; ± SE) decrease of steady-state kAE1 protein in these cells after the intensity of kAE1 bands was normalized to the internal control actin. These results indicate that the knockdown of either mu-1A or mu-1B results in decreased expression of kAE1.
To confirm these findings, we investigated the subcellular location of kAE1 WT protein in cells where mu-1A and to a lesser extent mu-1B were knocked down by immunostaining. MDCK cells expressing kAE1 WT were transiently transfected with siRNA against mu-1A or luciferase and grown for 48 or 72 h before detecting kAE1 (red staining) and CD-MPR (Fig. 3D; green staining). CD-MPR binds to newly synthesized hydrolases carrying mannose-6-phosphate in the TGN and target them to endosomes before they reach their final destination in lysosomes (17). AP-1A interacts with CD-MPR via the mu-1A subunit. In MDCK cells transfected with siRNAs against luciferase and incubated for either 48 or 72 h, kAE1 WT protein (red) localized predominantly at the plasma membrane, and CD-MPR was located in a perinuclear compartment, likely the TGN. In contrast, 48 h after transfection with siRNA against mu-1A, the staining from kAE1 WT protein was dramatically decreased and plasma membrane staining was no longer detected (Fig. 3D). In the same cells, the CD-MPR TGN marker (green) showed a diffuse, nonperinuclear staining, in agreement with previous findings in mu-1A−/− mouse embryonic fibroblasts (23). Seventy-two hours posttransfection, there was almost no more red staining corresponding to kAE1 protein in the three cells transfected with siRNAs against mu-1A (as verified using fluorescein dye attached to the siRNA, data not shown) (Fig. 3D, white stars). In these three cells, CD-MPR also showed diffuse staining in the periplasm, but the top cell that was not transfected displayed normal plasma membrane red staining for kAE1 protein and normal perinuclear staining of CD-MPR. These data are consistent with the degradation of kAE1 WT protein in MDCK cells expressing reduced levels of mu-1A/B.
Expression of human mu-1A-HA and mu-1B-HA rescues kAE1 stability in mu-1A/B siRNA-transfected cells.
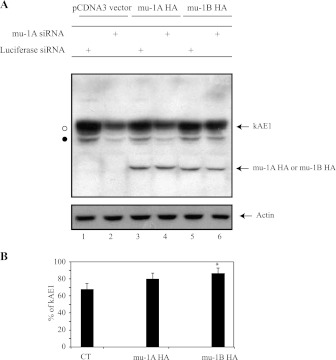
Since RT-PCR results indicated that the siRNAs we used were not exclusively specific to canine mu-1A but also significantly knock down mu-1B, we determined what effect siRNA-resistant human mu-1A or mu-1B proteins expression would have on kAE1 stability in cells knocked down for endogenous canine mu-1A/B. Figure 4A shows that human mu-1A HA and mu-1B HA proteins are unaffected by the mu-1A/B siRNAs since there is no significant decrease of human mu1A HA or mu-1B HA in cells either transfected with siRNA against luciferase or against canine mu-1A/B. Furthermore, in mu-1A/B siRNA-transfected cells, 80 ± 6% (n = 4; ±SE) of kAE1 was detected after expressing human mu-1A-HA while only 67 ± 7% (n = 4; ±SE) kAE1 was present after pcDNA3 transfection (see experimental procedures for detailed calculation; Fig. 4B). Similarly, 86 ± 6% (n = 4; ±SE) of kAE1 was detectable after transfection with human mu-1B HA in the mu-1A/B siRNA transfected cells. These findings indicate that transfection with siRNA-resistant human mu-1A or mu-1B stabilizes kAE1 in mu-1A/B siRNA transfected cells. Thus both mu-1A and mu-1B are involved in the stability of kAE1 at the steady state in epithelial cells.
