Abstract
Selectin-mediated interactions in the vasculature promote metastatic spread by facilitating circulating tumor cell binding to selectin-expressing host cells. Therefore, identifying the selectin ligand(s) on tumor cells is critical to the prevention of blood-borne metastasis. A current challenge is to distinguish between structures expressed by circulating tumor cells that can bind selectins in vitro from the functional ligands whose depletion suppresses selectin-dependent binding under flow in vivo. Interestingly, podocalyxin (PODXL), which can bind E- and L-selectin, is upregulated in a number of cancers, including those of the breast, colon, and pancreas. In this work, we show that metastatic pancreatic cancer cells overexpress PODXL compared with nonmalignant pancreatic epithelial cells. We further demonstrate via tissue microarray that 69% of pancreatic ductal adenocarcinomas stain positive for PODXL. In cases of focal expression, positive staining is restricted to the invasive front of primary tumors. By combining immunoblot, immunodepletion, short-hairpin RNA-mediated gene silencing, and flow-based adhesion assays, we evaluated the functional role of sialofucosylated PODXL in selectin-mediated adhesion under flow. Our data indicate that sialofucosylated PODXL is a functional E- and L-selectin ligand expressed by metastatic pancreatic cancer cells, as specific depletion of this molecule from the cell surface significantly interferes with selectin-dependent interactions. Cumulatively, these data support a correlation between sialofucosylated PODXL expression and enhanced binding to selectins by metastatic pancreatic cancer cells and offer additional perspective on the upregulation of PODXL in aggressive cancers.
Keywords: metastasis, fluid shear, selectins
the selectins are a family of three transmembrane glycoproteins expressed by endothelial cells, leukocytes, and platelets (E-, L-, and P-selectin) which serve primarily as receptors for sialofucosylated oligosaccharides such as sialyl Lewis x (sLex) and its isomer sLea expressed by leukocytes and tumor cells (23, 29, 39). Selectins play a pivotal role in cancer metastasis, as interfering with E-, L-, or P-selectin through function-blocking antibodies or genetic intervention limits the formation of metastatic colonies (4, 5). It is believed that a variety of tumor cells, including those from pancreatic cancers, express sialofucosylated molecules that can bind to selectins under shear flow (26, 33, 42, 46). While sLex is absent from normal pancreatic tissue (35, 37), its expression progressively increases with higher-grade pancreatic intraepithelial neoplasia and pancreatic adenocarcinoma and correlates with massive metastasis and poor prognosis in humans (1, 2, 36, 43).
Podocalyxin (PODXL) is a transmembrane protein that is expressed by a number of human cell types such as hematopoietic progenitors, platelets, and vascular endothelial cells (11, 15, 30, 32). PODXL was first identified in the kidney, where it helps form a highly negatively charged cell surface coat that maintains the filtration slit in the glomerulus (21). The charge repulsion effect responsible for filtration maintenance has also been reported to disrupt cell-cell contact in monolayers of canine kidney and breast cancer cells expressing ectopic PODXL (41, 44).
The clinical significance of PODXL in cancer progression has been investigated in numerous tumor types, including breast, colon, and uterine carcinoma. In uterine endometrioid adenocarcinoma, PODXL expression is correlated with tumor grade (49), while its overexpression is an independent indicator of poor outcome in breast and colorectal carcinoma (24, 41). Specifically, in colorectal cancer, PODXL expression was observed predominantly on the invasive tumor front, suggesting its importance in the metastatic spread of this disease (24). PODXL expression has also been shown to be a diagnostic marker for distinguishing pancreatic ductal adenocarcinomas from carcinomas arising from the biliary and gastrointestinal tracts (34).
Previous work in our laboratory has shown that PODXL expressed by colorectal carcinoma cells can support E- and L-, but not P-, selectin-mediated adhesion. The selectin-binding determinants displayed on PODXL in these cells are located on O-linked glycans but are distinct from those found on PODXL expressed by high endothelial venules (45), suggesting alternative glycosylation patterns of cancer-associated PODXL. However, as argued in the literature, a major challenge for investigators studying selectin ligands is the need to distinguish between structures that can bind selectins in vitro and the functional selectin ligands that actually do support selectin binding under flow conditions in vivo (47). A functional selectin ligand should be expressed in the right place at the right time and bind selectins with relatively high affinity, and its removal should abrogate selectin-mediated adhesive interactions. To date, there is no direct evidence showing that PODXL is functionally important in tumor cell-selectin binding.
