Abstract
The proton-coupled folate transporter (PCFT) mediates intestinal folate absorption, and loss-of-function mutations in this gene result in the autosomal recessive disorder hereditary folate malabsorption. The current study, focused on a structure-functional analysis of this transporter, identified Gly-189 and Gly-192 (a GxxG motif) located in the fifth transmembrane domain as residues that could not be replaced with alanine without a loss of function. In contrast, function was preserved when Gly-56 and Gly-59 (the other conservative GXXG motif in human PCFT) were replaced with alanine. Similarly, Gly-93 and Gly-97, which constitute the only conserved GXXXG dimerization motif in human PCFT, tolerated alanine substitution. To explore the role of this region in folate binding, the residues around Gly-189 and Gly-192 were analyzed by the substituted cysteine accessibility method. Both I188C and M193C mutants were functional and were inhibited by membrane-impermeable sulfhydryl-reactive reagents; this could be prevented with PCFT substrate, but the protection was sustained at 0°C only for the I188C mutant, consistent with localization of Ile-188 in the PCFT folate binding pocket. The functional role of residues around Gly-189 and Gly-192 is consistent with a molecular structural model in which these two residues along with Ieu-188 are accessible to the PCFT aqueous translocation pathway.
Keywords: intestinal folate transport, homology modeling, heme carrier protein 1
folates are a family of B9 vitamins that require highly specific membrane transporters for cellular internalization. The proton-coupled folate transporter (PCFT), a member of the superfamily of solute carriers (SLC46A1), plays an essential role in folate homeostasis by mediating transport of folates across the apical brush-border membrane of the duodenum and proximal jejunum, a critical first step in intestinal folate absorption (23). Loss-of-function mutations in the pcft gene result in the autosomal recessive disorder hereditary folate malabsorption (HFM; Refs. 4, 23, 39). The basic properties of PCFT-mediated transport and its roles in folate homeostasis, antifolate activities, and folate receptor-mediated endocytosis have been the subject of recent reviews (39, 41). The development of novel antifolate compounds designed specifically for transport mediated by PCFT is an area of current interest in cancer therapeutics (3).
Due to its physiological and pharmacological importance, studies have been directed to the identification of structural elements of PCFT that are required for its function. Two N-glycosylation sites (Asn-58 and Asn-68) suggested that the long loop harboring these residues is located extracellularly (37). The intracellular orientation of hemagglutin-tagged COOH and NH2 termini was determined based on their accessibility to a hemagglutinin-specific antibody (24, 37). The location of the remaining loops was established by the substituted cysteine accessibility method (45). Recently, it was reported that PCFT exists as a homo-oligomer (9); however, a dimerization motif has not been identified.
Information on PCFT structure/function has derived from the identification of mutations associated with HFM that result in impaired transport function. In all known point mutations, either charged residues were replaced by neutral residues (R113C, R113S, R376W, R376Q, and D156Y) or noncharged residues were replaced with charged residues (G147R, S318R, A335D, G338R, and P425R; Refs. 14, 18, 29, 30, 42). Detailed analysis of Arg-113 indicated that the integrity of this residue is critical for function (13). When Arg-376 was replaced with Trp, protein expression at the plasma membrane was preserved but function was not detected. Substitution of Arg-376 with Gln, however, did allow residual function but the affinity for substrate, as well as rates of conformational change associated with substrate translocation, were markedly decreased (18). Replacement of Asp-156 with Tyr or most other amino acids resulted in an unstable protein (30).
Another source of information on PCFT structure-function has come from site-directed mutagenesis. Of particular interest have been studies directed to conserved charged residues within transmembrane domains. His and Glu residues in these regions were substituted with Ala. Glu-185 was found to be required for proton coupling, since the E185A mutant retained all function at neutral pH but lost virtually all function at acidic pH. The kinetic basis for the defect was a marked decrease in a rate-limiting step in the transport cycle without a change in folate binding to the carrier (36). His-281 appears to play an important role in proton binding which, in turn, allosterically alters folate substrate binding since the decrease in affinity for folic acid caused by the H281A mutation can be corrected, in part, by lowering the extracellular pH (35).
PCR-based random mutagenesis has also been used as an unbiased approach to identify important PCFT amino acid residues (44). These studies identified hot spots in the pcft coding sequence for base deletion and insertion mutations; several have been detected in subjects with HFM (4). Amino acid substitutions that resulted in loss of PCFT function, but did not involve charged residues, were also identified (44). The current study describes a PCFT mutant generated by random mutagenesis and reveals functionally critical Gly-189 and Gly-192 residues that bracket a GXXG motif. Amino acid residues in this region were further probed by the substituted cysteine accessibility method. The functional and structural roles of the other conserved GXXG motif (Gly-56 and Gly-59) as well as the only conserved dimerization GXXXG motif (Gly-93 and Gly-97) were also assessed. The data are consistent with localization of the G(189)XXG(192) motif in, or near to, the folate binding site.
EXPERIMENTAL PROCEDURES
Chemicals and cell lines.
[3′,5′,7-3H(N)]methotrexate (MTX) was obtained from Moravek Biochemicals (Brea, CA). [2-(Trimethylammonium)ethyl]methanethiosulfonate bromide (MTSET) and 1,1-methanediyl bismethanethiosulfonate (MTS-1-MTS) were purchased from Toronto Research Chemicals (North York, Ontario, Canada), 2-[(biotinoyl)amino]ethyl methanethiosulfonate (MTSEA biotin) from Biotium (Hayward, CA), and EZ-Link Sulfo-NHS-LC-Biotin from Thermo Scientific (Rockford, IL). HeLa R1–11 cells were derived from HeLa cells and express neither the reduced folate carrier nor PCFT, due to deletion of the former gene (40) and methylation of the latter gene promoter (5). HeLa R1–11 cells served as transient transfection recipients and were maintained in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. HeLa R1–11 cells were thawed regularly from liquid nitrogen to ensure that PCFT expression was absent (38).