Fig. 4.
siRNA-resistant mu-1A stabilizes kAE1 in cells knocked down for mu-1A and/or B. A: MDCK cells expressing kAE1 WT were transiently transfected with either 5 μg of mu-1A HA, mu-1B HA or pCDNA3 as control. Twenty-four hours later, cells were transfected with 200 nM of siRNA targeting mu-1A or 200 nM of control siRNA targeting luciferase and grown for 48 h. Cell lysates (20 μg of proteins) were loaded on SDS-PAGE gel, and proteins were detected by immunoblotting using mouse anti-HA antibody and mouse anti-actin antibody. Blot represents 3 independent experiments. B: histogram representing the percentage of kAE1 present in cells transfected with siRNA against mu-1A or luciferase and either subsequently transfected with pCDNA3 vector, mu-1A HA or mu-1B HA. Amounts were calculated by densitometric analysis of 4 independent experiments; see experimental procedures for calculations details. Intensity of the bands was measured using the ImageJ software. Error bars correspond to means ± SE. *P < 0.05 vs. control.
In presence of AP-1A, newly synthesized kAE1 does not traffic through recycling endosomes.
AP-1B, which is predominantly located in REs, is required for basolateral trafficking of some newly synthesized membrane proteins such as the VSV-G (1). Since kAE1 WT binds to both AP-1A and AP-1B, we wondered whether AP-1B is necessary for newly synthesized kAE1 to reach the plasma membrane in renal epithelial cells. Since kAE1 reaches the basolateral membrane in polarized LLC-PK1 cells (10, 31, and our own data), we suspected that this hypothesis would be wrong. Nevertheless, we asked whether newly synthesized kAE1 traffics to the plasma membrane when REs, which contain AP-1B, are inactivated.
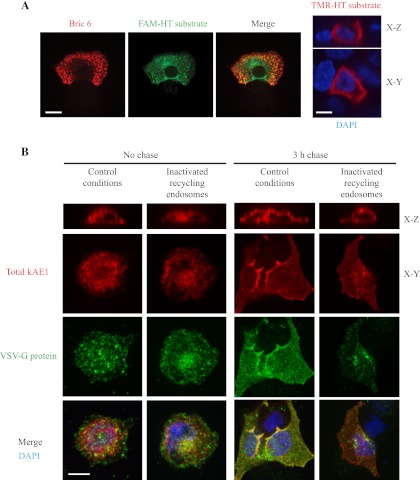
We obtained a construct encoding a chimeric protein where the modified haloallcane dehalogenase haloTag (HT) protein (Promega) is fused to the kAE1 amino terminus (kAE1 WT HT). We first confirmed that this fusion protein is properly targeted to the plasma membrane and to the basolateral membrane of polarized MDCK cells, despite some intracellular retention of the fusion protein (Fig. 5A). The HT system allows studying trafficking of a single pool of proteins, using pulse-chase-like protocols with multiple membrane-permeant, fluorescent HT substrates. We transiently cotransfected MDCK cells with VSV-G, which traffics through REs on its way to the plasma membrane (1), and kAE1 WT HT protein in MDCK cells. After transfection, we stopped trafficking of newly synthesized VSV-G in the Golgi by incubating the cells at 19°C for 18 h. We then blocked all preexisting kAE1-HT with a first coumarin HT ligand, labeled newly synthesized kAE1 WT-HT with a TMR (red) fluorescent HT substrate, and allowed its trafficking to the Golgi at 19°C. We inactivated REs using transferrin coupled to horseradish peroxidase (Tfn-HRP; see experimental procedures for details) and then released trafficking of proteins from the TGN by transferring cells from 19°C to 30°C for 0 (no chase) or 3 h (3 h chase). This protocol was adapted from Ang et al. (1) and exploits the reaction catalyzed by HRP, which forms an insoluble precipitate with DAB and H2O2. We then immunolocalized VSV-G (green), the kAE1 WT-HT being already labeled with the red fluorescent substrate in side views (X–Z sections) of the cells (Fig. 5B). If newly synthesized kAE1 traffics through AP-1B-positive REs before reaching the plasma membrane, it should be retained intracellularly in inactivating conditions, along with VSV-G. In contrast, if kAE1 traffics independently from AP-1B-positive REs, the REs inactivation should only retain VSV-G intracellularly without affecting kAE1 cell surface localization. As seen in Fig. 5B, in contrast with control conditions, kAE1 was detected at the plasma membrane in cells where VSV-G was retained intracellularly due to REs inactivation. We observed that after a 3-h chase in control conditions (active recycling endosomes), 81% (n = 121) of the kAE1 transfected cells displayed kAE1 at the cell surface and 76% (n = 83) of VSV-G transfected cells showed VSV-G at the plasma membrane. In contrast, when REs were inactivated, 75% (n = 86) of the kAE1-transfected cells displayed kAE1 at the cell surface while only 45% (n = 86) of VSV-G-transfected cells showed VSV-G at the plasma membrane. This result indicates that newly synthesized kAE1 does not traffic through REs before reaching the plasma membrane and suggests that kAE1 preferentially use another adaptor protein complex than AP-1B.