In this work, we demonstrate that sialofucosylated PODXL is overexpressed by metastatic pancreatic cancer lines that display E- and L-, but not P-, selectin binding activity, but not by nonmalignant pancreatic epithelial cells. Additionally, we show that 69% of pancreatic ductal adenocarcinomas stain positive for PODXL. The depletion of PODXL from pancreatic tumor cell lysate by either serial immunodepletion or short-hairpin RNA-mediated knockdown effectively eliminates selectin-mediated cell adhesion to immobilized lysate. We also demonstrate that selective knockdown of PODXL significantly affects metastatic pancreatic tumor cell interactions with both E- and L-selectin under physiologically relevant shear flow, thereby showing its functional role in selectin binding. Taken together, these results shed new light on the overexpression of PODXL in cancer and its role in metastatic spread. Additionally, our data will aid in the design of therapeutics aimed at blocking tumor-host cell binding, thereby interfering with metastatic spread without impairing selectin-dependent physiological processes.
EXPERIMENTAL PROCEDURES
Cell culture.
The nonmalignant human pancreatic epithelioid cell line hTERT-HPNE and pancreatic adenocarcinoma cell lines SW1990 and CFPAC-1 were obtained from the American Type Culture Collection (Manassas, VA). The human pancreatic ductal epithelial (HPDE) cell line and pancreatic adenocarcinoma cell lines Pa03C and Pa07C have been previously described (20). Chinese hamster ovary (CHO) cells expressing E-selectin were generously provided by Affymax. hTERT-HPNE cells were cultured in medium containing 3 vol of glucose-free DMEM, 1 vol M3:BaseF (INCELL, San Antonio, TX), 5% FBS, 5.5 mM glucose, 10 ng/ml human recombinant EGF, and 50 μg/ml gentamicin. HPDE cells were cultured in keratinocyte-SFM supplemented with 0.1 ng/ml epidermal growth factor and 30 μg/ml bovine pituitary extract (Invitrogen, Carlsbad, CA). CFPAC-1 cells were cultured in DMEM with 10% FBS and 50 μg/ml gentamicin. SW1990, Pa03C, and Pa07C cells were cultured in DMEM supplemented with 10% FBS and 50 μg/ml gentamicin. CHO-E cells were cultured in DMEM/F-12 (Invitrogen) supplemented with 5% FBS. Before use, cells were harvested via mild trypsinization (0.25% trypsin/EDTA·4Na for 5 min at 37°C) and incubated at 37°C for 2 h to regenerate surface glycoproteins (18, 26, 28).
Flow cytometry.
Surface expression of PODXL was monitored using FITC-conjugated anti-PODXL (clone 53D11; MBL International, Woburn, MA) and matched isotype control monoclonal antibodies (mAbs). All flow cytometry assays were performed using a BD FACSCalibur; data analysis was accomplished using BD CellQuest Pro software (BD Biosciences, San Jose, CA).
Cell lysis and immunodepletion.
Cell lysate was prepared by membrane disruption using 2% Nonidet P-40 followed by differential centrifugation. PODXL was depleted from lysate via serial immunoprecipitation with an anti-PODXL mAb (clone 3D3; Santa Cruz Biotechnology, Santa Cruz, CA) and protein G agarose spheres (Invitrogen; Refs. 9, 12, 13, 33, 45, 46).
SDS-PAGE and immunoblotting.
Protein from cell lysates was diluted with reducing sample buffer and separated via 4–20% SDS PAGE Tris-HCl gels (Bio-Rad Laboratories, Hercules, CA). Resolved proteins were transferred to Immun-blot polyvinylidene difluoride and blocked with StartingBlock (Pierce Biotechnology, Rockford, IL) for 15 min. Immunoblots were stained with anti-PODXL (3D3), anti-sLea (KM231, Millipore), HECA-452 (BD Pharmingen), or anti-β-actin (C4/actin; BD Transduction Laboratories) mAbs, rinsed with TBS/0.1% Tween 20, and incubated with appropriate alkaline phosphatase (AP)- or horseradish peroxidase (HRP)-conjugated secondary antibodies as previously described (13, 33, 45, 46). Western Blue AP substrate (Promega, Madison, WI) or SuperSignal West Pico chemiluminescent substrate (Pierce Biotechnology) was used to develop immunoblots. In select experiments, staining intensity was quantified using ImageJ (National Institutes of Health, Bethesda, MD).