Random and site-directed mutagenesis of pcft.
A random mutagenesis protocol (Diversify PCR random mutagenesis kit; Clontech, Mountain View, CA) reported previously (44) was used to generate the PFCT 5–91 mutant. Specific individual mutations were introduced using the QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) in a human PCFT expression vector. Wild-type (WT) and mutated PCFTs were HA-tagged at the COOH terminus of the protein. The coding region of all expression vectors was sequenced by the Albert Einstein Cancer Center Genomics Shared Resource.
Transient transfection.
HeLa R1–11 cells were seeded in 20-ml low background glass scintillation vials (Research Products International, Prospect IL; 0.35 million cells per vial) for transport studies or in sixwell plates (0.6 million cells per well) for biotinylation and dimerization experiments. In both cases, transfections were performed 2 days later with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Transport and labeling assays were performed 2 days after transfection.
Membrane transport.
[3H]MTX was used as an inexpensive and stable surrogate for folates in all membrane transport assays. Cells were washed twice with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered saline (HBS; 20 mM 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid, 5 mM dextrose, 140 mM NaCl, 5 mM KCl, and 2 mM MgCl2, pH 7.4) and incubated in the same buffer at 37°C for 20 min. The incubation buffer was then aspirated, and transport was initiated by the addition of 0.5 ml of prewarmed (37°C) MBS [20 mM 2-(4-morpholino) ethanesulfonic acid, 5 mM dextrose, 140 mM NaCl, 5 mM KCl, and 2 mM MgCl2 pH 5.5] containing the desired [3H]MTX concentrations. Influx was assessed at 37°C over 1 min and stopped by the addition of 5 ml of ice-cold HBS. Cells were washed three times with ice-cold HBS and digested in 0.5 ml of 0.2 M NaOH at 65°C for 1 h. Radioactivity in 0.4 ml of lysate was measured on a liquid scintillation spectrometer and normalized to protein levels obtained with the BCA protein assay (Pierce, Rockford, IL). In most cases, the transport rate is expressed as a percentage of WT-PCFT activity. Otherwise, it is expressed in units of pmol·mg protein−1·min−1.
Modification of the substituted cysteine residue by MTSET.
Cells in transport vials were washed twice with HBS and incubated with 1 ml of freshly made MTSET solution in HBS, at a concentration of 1 mg/ml, for 30 min at room temperature following which the MTSET solution was removed. The cells were then washed twice with HBS, and transport was assessed. To evaluate substrate protection of the cysteine modification, cells were exposed for 30 min to an MTSET solution at 0.064 mg/ml for the I188C mutant or at 0.16 mg/ml for the M193C mutant in the presence or absence of 1 mM pemetrexed, an antifolate with a high affinity for PCFT particularly at neutral pH (43). This assay was performed both at room temperature and at 0°C in the same experiment.
Cell surface and cysteine biotinylation assays.
Cell surface- and cysteine- biotinylation assays were described previously and differed only in the first step (36, 45). For surface labeling, cells were treated with EZ-Link Sulfo-NHS-LC-biotin at a concentration of 1 mg/ml in HBS. For cysteine biotinylation, cells were treated with MTSEA biotin at a concentration of 0.2 mg/ml in HBS. Both treatments were performed at room temperature for 30 min. After two washes with HBS, cells were treated with 0.7 ml hypotonic buffer (0.5 mM Na2HPO4 and 0.1 mM EDTA, pH 7.0) containing protease inhibitor cocktail (Roche, Indianapolis, IN) on ice for 30 min. The cells were then detached from the plates with disposable cell lifters and centrifuged at 16, 000 g and 4 °C for 10 min. The pellet was then resuspended in 0.4 ml lysis buffer (50 mM Tris-base, 150 mM NaCl, 1% NP40, and 0.5% sodium deoxycholate pH 7.4) and mixed on a rotator for 1 h at 4°C. A 25-μl portion (identified as “crude membrane”) was collected and stored in a −20°C freezer. The remaining crude membrane fraction was centrifuged at 16,000 g and 4°C for 15 min, and the supernatant was mixed on a rotator overnight at 4°C with 50 μl of streptavidin agarose beads (Fisher Scientific, Pittsburg, PA) that were prewashed three times with the lysis buffer. The agarose beads were then washed two times with the lysis buffer and then two times with the lysis buffer containing 2% SDS, each with a 20-min mix on a rotator at room temperature. The precipitated proteins were released from the beads by heating at 95°C for 5 min in 2× SDS-PAGE sample loading buffer containing dithiothreitol.
Assessment of PCFT dimerization.