Fig. 5.
Newly synthesized kAE1 traffics in a mu-1B-independent pathway and does not travel through recycling endosomes. A: 24 h after transfection in MDCK cells, kAE1-HT was stained with 50 nM of FAM-HT substrate (green) for 10 min at 37°C. After 3 washes for 5, 5, and 10 min with culture medium without FBS or antibiotics on ice, cells were fixed, blocked with 1% BSA, and incubated with Bric 6 antibody followed by anti-mouse antibody coupled to Cy3 (red) before being mounted the coverslip on slides. Samples were examined using an Olympus spinning disk confocal microscope and a ×100 objective. Note that Bric 6 antibody recognizes an extracellular epitope of kAE1 on these nonpermeabilized cells. Right: kAE1-HT expression, stained in red with TMR-HT substrate, in polarized MDCK cells. Blue staining indicates nuclear staining with DAPI. Bar = 10 μm. B: MDCK cells were transiently transfected with cDNAs encoding vesicular stomatitis virus glycoprotein (VSV-G) and kAE1-HT, and newly synthesized proteins were blocked in the Golgi by incubating cells at 19°C (see experimental procedures for details). After blocking all the preexisting kAE1 with a first HT substrate, newly synthesized kAE1-HT was stained with TMR-HT substrate (red) and allowed to traffic to the Golgi by incubating cells at 19°C. During that incubation, Tfn-HRP was added to the medium at 19°C to allow its accumulation in REs. REs were then either inactivated as described in experimental procedures (“inactivated recycling endosomes”) or kept intact (“control conditions”). Newly synthesized proteins were then released from the Golgi by incubation at 30°C for 0 or 3 h before fixation. VSV-G protein was detected using an anti-GFP antibody (green), and total kAE1 is stained in red. Samples were examined using an Olympus spinning disk confocal microscope and a ×100 objective. Blue staining corresponds to DAPI nuclear staining. Yellow staining indicates colocalization between red and green colors. Bar = 10 μm.
Expression of mu-1A rescues trafficking of newly synthesized kAE1 to the plasma membrane.
Our previous data strongly suggest that, when AP-1A is present, AP-1B is not involved in trafficking of newly synthesized kAE1 to the plasma membrane. Since kAE1 does not interact with the TGN-located AP-4 adaptor complex that is involved in basolateral targeting of some proteins (29), we hypothesized that AP-1A may be involved in targeting of newly synthesized kAE1 to the plasma membrane.