Immunohistochemistry.
Formalin-fixed paraffin-embedded tissue microarrays (TMA) containing tissues obtained from 105 pancreatic ductal adenocarcinoma (PDAC) patients, who underwent surgical resection between 2004 and 2006 at Johns Hopkins Medical Institutions, were analyzed by immunohistochemistry. The tissue microarrays were constructed under the Johns Hopkins Medical Institutions Institutional Review Board and are delinked from any direct patient identifiers. Of each patient normal acinar, normal ducts, and PDAC tissues had been used for the construction of the TMAs. Five-micrometer sections of the TMAs were deparaffinized using routine techniques, and were quenched in 0.3% H2O2 in methanol for 20 min to block endogenous peroxidase. Antigen retrieval was performed in steaming EDTA buffer (pH 8.0) for 40 min. After blocking of nonspecific binding sites by using serum free protein-block (DAKO North America, Carpinteria, CA), the tissues were incubated with a primary antibody against PODXL (dilution 1:100, 1-h incubation at room temperature, HPA002110; Sigma Aldrich, St. Louis, MO). The tissues were then exposed to Post-antibody Blocking and PowerVision+ Poly-HRP-anti-Mouse/Rabbit IgG (Leica Biosystems, Newcastle, UK) for 15 and 30 min, respectively. 3.3-Diamino benzidine tetra-chloride (Leica Biosystems) was used as the chromogen for visualization. Finally, the slides were counterstained with hematoxylin, dehydrated in ethanol, and cleared in xylene. Immunohistochemical labeling was scored by A. Maitra and M. M. Streppel. The normal tissues adjacent to the PDACs functioned as negative controls. The staining was considered positive if the PDAC cells exhibited an intense brown membranous/cytoplasmic staining.
Flow-based adhesion assays.
Nonmalignant pancreatic cells or pancreatic tumor cells suspended at 1×106/ml in D-PBS/0.1% BSA were perfused over immobilized E-, L-, or P-selectin-coated dishes at prescribed wall shear stresses using a parallel plate flow chamber (250-μm channel depth, 5.0-mm channel width; Refs. 7, 13, 46). Where appropriate, the extent of adhesion was quantified by enumerating the total number of binding events in a single field of view during a 2-min period. Nonspecific adhesion was assessed by preincubating selectin-coated dishes with anti-E- or L-selectin function blocking mAb or addition of 5 mM EDTA to the perfusion medium.
Preparation of short interfering RNA oligonucleotides and vector construction.
Short interfering RNA (siRNA) oligonucleotides targeting PODXL were generated using the siRNA design program from Whitehead Institute (Massachusetts Institute of Technology, Cambridge, MA; Ref. 46). The siRNA sequences and a scramble control sequence were used to construct 60 base pair short hairpin RNA (shRNA) oligonucleotides, which were then synthesized (Invitrogen) and ligated into the pSUPER.neo (shRNA) or pSUPER.puro (scramble) expression vector (Oligoengine, Seattle, WA) under the control of the H1 promoter. The following oligonucleotide was used to generate knockdown (underlined, sense/antisense sequences; italicized, loop with linker): 5′-GATCCCCGAGCCAGGATGAGAACAAATTCAAGAGATTTGTTCTCATCCTGGCTCTTTTTA-3′. The ligated product was transformed into DH5α Escherichia coli cells, amplified in the presence of ampicillin, and purified using the EndoFree Maxi kit (Qiagen, Valencia, CA). Oligonucleotide insertion was confirmed by direct sequencing.
Stable PODXL knockdown.
SW1990 or Pa03C cells were plated in T75 flasks, grown to 90% confluence, and transfected with 6 μg of pSUPER.PODXL or pSUPER.scramble using Lipofectamine 2000 (Invitrogen) for 24 h. Upon reaching confluence, cells were passed and 1 × 106 cells were seeded per 15-cm dish in growth medium. After 24 h, the medium was replaced by a fresh aliquot containing 750 μg/ml G418 or 0.5 μg/ml puromycin. Cells were grown continually without passaging for 15 days, replenishing the G418- or puromycin-containing medium every 2–3 days. Single-cell colonies were isolated and maintained in medium containing G418 or puromycin at 375 or 0.25 μg/ml, respectively.
Blot rolling assays.