Cells in sixwell plates were washed twice with HBS before treatment with the cross-link reagent MTS-1-MTS. MTS-1-MTS powder was first dissolved in DMSO at a concentration of 3.6 mg/ml (15 mM) and then diluted with cold HBS to achieve a concentration of 0.036 mg/ml (0.15 mM). This solution was immediately added to cells and incubated on ice for 1 h. Cells were then washed twice with HBS and treated with 0.7 ml hypotonic buffer (0.5 mM Na2HPO4 and 0.1 mM EDTA, pH 7.0) containing protease inhibitor cocktail (Roche, Indianapolis, IN) on ice for 30 min. The cells were detached from the plates with disposable cell lifters and centrifuged at 16,000 g and 4°C for 10 min. The pellet was then dissolved in 0.4 ml lysis buffer (50 mM Tris-base, 150 mM NaCl, 1% NP40, and 0.5% sodium deoxycholate pH 7.4), and the resulting solution was centrifuged at 16,000 g and 4°C for 15 min. The supernatant was either stored in a freezer or diluted with dithiothreitol-free 2× SDS-PAGE sample loading buffer at room temperature for immediate SDS-PAGE analysis.
Western blot analysis.
Protein samples were resolved by standard 4–20% or 12.5% SDS-PAGE. The precipitated proteins (released from beads as described in the previous section) were loaded directly on gels while the crude membrane fraction was mixed (1:1) with dithiothreitol-containing, or dithiothreitol-free, 2× SDS-PAGE sample loading buffer at room temperature before loading on the gels. After SDS-PAGE, proteins were transferred to Amersham Hybond membranes (GE Healthcare, Piscataway, NJ) and were blocked with 10% dry milk in TBST (20 mM Tris, 135 mM NaCl, and 1% Tween 20 pH 7.6) overnight at 4°C. The blots were probed first with anti-HA antibody (Sigma, St. Lois, MO; 1:4,000 in TBST, 0.1% milk) or anti-actin antibody (Cell Signaling Technology, Danvers, MD; 1:2,000 in TBST, 0.1% milk) and then a second antibody, anti-rabbit IgG-horseradish peroxidase conjugate (Cell Signaling Technology; 1:5,000 in TBS Tween). The blots were developed with Amersham ECL Plus reagent (GE Healthcare, Piscataway, NJ) and quantified by ImageJ.
Statistical analysis.
Statistical analyses were conducted with the repeated-measures ANOVA option of GraphPad Software (La Jolla, CA).
Homology model of human PCFT.
A comparative protein model was generated to interpret experimental data in three-dimensional space. Experimentally solved structures were identified as suitable templates from the Protein Data Bank using the sequence of human PCFT with the PSIPRED and HHsearch fold recognition programs (2, 20, 33). Both approaches identified as the most suitable template the glycerol-3-phosphate (GlpT) transporter from Escherichia coli (PDB code 1pw4), sharing ∼14% sequence identity to PCFT (10). The optimal alignment between PCFT and 1pw4 was obtained directly from HHpred and served as input to comparative protein structure modeling with Modeller (27) within the MMM program (25, 26). The quality of the model was verified through energetic analysis using statistical pair potentials implemented in Prosa energy function (32). An additional quality check was performed by predicting the location of transmembrane segments using HMMTOP (34) and comparing it with the location of modeled transmembrane helices in the three-dimensional model.
RESULTS
Identification of an inactivating PCFT mutation with a Val substitution for Gly at position 192.
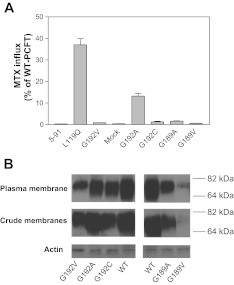
Random mutagenesis was employed by this laboratory for structure-function analyses of PCFT (44). One clone, “5–91,” lost all PCFT function as indicated in Fig. 1A. DNA sequencing revealed two point mutations in the pcft coding region: c.356T>A and c.575G>A. The former mutation resulted in the replacement of Leu with Gln at position 119 (p.L119Q), whereas the latter mutation led to a substitution of Gly by Val at position 192 (p.G192V). Expression vectors for the L119Q and G192V mutants were individually generated. and their functions were assessed separately. The L119Q mutant retained ∼30% of WT-PCFT activity, while the G192V mutant was not functional at all (Fig. 1A). Hence, the G192V mutation was responsible for the complete loss of PCFT function in clone 5–91.
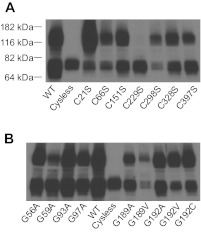
Fig. 1.
Expression and function of G192 and G189 proton-coupled folate transporter (PCFT) mutants. A: [3H]methotrexate ([3H]MTX) influx was assessed at a concentration of 0.5 μM and at pH 5.5 over 1 min. Influx is indicated as percentage of wild-type (WT)-PCFT activity. Data are the means ± SE from 3 separate experiments. B: expression of the G192V, G192C, G192 C, G189A, and G189V mutants and WT-PCFT in the crude membrane preparation and on the plasma membrane as assessed by surface biotinylation. Expression of actin serves as an internal control for the crude membrane samples. Image represents 2 independent blots.
Identification of a functionally critical GXXG motif.
Gly-192 is located in a highly conserved region of the fifth transmembrane domain, within a GXXG sequence motif (Fig. 2). Both Gly192 and Gly189 residues are fully conserved across species to xenopus and zebrafish. The GXXG motif has been established as a Nova Protein K homology (KH) domain, a key RNA binding segment (16) with no known role in the function of membrane transporters. Accordingly, Gly-192 and Gly-189 were further evaluated. First, the Gly-192 residue was replaced with Ala, the closest in physicochemical nature to Gly, with a difference of only one methyl group in the sidechain. However, with this substitution only 13% of WT activity was retained (Fig. 1A). Replacement with Cys is often a well-tolerated substitution that allows analysis of accessibility and modification with sulfhydryl-reacting reagents; however, substitution with this residue resulted in a complete loss of activity (Fig. 1A). Thus the integrity of Gly-192 is essential for PCFT function.