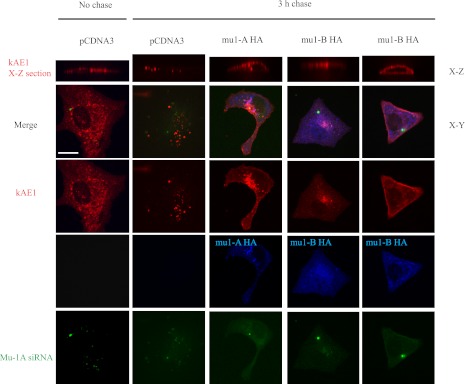
To test this hypothesis, MDCK cells with knocked down mu-1A/B were transfected with kAE1 HT and either human mu-1A HA, mu-1B HA or the empty vector. Forty-eight hours later, we performed a pulse-chase like experiment as follows: preexisiting kAE1 HT proteins were first blocked with coumarin HT substrate, and newly synthesized proteins were stained with TMR (red) fluorescent HT substrate and either allowed to traffic for 0 or 3 h (3 h chase) at 37°C (Fig. 6). After fixation, cells were examined using a confocal microscope. Cells transfected with the fluorescein coupled siRNA displayed green labeling and expression of mu-1A HA or mu-1B HA was confirmed using a mouse anti-HA antibody coupled to Cy5. To better assess plasma membrane targeting, we took multiple sections in the Z-axis through the cell and show X-Z views of kAE1 staining. Figure 6 shows that kAE1 is detectable at the plasma membrane of cells where mu-1A HA was transfected. In this experiment, 88% of the cells (n = 9) that were transfected with kAE1, mu1A/B siRNA, and mu-1A HA displayed cell surface kAE1. As seen in the “mu-1B HA” representative images on Fig. 6, only 53% of the cells rescued with mu-1B HA (n = 15) displayed cell surface kAE1 while the remaining 47% showed intracellular retention of kAE1. This last finding suggests that mu-1B can partially compensate for the absence of mu-1A, in agreement with previously published works (18). Based on these results, we propose that mu-1A from AP-1A adaptor complexes facilitates kAE1 targeting to the plasma membrane of nonpolarized epithelial cells.
Fig. 6.
mu-1A HA allows newly synthesized kAE1 to reach the plasma membrane. MDCK cells were transfected with siRNA against either luciferase or fluorescein-labeled siRNA against mu-1A/B. Twenty-four hours later, the cells were transfected with kAE1-HT and either mu-1A HA, mu-1B HA, or pcDNA3 vector as a control. Forty-eight hours after this second transfection, preexisting kAE1 HT proteins were blocked with coumarin-HT substrate, and cells were incubated at 37°C for 30 min to allow synthesis of new kAE1-HT. Newly synthesized kAE1-HT were then stained with TMR-HT substrate and incubated at 37°C for 0 (no chase) or 3 h to allow trafficking of the protein. Cells were then fixed, permeabilized, blocked with 1% BSA, and incubated with mouse anti-HA antibody to detect mu-1A HA or mu-1B HA. Slides were examined using an Olympus IX81 microscope equipped with a Nipkow spinning-disk optimized by Quorum Technologies and a ×100 objective. Bar = 10 μm. Top: kAE1-HT localization on side (X-Z) views of the cells. Green staining corresponds to cells transfected with the mu-1A/B siRNA, blue staining indicates the location of human mu-1A HA or mu-1B HA, and red staining corresponds to cells expressing kAE1-HT; 2 right columns correspond to the 2 phenotypes observed with cells rescued with mu-1B HA.