Immunoblots of cell lysate from SW1990, scramble control, or PODXL-knockdown cells were stained with HECA-452 and rendered translucent by immersion in 90% D-PBS/10% glycerol. The blots were placed under a parallel plate flow chamber and CHO transfectants expressing E-selectin, resuspended at 5×106 cells/ml in D-PBS/0.1% BSA, and perfused at a shear stress of 0.5 dyn/cm2 (9, 13, 33). Molecular mass markers were used as guides to aid placement of the flow chamber overstained bands of interest. The number of interacting cells per lane was averaged over five fields of view (0.55 mm2 each) within each stained region.
Statistical analysis.
Data are presented as means ± SE of n ≥ 3 independent experiments. Two-tailed Student's unpaired t-test was used to determine significance. The statistical significance minimum was set at P < 0.05.
RESULTS
Podocalyxin is overexpressed by metastatic pancreatic tumor cells.
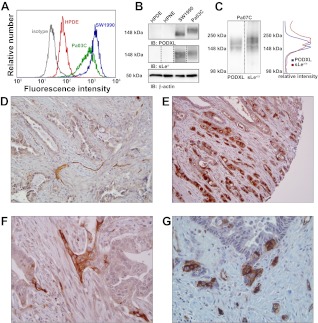
A panel of metastatic pancreatic tumor cell lines was tested for expression of PODXL via flow cytometry using anti-PODXL and corresponding isotype control mAbs. The expression levels were compared with those of two human pancreatic epithelial cell lines, HPDE and hTERT-HPNE. The metastatic lines tested were CFPAC-1 and Pa03C, both isolated from liver metastases of pancreatic tumors; Pa07C, derived from a peritoneal pancreatic metastasis; and SW1990, isolated from the splenic metastasis of a pancreatic tumor. As shown by flow cytometry, SW1990, Pa07C, and Pa03C, but not CFPAC-1, pancreatic cancer cells express high levels of PODXL relative to the HPDE and hTERT-HPNE cells (Table 1; Fig. 1A). These results were confirmed by immunoblot with anti-PODXL and -β-actin mAbs (Table 1; Fig. 1B).
Table 1.
Expression of sialofucosylated PODXL correlates with E- and L-selectin binding
| PODXL Expression |
||||||
|---|---|---|---|---|---|---|
| Cell Line | Origin | FCM | IB | E-Selectin | L-Selectin | P-Selectin |
| HPDE | Normal pancreatic ductal epithelium | 80 | − | + | − | − |
| hTERT-HPNE | Normal pancreatic ductal tissue | 180 | − | − | − | − |
| CFPAC-1 | Liver metastasis from pancreatic tumor | 110 | + | + | + | − |
| Pa07C | Peritoneal metastasis from pancreatic tumor | 310 | + | ++ | ++ | − |
| Pa03C | Liver metastasis from pancreatic tumor | 730 | +++ | ++ | + | − |
| SW1990 | Splenic metastasis from pancreatic tumor | 1,400 | +++ | +++ | +++ | − |
Various pancreatic tumor cell lines were assessed for expression of podocalyxin (PODXL) by flow cytometry (FCM) and immunoblot (IB; examples depicted in Fig. 1). The same cell lines were then exposed to immobilized E-, L-, or P-selectin at 10 μg/ml in a parallel-plate flow chamber to determine their ability to interact with selectins under physiological shear stress (0.5 dyn/cm2). Numbers in FCM column indicate geometric mean of PODXL staining in representative flow cytometry experiment. HPDE, human pancreatic ductal epithelial. +++, Very strong interaction or staining; ++, strong interaction or staining; +, weak interaction or staining; −, no interaction or staining.
Fig. 1.
Podocalyxin (PODXL) is overexpressed by a subset of pancreatic cancer cells. A and B: pancreatic epithelial cell lines human pancreatic ductal epithelial (HPDE; red curve) and hTERT-HPNE and the metastatic lines SW1990 (blue curve) and Pa03C (green curve) were assayed for PODXL expression by flow cytometry (A) or immunoblot (IB; B). Sialofucosylation was probed with sLea immunostaining, while equal loading was verified via β-actin immunostaining. Dotted lines indicate that blots were developed independently. Developing times for sLea immunoblots, moving left to right, were as follows: 30, 30, 10, and 30 min. C: whole cell lysate from Pa07C metastatic pancreatic tumor cells was separated via SDS-PAGE and immunoblotted with anti-PODXL or HECA-452 mAbs. Staining intensity was quantified with ImageJ. D: immunohistochemistry of archival pancreatic ductal adenocarcinoma (PDAC) negative for PODXL (×10 magnification). E: PDAC specimen revealing robust immunolabeling for PODXL. PODXL-expressing cells are seen infiltrating into the pancreatic parenchyma (10× magnification). F and G: upregulation of PODXL is observed specifically in clusters of cells that extend into the peritumoral stroma (incipient invasion; 20× magnification).