Fig. 2.
Alignment of partial PCFT protein sequences across 8 species. Numbers indicate the position of Gly residues in human PCFT.
PCFT expression in the crude membrane fraction and accessibility on the plasma membrane were determined for the G192V, G192A, and G192C mutants along with WT-PCFT. As indicated in Fig. 1B, all these mutants were expressed in the crude membrane preparation and were accessible to biotinylation at the plasma membrane, indicating that these proteins are synthesized, stable, and traffic to the plasma membrane. The intensity of the protein band from the G192A mutant, which retained partial function, was ∼80% that of WT-PCFT based on quantification of two independent Western blots.
Next, Gly-189 was replaced with either Ala or Val but neither mutant was functional (Fig. 1A). The G189A mutant was expressed but at a lower level that that of WT-PCFT in the plasma membrane and the crude membrane preparation (Fig. 1B). Expression of the G189V mutant was substantially lower than that of WT-PCFT in the crude membrane and plasma membrane preparations. Since replacement of Gly by Ala represents the smallest change possible, the integrity of Gly-189 also appeared to be essential for PCFT function.
Functional roles of a second conserved GXXG and a GXXXG motifs.
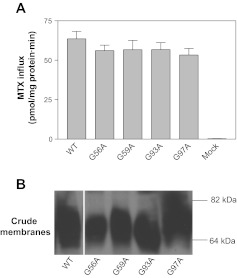
Examination of human PCFT and its alignments to PCFTs from seven other species revealed only one additional fully conserved GXXG motif (Gly-56 to Gly-59). This motif is located in the first extracellular loop connecting the first and second transmembrane domains, based on the established PCFT membrane topology (45). Replacement of either Gly-56 or Gly-59 with Ala did not result in a significant decrease in function relative to WT-PCFT (Fig. 3A) nor did this alter PCFT expression in the crude membrane fraction (Fig. 3B). Therefore, both Gly residues, unlike Gly-189 and Gly-192, are not required for PCFT function.
Fig. 3.
Impact of substitutions of Gly-56, -59,-93 and -97 with Ala on the function and expression of PCFT. A: [3H]MTX (0.5 μM) influx was assessed at pH 5.5 over 1 min. Data are means ± SE from 3 separate experiments. B: expression of the PCFT mutants in the crude membrane preparation. The blot is representative of 3 independent analyses. The white line on the image indicates lanes that have been repositioned.
A related motif GXXXG (two Gly residues separated by any three amino acid residues) has been identified in transmembrane domains of membrane proteins as a dimerization motif (15, 28). PCFT has been reported to form an oligomer (9). PCFT contains only one such motif, Gly-93 to Gly-97, in which both Gly residues are fully conserved among the eight species (Fig. 2). This motif is located within the second transmembrane domain. As indicated in Fig. 3, A and B, substitution of either Gly-93 or Gly-97 with Ala had no impact on PCFT function and expression; the role of these residues in PCFT dimerization is addressed below.
Kinetics of transport mediated by the G192A mutant.
The G192A mutant retained sufficient transport activity to allow analysis of influx kinetics. The MTX influx Vmax mediated by the G192A mutant was 159 ± 26 pmol·mg protein−1·min−1, about half the level of WT-PCFT (305 ± 69 pmol·mg protein−1·min−1). The MTX influx Kt for G192A (15.2 ± 4.8 μM) was increased nine times that of WT-PCFT (1.74 ± 0.3 μM) based on three independent measurements. The 50% decrease in the MTX influx Vmax for the G192A mutant could be explained at least in part by the decrease (∼20%) in protein expressed at the plasma membrane. The marked increase in the MTX influx Kt suggested involvement of the Gly-192 residue in folate substrate binding to the carrier.
Substitution of residues in proximity to Gly-189 and Gly-192 with cysteine.
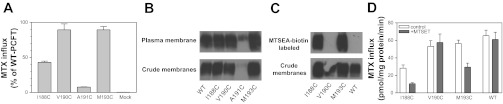
Due to the critical roles of Gly-189 and Gly-192 in PCFT function, residues contiguous to Gly-189 and Gly-192, Ile-188, Val-190, Ala-191, and Met-193 were individually substituted with Cys. Since none of the seven native cysteine residues in PCFT react with membrane-impermeant sulfhydryl-reacting reagents, these mutations could be generated in WT-PCFT for subsequent study with sulfhyryl reagents. As indicated in Fig. 4A, the I188C mutant retained about 50% of WT activity, the V190C and M193C mutants were fully functional, while the A191C mutant had only ∼10% of WT-PCFT activity. As indicated in Fig. 4B, expression of I188C, V190C, and M193C was comparable to that of WT-PCFT in both the crude membrane and the plasma membrane preparations. However, expression of the A191C mutant was markedly decreased.
Fig. 4.
Accessibility and functional consequences of modifications of Cys-substituted residues at position 188, 190, 191, and 193 of human PCFT. A: [3H]MTX (0.5 μM) influx at pH 5.5 over 1 min. Influx mediated by the mutants is indicated as percentage of WT-PCFT activity. B: expression of the mutants in the crude membrane preparation and biotinylated on the plasma membrane. C, Top: labeling of the Cys-substituted PCFT residues with the membrane-impermeable 2-[(biotinoyl)amino]ethyl methanethiosulfonate (MTSEA biotin). Bottom: expression levels of the mutant PCFTs in the crude membrane preparation. D: impact of modification of the Cys-substituted residues with [2-(trimethylammonium)ethyl]methanethiosulfonate bromide (MTSET) on PCFT function. Cysteine modification with MTSET (1 mg/ml) was carried out at room temperature for 30 min. After the modification, [3H]MTX influx (0.5 μM) was assessed at pH 5.5 over 1 min. Influx is expressed as pmol MTX/mg protein/min. For A and D, data are the means ± SE from three independent experiments. For B and C, The blots are representative of 2 independent experiments.