DISCUSSION
The physical interaction between kAE1 carboxyl terminus, via a canonical tyrosine/aspartate/glutamate/valine (YDEV) sequence, and mu-1A protein from the adaptor complex 1A was recently reported (27). In our study, we characterized the novel interaction between kAE1 protein and mu subunits from the AP-1 adaptor complexes in a physiologically relevant model cell line, the renal epithelial MDCK cells. In this cell line, kAE1 protein behaves similarly as when expressed in renal ICC: kAE1 protein is properly folded (9), and traffics to the basolateral membrane (7, 10, 31). When expressed in porcine LLC-PK1 cell line that is devoid of endogenous mu-1B, kAE1 WT was also predominantly located at the basolateral membrane (10, 31). In both MDCK and LLC-PK1 cells, we were able to coimmunoprecipitate heterologously expressed kAE1 WT with endogenous mu-1A and/or B and with endogenous gamma-adaptin, indicating that kAE1 WT physically interacts with AP-1 adaptor protein complexes. In addition, in MDCK cells, we show that kAE1 WT immunoprecipitates with heterologously expressed mu-1A-HA and mu-1B-HA subunits. Using siRNA knockdown, we confirmed that AP-1 adaptor complexes are required for normal stability of kAE1 in MDCK cells (Fig. 3). Furthermore, introducing siRNA-resistant human mu-1A or mu-1B stabilized kAE1 in these cells (Fig. 4). In support of these findings, we were able to coimmunoprecipitate kAE1 with mu-1A from freshly dissected mouse kidneys (Fig. 2B) and we observed a colocalization of endogenous kAE1 and AP-1A and/or B in intracellular vesicles of mouse kidney sections (Fig. 2A). Thus our results strongly support that AP-1A is required for processing of the polytopic basolateral protein kAE1 to the plasma membrane in renal epithelial cells. Although further work is needed in polarized epithelial cells, our results highlight the unsuspected key role of AP-1A adaptor complex for targeting of this basolateral membrane protein. Our results are in agreement with recent findings from Gravotta et al. (18) who showed that AP-1A is involved in basolateral targeting of membrane proteins and that AP-1B can partially compensate for the absence of AP-1A. Of note, had our mu-1A/B siRNA been exclusively specific to mu-1A subunit, we would likely have been unable to see any effect on kAE1 protein stability as endogenous mu-1B may have compensated for the loss of endogenous mu-1A.
Interestingly, only AP-3A but not AP-4 was also found to coimmunoprecipitate with kAE1 (Fig. 1, C and D). AP-4 is located in subdomains of the TGN and is involved in basolateral targeting of LDL receptor or MPR46 but not of Tfn receptor in epithelial cells (29). The lack of interaction that we observed in our experiments suggests that the AP-4 adaptor is not involved in kAE1 targeting to the plasma membrane. AP-3A is ubiquitously expressed and is found in the TGN and in endosomes. We hypothesize that endocytosed kAE1, targeted for lysosomal degradation, traffics via AP-3A-positive endosomes.
Mu-1B from AP-1B protein complex was found to physically interact with kAE1 protein. Since both kAE1 and AP-1B are expressed in epithelial cells (25), such interaction between kAE1 and AP-1B was not surprising. AP-1B is involved in the targeting of some basolateral membrane proteins such as VSV-G to the basolateral membrane (1): newly synthesized VSV-G proteins exit the TGN, and within a few minutes, enter transferrin-positive RE before reaching the cell surface. Therefore, we tested whether AP-1B, which colocalizes with Tfn receptor (16), is also involved in kAE1 targeting to the plasma membrane by inactivating Tfn receptor-positive RE (Fig. 5). We found that inactivating REs did not affect cell surface trafficking of newly synthesized kAE1 to the cell surface, in contrast with trafficking of VSV-G. Thus newly synthesized kAE1 protein, a protein normally expressed in epithelial ICC, traffics to the basolateral membrane in a fashion independent of the epithelial-specific mu-1B adaptor protein. Since in MDCK cells, endocytosed kAE1 colocalizes with Tfn receptor (our own unpublished data), a protein that colocalizes with AP-1B adaptor complex (16), we hypothesize that endocytosed kAE1 interacts with AP-1B in transferrin receptor-positive RE. Indeed, plasma membrane kAE1 may be constitutively endocytosed and targeted to recycling endosomes where it may interact with AP-1B protein complex before returning to the cell surface. This hypothesis is consistent with the clear stabilization of kAE1 after expression of siRNA-resistant mu-1B in cells where endogenous mu-1A and B were knocked down (Fig. 4) but remains contradictory with the predominant basolateral targeting of kAE1 at the basolateral membrane of LLC-PK1 cells. One would expect that if AP-1B is required for proper recycling of endocytosed kAE1 to the basolateral membrane, kAE1 would either be prematurely degraded or apically mistargeted in polarized LLC-PK1 cells.
The physical interaction of kAE1 with mu-1A from adaptor complex 1A was more unexpected. To date, AP-1A has been reported to be important for CD-MPR trafficking between the TGN and endosomes (23). In agreement with our findings, two recent works (3, 18) reported the key role of AP-1A for basolateral targeting of various membrane proteins, suggesting that AP-1A may play an underestimated role for normal processing of a wide number of basolateral membrane proteins. In our study, we observed that kAE1 is destabilized and degraded via a lysosomal pathway in cells where mu-1A and to a lesser extent mu-1B were knocked down (data not shown) and that expression of siRNA-resistant mu-1A restored stability of the protein (Fig. 4). This last finding confirms that AP-1A is important for kAE1 stability and trafficking. It also suggests that kAE1 is degraded via specific mechanisms that differ from CD-MPR, which is not rapidly degraded when mis-sorted in mu-1A-deficient cells (23).
Our results from Fig. 6 suggest that mu-1B can occasionally compensate for the absence of mu-1A for kAE1 processing. This is in agreement with the compensatory action of AP-1B in absence of AP-1A for sorting of mannose 6-phosphate receptors and coxsackie and adenovirus receptor (3, 11); however, the clear rescue of kAE1 trafficking in mu-1A HA-transfected cells strongly suggests that mu-1A is the major adaptor complex involved in kAE1 processing. Thus the role of the interaction between kAE1 and AP-1B remains unclear and will require further investigations. Interestingly, kAE1 is not the only protein reported to traffic to the basolateral membrane in a mu-1B-independent way. Like kAE1, the Na+-K+-ATPase that resides at the basolateral membrane of epithelial cells also traffics in an AP-1B independent way to the basolateral membrane of MDCK cells (12).
In addition to adaptor proteins, the carboxyl terminus of kAE1 is the site of interaction with other proteins (35). Recently, the GAPDH was also reported to interact with the D902EYDE motif in the kAE1 carboxyl terminal domain, which includes tyrosine 904 from the Y904DEV907 motif that interacts with mu-1A protein (30). The Y904DEV907 motif contains tyrosine 904 that can be phosphorylated and its phosphorylation status determines whether the protein remains at the plasma membrane or undergoes endocytosis (33). Further studies will be needed to investigate whether binding of GAPDH affects the interaction of the carboxyl terminus of kAE1 with adaptor proteins and whether these overlapping interactions are part of a regulatory mechanism for kAE1 targeting to the cell surface.
Two dRTA patients have been reported to carry mutations that cause carboxyl-terminal truncations of kAE1 protein by 11 (R901X) or 23 (A888L/D889X) amino acids (4, 22). The kAE1 R901X mutant either mis-trafficked to both basolateral and apical membranes in polarized MDCK cells or exclusively to the apical membrane in highly polarized MDCK I cells (10, 31). We postulate that mis-trafficking of the R901X mutant and possibly of the A888L/D889X mutant cause dRTA due to the lack of interaction with mu-1 subunits from adaptor protein complexes 1A or B and perhaps with GAPDH. This would result in loading of newly synthesized kAE1 proteins in the wrong trafficking vesicles en route to the apical membrane instead of the basolateral membrane. It is possible that the phosphorylation status of this tyrosine potentially regulates kAE1 interaction with mu-1A adaptin.
All together, our study strongly supports that AP-1A regulates normal trafficking of newly synthesized kAE1 to the plasma membrane. Further studies are required to fully understand the respective physiological role of AP-1A and B on stability and polarized trafficking of newly synthesized or endocytosed kAE1 to the basolateral membrane of type-A ICC in the kidney and the consequences of its mis-sorting on the development of distal renal tubular acidosis.