Labeling of the I188C, V190C, or M193C mutants with MTSEA biotin and functional consequences of the MTSET modification.
Since the I88C, V190C, and M193C mutants were functional, it was possible to assess the accessibility of these cysteine residues to the aqueous translocation pathway. The key step in this experimental approach is the labeling of cysteine residues with membrane-impermeable methanethiosulfonate coupled to biotin (MTSEA biotin; Ref. 45). As previously reported and further confirmed in this study, WT-PCFT was not labeled with MTSEA biotin (Fig. 4C). While the I188C and M193C mutants were heavily labeled, the V190C mutant was not labeled at all. Hence, both Ile-188 and Met-193 are accessible to the aqueous extracellular environment despite their location in the mid-region of the fifth transmembrane domain (see below).
Further experiments were conducted to determine whether modification of cysteine residues by MTSET (also a membrane-impermeant methanethiosulfonate compound) in the I188C, V190C, and M193C mutants resulted in a decrease in transport activity. As indicated in Fig. 4D, modification of I188C with MTSET at a concentration of 1 mg/ml reduced activity by ∼60% whereas modification of M193C by this reagent at the same concentration decreased activity by ∼50%. There was no alteration in activity when the V190C mutant was treated with MTSET in a similar way. As expected, WT-PCFT activity was not affected by MTSET. These findings were consistent with the results of labeling with MTSEA biotin, indicating that the substituted cysteine residues of the I188C and M193C mutants can be modified by methanethiosulfonate reagents. This places these residues in a functionally sensitive part of the PCFT protein.
Substrate protection from MTSET inhibition: effect of temperature.
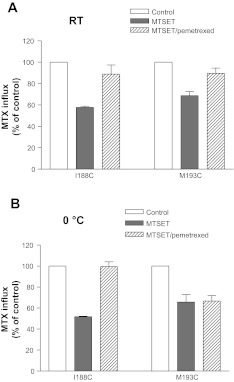
The reduction in activity when the I188C and M193C mutants were modified by MTSET afforded an opportunity to assess if this could be prevented or diminished by PCFT substrate to determine whether these residues are located in or near the folate binding pocket. First, the minimal concentration of MTSET was assessed at which a significant reduction in activity was still achieved for the I188C and M193 PCFT mutants after modification of cysteine residues. This was determined to be 0.064 mg/ml for the I188C mutant and 0.16 mg/ml for the M193C mutant, concentrations 1/16th and 1/6th, respectively, of what was used in the experiments indicated in Fig. 4D. Pemetrexed was employed in this protection assay since it has the highest affinity for PCFT among the folates and antifolates at neutral pH (43). As indicated in Fig. 5A, modification of the I188C mutant with MTSET at room temperature reduced the activity by 40% (P < 0.001) in the absence of pemetrexed; in the presence of pemetrexed the inhibitory effect of MTSET was abolished. Similarly, modification of the M193C mutant with MTSET decreased activity by 30% (P < 0.01) in the absence of pemetrexed but only ∼10% (>0.05) in the presence of pemetrexed compared with the control cells not exposed to either agent. Hence, sulfhydryl modification of the I188C and M193C mutants could be suppressed by the presence of pemetrexed substrate at room temperature.
Fig. 5.
Impact of pemetrexed protection on modification of the Cys-substituted PCFT residues with MTSET at room temperature and at 0°. Cells were incubated with MTSET (0.064 mg/ml for the I188C mutant or 0.16 mg/ml for the M193C mutant) in the presence or absence of 1 mM pemetrexed for 30 min either at room temperature (A) or at 0° (B) before MTX influx was assessed at 0.5 μM and pH 5.5 over 1 min. Control represents cells incubated with buffer. Data are means ± SE from 3 independent experiments.
There are two possible explanations for the pemetrexed protection. Substrate binding could physically prevent access of MTSET to the substituted cysteine residue. Alternatively, a conformational change that occurs after pemetrexed binding could occlude the substituted cysteine residue and make it inaccessible to MTSET, as reported for other transporters (1, 8, 17, 22). Since conformational changes in transporters are reduced at low temperature, the MTSET modification was assessed at ice-water temperature to differentiate between these two possibilities. As indicated in Fig. 5B, modification of the I188C mutant with MTSET under these conditions reduced transport activity by ∼50% (P < 0.001) in the absence of pemetrexed, but there was no inhibition in the presence of pemetrexed. Therefore, there was no difference in substrate protection at the two temperatures. This observation is consistent with a direct interaction between Cys at position 188 and pemetrexed. On the other hand, while MTSET decreased activity of M193C PCFT by ∼40% at 0°C (P < 0.001), there was no difference in the degree of inhibition in the presence pemetrexed. This suggested that a conformational change occurred upon pemetrexed binding to the transporter that prevents access of MTSET to the substituted cysteine residue at position 193.
Assessment of the role of the GXXXG and GXXG motifs in PCFT dimerization.
One common method for assessing oligomerization of transporters is to determine if cysteine residues from different monomers can be cross-linked. For PCFT, none of native cysteine residues are labeled with the hydrophilic methanethiosulfonates since only Cys-66 and Cys-298 are located extracellularly and these residues form a disulfide bridge (45). Therefore, a membrane permeable reagent, MTS-1-MTS, was used in cross-link experiments (9). As indicated in Fig. 6A, a protein band at a molecular size of ∼140 kDa was detected in the WT-PCFT preparation; the intensity of this band was similar to that of the monomer at 70 kDa. This observation is consistent with the formation of a PCFT dimer. As expected, the higher molecular mass band was absent in cysteine-less PCFT, which is functional (45), indicating that one or more of the cysteine residues were cross-linked during the reaction.
Fig. 6.
Cross-linking of human PCFT with 1,1-methanediyl bismethanethiosulfonate (MTS-1-MTS). A: identification of a Cys residue cross-linked in human PCFT. WT- and Cysless-PCFT, along with PCFT mutants in which one of the native Cys residues was replaced with serine, were subjected to the cross-link reaction. Molecular size markers are indicated at left. B: Gly mutants, along with WT-PCFT and Cysless-PCFT, were subjected to the cross-link reaction. The blots are representative of 2 independent experiments.
Studies were conducted to determine which native cysteine residues were responsible for the observed cross-link reaction. The C66S and C298S mutants, which are fully functional, have been generated previously (45). PCFT mutants, in which one of five remaining native cysteine residues were individually replaced with serine, were produced; all had activity comparable to WT-PCFT (data not shown). As shown in Fig. 6A, only the C229S PCFT mutant resulted in the loss of the high molecular mass band, suggesting that only Cys-229 is involved in the cross-linking of PCFT monomers.
GXXXG is a dimerization motif that has been demonstrated in numerous membrane proteins (15, 28). The G93A and G97A PCFT mutants, that are fully functional, were subjected to the cross-linking reaction. As indicated in Fig. 6B, a higher molecular band at ∼140 kDa was present for both mutants implying that neither Gly-93 nor Gly-97 is involved in PCFT dimerization or the change from glycine to alanine is too subtle to disrupt dimerization of the PCFT mutants. The same analysis was also performed for the G56A and G59A PCFT; cross-link products were seen for both mutants. Further, dimerization was also detected for the mutants generated at position 189 and 192. It is of interest that the cross-linking was unperturbed for the G189V mutant despite the much lower level of protein expression. Hence, neither of the conservative GXXG and GXXXG motifs appears to be involved in the dimerization of PCFT. These observations suggest further that gross structural defects were unlikely to be the basis for the complete lack of function of the G189A, G192V, and G192C mutants, since there could be changes in tertiary structure independent of changes in dimerization.
DISCUSSION
These studies identified two Gly residues that play a key role in PCFT function: the Gly-192A mutant retained only 12% of WT-PCFT activity while the G192V mutant was completely inactive. Likewise, the G189A mutant was inactive. Gly residues in polypeptides provide conformational flexibility due to the lack of a side chain and therefore can have important structural and/or functional roles. Gly-80 in the neurotransmitter transporter GAT-1 is involved in conformational transitions during the transport cycle (46), while Gly-121 in the rhodopsin photoreceptor forms part of the retinal binding pocket and interacts directly with the retinal ligand (7). The data presented in the current study suggest that Gly-192 interacts directly with folate substrate. 1) The G192A mutant retained ∼12% the transport activity of WT-PCFT. Kinetic analysis indicated that MTX binding to this mutant was markedly impaired with only a small, if any, effect on the rate of conformational changes associated with the transport cycle. 2) Although Gly-192 is three residues away from Ile-188, it is spatially next to this residue in the helical transmembrane structure. Ile-188 appears to be located in the folate binding site. Substitutions at Gly-189 resulted in complete loss of activity despite its expression at the cell membrane, so that the role of this residue in folate binding could not be determined. However, based on its proximity to Ile-188, Gly-189 may also be critical to folate binding. This residue may play an additional role in the maintenance of PCFT structural integrity since, unlike G192V, expression of the G189V mutant was barely detectable.
Another structural role of Gly residues relates to the GXXXG sequence, which can serve as a dimerization motif in membrane transporters (15, 28). Substitution of either Gly residue of this motif with Ala disrupts or impairs dimerization of glycophorin A (12), a yeast G protein-coupled receptor and a membrane channel formed by Helocobacter pylori vacuolating toxin (19, 21). There is only one such motif in human PCFT (Gly-93 to Gly-97) in which these two Gly residues are conserved across eight species (Fig. 2). This motif in human PCFT does not appear to be involved in dimerization, since substitution of either Gly residue with Ala does not alter formation of a high molecular mass species. However, the possibility that substitution of Gly by Ala does not compromise dimerization of the PCFT mutants, could not be excluded since it has also been reported that an AXXXA motif may stabilize protein folding (11). Further, the substitution of Gly residues with Ala did not have any functional impact; hence, despite their conservation and location in a highly conserved segment of the PCFT protein, these Gly residues do not appear to be essential for the functional integrity of the carrier.
PCFT is reported to form an oligomer (9). The current study identified Cys-229 as the only Cys residue involved in the cross-linking of PCFT since the cross-link was absent in the C229S mutant as well as Cysless-PCFT. This suggests that transmembrane domain 6, harboring Cys-229, in two PCFT molecules interacts to form a dimer. It is not possible, at this point, to address whether dimerization is required for PCFT function since no PCFT mutant has been identified that retains function but lacks a dimer structure.