GRANTS
This work was supported by fundings from the Kidney Foundation of Canada, the Canadian Institutes of Health Research and the Banting Research Foundation. E. Y. Almomani is supported by a Women and Children's Health Research Institute graduate studentship. P. Yenchitsomanus is supported by the Senior Research Scholar Grant from the Thailand Research Fund and by the Pre-Clinic Staff Development Fund from the Faculty of Medicine Siriraj Hospital, Mahidol University. E. Cordat and R. T. Alexander are supported by a KRESCENT New Investigator award. R. T. Alexander is an Alberta Heritage Foundation for Medical Research clinical scientist.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.Y.A., J.C.K., and J.N. performed experiments; E.Y.A., J.C.K., J.N., and E.C. analyzed data; E.Y.A., J.C.K., R.T.A., and E.C. interpreted results of experiments; E.Y.A. and E.C. prepared figures; E.Y.A. and E.C. drafted manuscript; E.Y.A., P.-T.Y., P.M., T.L., R.T.A., and E.C. edited and revised manuscript; E.Y.A., P.-T.Y., P.M., T.L., R.T.A., and E.C. approved final version of manuscript; E.C. conception and design of research.
ACKNOWLEDGMENTS
We gratefully thank Dr. Reinhart Reithmeier and Jing Li for providing the PFB-Neo-kAE1 constructs, Dr. Yves Sauve for help with mouse kidney collection and Wanling Pan for technical help with the quantitative RT-PCR experiments.
REFERENCES
- 1. Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol 167: 531–543, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cancino J, Torrealba C, Soza A, Yuseff MI, Gravotta D, Henklein P, Rodriguez-Boulan E, Gonzalez A. Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes. Mol Biol Cell 18: 4872–4884, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carvajal-Gonzalez JM, Gravotta D, Mattera R, Diaz F, Perez Bay A, Roman AC, Schreiner RP, Thuenauer R, Bonifacino JS, Rodriguez-Boulan E. Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXϕ motif with the clathrin adaptors AP-1A and AP-1B. Proc Natl Acad Sci USA 109: 3820–3825, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheidde L, Vieira TC, Lima PR, Saad ST, Heilberg IP. A novel mutation in the anion exchanger 1 gene is associated with familial distal renal tubular acidosis and nephrocalcinosis. Pediatrics 112: 1361–1367, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Christensen BM, Kim YH, Kwon TH, Nielsen S. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Cordat E, Casey JR. Bicarbonate transport in cell physiology and disease. Biochem J 417: 423–439, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Cordat E, Kittanakom S, Yenchitsomanus PT, Li J, Du K, Lukacs GL, Reithmeier RA. Dominant and recessive distal renal tubular acidosis mutations of kidney anion exchanger 1 induce distinct trafficking defects in MDCK cells. Traffic 7: 117–128, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Cordat E, Li J, Reithmeier RA. Carboxyl-terminal truncations of human anion exchanger impair its trafficking to the plasma membrane. Traffic 4: 642–651, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Cordat E, Reithmeier RA. Expression and interaction of two compound heterozygous distal renal tubular acidosis mutants of kidney anion exchanger 1 in epithelial cells. Am J Physiol Renal Physiol 291: F1354–F1361, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Devonald MA, Smith AN, Poon JP, Ihrke G, Karet FE. Non-polarized targeting of AE1 causes autosomal dominant distal renal tubular acidosis. Nat Genet 33: 125–127, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Eskelinen EL, Meyer C, Ohno H, von Figura K, Schu P. The polarized epithelia-specific mu 1B-adaptin complements mu 1A-deficiency in fibroblasts. EMBO Rep 3: 471–477, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farr GA, Hull M, Mellman I, Caplan MJ. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J Cell Biol 186: 269–282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fields IC, King SM, Shteyn E, Kang RS, Folsch H. Phosphatidylinositol 3,4,5-trisphosphate localization in recycling endosomes is necessary for AP-1B-dependent sorting in polarized epithelial cells. Mol Biol Cell 21: 95–105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fields IC, Shteyn E, Pypaert M, Proux-Gillardeaux V, Kang RS, Galli T, Folsch H. v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells. J Cell Biol 177: 477–488, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99: 189–198, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Folsch H, Pypaert M, Schu P, Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J Cell Biol 152: 595–606, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glickman JN, Conibear E, Pearse BM. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J 8: 1041–1047, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gravotta D, Carvajal-Gonzalez JM, Mattera R, Deborde S, Banfelder JR, Bonifacino JS, Rodriguez-Boulan E. The clathrin adaptor AP-1A mediates basolateral polarity. Dev Cell 22: 811–823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gravotta D, Deora A, Perret E, Oyanadel C, Soza A, Schreiner R, Gonzalez A, Rodriguez-Boulan E. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci USA 104: 1564–1569, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirst J, Barlow LD, Francisco GC, Sahlender DA, Seaman MN, Dacks JB, Robinson MS. The fifth adaptor protein complex. PLoS Biol 9: e1001170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta 1404: 173–193., 1998 [DOI] [PubMed] [Google Scholar]
- 22. Karet FE, Gainza FJ, Gyory AZ, Unwin RJ, Wrong O, Tanner MJ, Nayir A, Alpay H, Santos F, Hulton SA, Bakkaloglu A, Ozen S, Cunningham MJ, di Pietro A, Walker WG, Lifton RP. Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci USA 95: 6337–6342, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, von Figura K, Schu P. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J 19: 2193–2203, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakatsu F, Ohno H. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Func 28: 419–429, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Foelsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett 449: 215–220, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282: 1327–1332, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sawasdee N, Junking M, Ngaojanlar P, Sukomon N, Ungsupravate D, Limjindaporn T, Akkarapatumwong V, Noisakran S, Yenchitsomanus PT. Human kidney anion exchanger 1 interacts with adaptor-related protein complex 1 mu1A (AP-1 mu1A). Biochem Biophys Res Commun 401: 85–91, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Schreiner R, Frindt G, Diaz F, Carvajal-Gonzalez JM, Perez Bay AE, Palmer LG, Marshansky V, Brown D, Philp NJ, Rodriguez-Boulan E. The absence of a clathrin adapter confers unique polarity essential to proximal tubule function. Kidney Int 78: 382–388, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simmen T, Honing S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol 4: 154–159, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Su Y, Blake-Palmer KG, Fry AC, Best A, Brown AC, Hiemstra TF, Horita S, Zhou A, Toye AM, Karet FE. Glyceraldehyde 3-phosphate dehydrogenase is required for band 3 (anion exchanger 1) membrane residency in the mammalian kidney. Am J Physiol Renal Physiol 300: F157–F166, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toye AM, Banting G, Tanner MJ. Regions of human kidney anion exchanger 1 (kAE1) required for basolateral targeting of kAE1 in polarised kidney cells: mis-targeting explains dominant renal tubular acidosis (dRTA). J Cell Sci 117: 1399–1410, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114: 299–310, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Williamson RC, Brown AC, Mawby WJ, Toye AM. Human kidney anion exchanger 1 localisation in MDCK cells is controlled by the phosphorylation status of two critical tyrosines. J Cell Sci 121: 3422–3432, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Wrong O, Bruce LJ, Unwin RJ, Toye AM, Tanner MJ. Band 3 mutations, distal renal tubular acidosis, and Southeast Asian ovalocytosis. Kidney Int 62: 10–19, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Wu F, Saleem MA, Kampik NB, Satchwell TJ, Williamson RC, Blattner SM, Ni L, Toth T, White G, Young MT, Parker MD, Alper SL, Wagner CA, Toye AM. Anion exchanger 1 interacts with nephrin in podocytes. J Am Soc Nephrol 21: 1456–1467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]