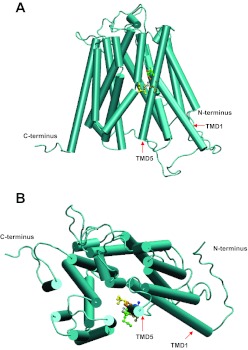
A homology model of PCFT (31, 35), based on the crystal structure of the glycerol-3 phosphate transporter from Escherichia coli, provides a structural explanation for the functional impact of the replacement of the Ile-188, Val-190, Ala-191, and Met-193 residues with Cys. According to this model, PCFT consists of 12 transmembrane domains with its COOH and NH2 termini directed to the cytoplasm (Fig. 7). This is consistent with the topological analysis of this transporter (45). In this model, the transporter is in the inward facing conformation that opens to the cytoplasm (Fig. 7A). Residues 188 to 193 are located in the middle of transmembrane domain 5. 1) Ile-188 (yellow), which was determined experimentally to be in the folate binding pocket, is predicted to be in proximity to the central aqueous translocation pathway. Hence, replacement of this residue with Cys reduced activity by 60% and further modification with MTSET produced an additional decrease in activity to ∼10% that of WT-PCFT. This inhibition by MTSET could be completely obviated, regardless of temperature, by the presence of the PCFT substrate, pemetrexed. 2) Val-190 (brown) appears to be in proximity to the bilayer membrane; replacement of this residue had no impact on function. Consistent with this location, the substituted cysteine residue was not accessible to membrane-impermeant reagents. 3) Ala-191 (blue) is in proximity to the first transmembrane domain. Hence, it appears to be located in a position that would allow participation in helix packing between the fifth and first transmembrane domains. Substitution of this residue with Cys, essentially an addition of a sulfhydryl group on the side chain, may disrupt the interaction between transmembrane helices and reduce stability/expression of this mutant. 4) Met-193 (green) is located more extracellularly also in proximity to the aqueous translocation pathway. It is still accessible to membrane-impermeant reagents. Substitution of this residue with Cys, a change of only a methyl group, had no functional impact. However, modification of the substituted Cys residue with MTSET, with its much larger size and positive charge, reduced the activity of the M193C mutant. Protection of the Cys modification by pemetrexed appeared to require preservation of the mobility of the carrier since it was observed at room temperature but not at 0°. Hence, this residue does not appear to be directly involved in folate binding.
Fig. 7.
A homology model of PCFT showing transmembrane domains (TMD), COOH and NH2 termini, and the locations of the I188, V190, A191, G192, and M193 residues. A: lateral view of the carrier. B: view looking into the aqueous translocation pathway from the extracellular compartment. I188, yellow; G189, grey; V190, brown; A191, blue; G192, orange; and M193, green.
The identification of Ile-188 as a residue that directly interacts with folate substrate, along with the previous identification of Glu-185 as a residue required for proton coupling (36), indicates that the fifth transmembrane domain of human PCFT is directly involved in the binding and translocation of folate and proton. This is consistent with the homology model of PCFT, which places the fifth transmembrane domain as an important contributor to the aqueous translocation pathway. The location of Ile-188 in the middle of this transmembrane domain is consistent with its role in the release of bound folate into either the intracellular or extracellular aqueous pathway as the carrier undergoes conformational changes intrinsic to the alternative access model for facilitative transporters (6).
GRANTS
This work was supported by National Cancer Institute Grant CA-82621.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.Z., D.S.S., and I.D.G. conception and design of research; R.Z. and D.S.S. performed experiments; R.Z., D.S.S., A.F., and I.D.G. analyzed data; R.Z., D.S.S., A.F., and I.D.G. interpreted results of experiments; R.Z. and A.F. prepared figures; R.Z. and A.F. drafted manuscript; I.D.G. edited and revised manuscript; I.D.G. approved final version of manuscript.
REFERENCES
- 1. Androutsellis-Theotokis A, Ghassemi F, Rudnick G. A conformationally sensitive residue on the cytoplasmic surface of serotonin transporter. J Biol Chem 276: 45933–45938, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res 28: 235–242, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desmoulin SK, Wang L, Hales E, Polin L, White K, Kushner J, Stout M, Hou Z, Cherian C, Gangjee A, Matherly LH. Therapeutic targeting of a novel 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate to human solid tumors based on selective uptake by the proton-coupled folate transporter. Mol Pharmacol 80: 1096–1107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diop-Bove N, Kronn D, Goldman ID. Hereditary folate malabsorption (Online). In: GeneReviews, edited by Pagon RA, Bird TD, Dolan CR, Stephens K. Seattle, WA: Unverisity of Washington, Seattle, 2011 [Google Scholar]
- 5. Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther 8: 2424–2431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Forrest LR, Rudnick G. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology (Bethesda) 24: 377–386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han M, Groesbeek M, Sakmar TP, Smith SO. The C9 methyl group of retinal interacts with glycine-121 in rhodopsin. Proc Natl Acad Sci USA 94: 13442–13447, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henry LK, Adkins EM, Han Q, Blakely RD. Serotonin and cocaine-sensitive inactivation of human serotonin transporters by methanethiosulfonates targeted to transmembrane domain I. J Biol Chem 278: 37052–37063, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Hou Z, Desmoulin SK, Etnyre E, Olive M, Hsiung B, Cherian C, Wloszczynski PA, Moin K, Matherly LH. Identification and functional impact of homo-oligomers of the human proton-coupled folate transporter. J Biol Chem 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 301: 616–620, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Kleiger G, Grothe R, Mallick P, Eisenberg D. GXXXG and AXXXA: common alpha-helical interaction motifs in proteins, particularly in extremophiles. Biochemistry 41: 5990–5997, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Langosch D, Brosig B, Kolmar H, Fritz HJ. Dimerisation of the glycophorin A transmembrane segment in membranes probed with the ToxR transcription activator. J Mol Biol 263: 525–530, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Lasry I, Berman B, Glaser F, Jansen G, Assaraf YG. Hereditary folate malabsorption: a positively charged amino acid at position 113 of the proton-coupled folate transporter (PCFT/SLC46A1) is required for folic acid binding. Biochem Biophys Res Commun 386: 426–431, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Lasry I, Berman B, Straussberg R, Sofer Y, Bessler H, Sharkia M, Glaser F, Jansen G, Drori S, Assaraf YG. A novel loss of function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood 112: 2055–2061, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Lemmon MA, Treutlein HR, Adams PD, Brunger AT, Engelman DM. A dimerization motif for transmembrane alpha-helices. Nat Struct Biol 1: 157–163, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Lewis HA, Musunuru K, Jensen KB, Edo C, Chen H, Darnell RB, Burley SK. Sequence-specific RNA binding by a Nova KH domain: implications for paraneoplastic disease and the fragile X syndrome. Cell 100: 323–332, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Lopez-Corcuera B, Nunez E, Martinez-Maza R, Geerlings A, Aragon C. Substrate-induced conformational changes of extracellular loop 1 in the glycine transporter GLYT2. J Biol Chem 276: 43463–43470, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Mahadeo K, Diop-Bove N, Shin D, Unal E, Teo J, Zhao R, Chang MH, Fulterer A, Romero MF, Goldman ID. Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. Am J Physiol Cell Physiol 299: C1153–C1161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McClain MS, Iwamoto H, Cao P, Vinion-Dubiel AD, Li Y, Szabo G, Shao Z, Cover TL. Essential role of a GXXXG motif for membrane channel formation by Helicobacter pylori vacuolating toxin. J Biol Chem 278: 12101–12108, 2003 [DOI] [PubMed] [Google Scholar]
- 20. McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics 16: 404–405, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Overton MC, Chinault SL, Blumer KJ. Oligomerization, biogenesis, and signaling is promoted by a glycophorin A-like dimerization motif in transmembrane domain 1 of a yeast G protein-coupled receptor. J Biol Chem 278: 49369–49377, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Pajor AM, Randolph KM. Conformationally sensitive residues in extracellular loop 5 of the Na+/dicarboxylate co-transporter. J Biol Chem 280: 18728–18735, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127: 917–928, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, Matherly LH, Zhao R, Akabas MH, Goldman ID. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol 293: C1669–C1678, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Rai BK, Fiser A. Multiple mapping method: a novel approach to the sequence-to-structure alignment problem in comparative protein structure modeling. Proteins 63: 644–661, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Rai BK, Madrid-Aliste CJ, Fajardo JE, Fiser A. MMM: a sequence-to-structure alignment protocol. Bioinformatics 22: 2691–2692, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234: 779–815, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Senes A, Engel DE, DeGrado WF. Folding of helical membrane proteins: the role of polar, GxxxG-like and proline motifs. Curr Opin Struct Biol 14: 465–479, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Shin DS, Mahadeo K, Min SH, Diop-Bove N, Clayton P, Zhao R, Goldman ID. Identification of novel mutations in the proton-coupled folate transporter (PCFT-SLC46A1) associated with hereditary folate malabsorption. Mol Genet Metab 103: 33–37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shin DS, Min SH, Russell L, Zhao R, Fiser A, Goldman ID. Functional roles of aspartate residues of the proton-coupled folate transporter (PCFT; SLC46A1); a D156Y mutation causing hereditary folate malabsorption. Blood 116: 5162–5169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shin DS, Zhao R, Yap EH, Fiser A, Goldman ID. A P425R mutation of the proton-coupled folate transporter causing hereditary folate malabsorption produces a highly selective alteration in folate binding. Am J Physiol Cell Physiol 302: C1405–C1412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sippl MJ. Recognition of errors in three-dimensional structures of proteins. Proteins 17: 355–362, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Soding J. Protein homology detection by HMM-HMM comparison. Bioinformatics 21: 951–960, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics 17: 849–850, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J Biol Chem 284: 17846–17857, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. Am J Physiol Cell Physiol 297: C66–C74, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT). Biochim Biophys Acta 1178: 1407–1414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao R, Chattopadhyay S, Hanscom M, Goldman ID. Antifolate resistance in a HeLa cell line associated with impaired transport independent of the reduced folate carrier. Clin Cancer Res 10: 8735–8742, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 31: 177–201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-pH methotrexate transport activity in human solid tumor cells: Contribution to the preservation of methotrexate pharmacological activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res 10: 718–727, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med 11: e4, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, Malatack JJ, Rosenblatt DS, Goldman ID. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood 110: 1147–1152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter (PCFT): impact on pemetrexed transport and on antifolate activities compared with the reduced folate carrier. Mol Pharmacol 74: 854–862, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao R, Shin DS, Diop-Bove N, Ovits CG, Goldman ID. Random mutagenesis of the proton-coupled folate transporter (PCFT, SLC46A1), clustering of mutations and the bases for associated losses of function. J Biol Chem 286: 24150–24158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao R, Unal ES, Shin DS, Goldman ID. Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry 49: 2925–2931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou Y, Kanner BI. Transporter-associated currents in the gamma-aminobutyric acid transporter GAT-1 are conditionally impaired by mutations of a conserved glycine residue. J Biol Chem 280: 20316–20324, 2005 [DOI] [PubMed] [Google Scholar]