Interestingly, immunoblots of SW1990 and Pa03C lysate demonstrated sialofucosylation at a similar molecular weight as PODXL, while HPDE and hTERT-HPNE cells were devoid of such reactivity. As evidenced by the shorter developing time required to generate an approximately equivalent signal, sLea reactivity on PODXL is stronger in SW1990 than Pa03C cells (Fig. 1B). Pa07C metastatic pancreatic tumor cells also displayed sialofucosylation at the same molecular weight as PODXL (Fig. 1C).
To complement these cell line-based results, we evaluated a panel of 105 archival pancreatic ductal adenocarcinomas in a tissue microarray format for PODXL expression. Notably, 72 of these 105 (69%) cases demonstrated robust immunolabeling for PODXL, with membranous localization, consistent with the predicted function of the protein. Most importantly, PODXL immunolabeling was strongest in the clusters of invasive adenocarcinoma cells that permeated into the pancreatic parenchyma (Fig. 1E). Additionally, in ∼34% of cases where PODXL is overexpressed, we observed specific upregulation of within foci of incipient stromal invasion (Fig. 1, F and G), underscoring a putative role for this protein in facilitating tumor progression.
Overexpression of sialofucosylated PODXL correlates with E- and L-selectin binding.
Accumulating evidence reveals the importance of vascular selectins in cancer metastasis (10). We therefore sought to assess the ability for pancreatic tumor cells to interact with selectins under physiologically relevant shear conditions. Both “normal” and pancreatic tumor cells were perfused over immobilized E-, L-, or P-selectin at a shear stress of 0.5 dyn/cm2 in a parallel-plate flow chamber. As summarized in Table 1, cells derived from normal pancreatic tissue expressed low levels of PODXL lacking sialofucosylation. These cells were unable to interact with selectins except for HPDE cells, which exhibited modest binding to immobilized E-, but not L- or P-, selectin. On the other hand, metastatic pancreatic cell lines expressing sialofucosylated PODXL possess E- and L-, but not P-, selectin binding activity. Notably, the presence of EDTA or preincubation with E- or L-selectin antibodies prevented selectin-mediated adhesion (data not shown). Cumulatively, our data suggest a correlation between sialofucosylated PODXL-overexpression on metastatic pancreatic cancer cells and E-/L-selectin binding in shear flow.
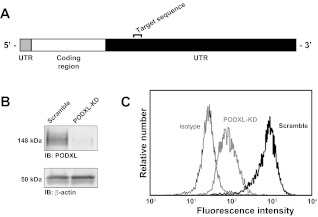
Due to the extensive binding of SW1990 cells with immobilized selectins, these cells were used to further investigate the role of PODXL in tumor cell-selectin binding. We generated stable PODXL-knockdown SW1990 cell lines by transfecting wild-type cells with a PODXL-specific shRNA plasmid, isolating single cell clones, and propagating these clones in G418-containing medium. As depicted in Fig. 2A, the shRNA sequence used to silence PODXL targeted the 3′-untranslated region of the mRNA transcript. Individual clones were screened for PODXL-knockdown via flow cytometry using FITC-conjugated anti-PODXL mAbs. Clones in which PODXL expression was reduced by ≥90% were pooled together and used for subsequent experiments. Knockdown in the clone pool was confirmed through immunoblot (Fig. 2B) and flow cytometry (Fig. 2C). Clones transfected to express a nonspecific, scramble shRNA sequence were used as controls in all experiments alongside PODXL-knockdown cells. With the use of this same strategy, PODXL-knockdown was also established in Pa03C cells (data not shown).
Fig. 2.
Knockdown of PODXL in metastatic pancreatic tumor cells. A: stable knockdown of PODXL in SW1990 cells was achieved through targeting of the 3′-untranslated region (UTR) of the human PODXL mRNA transcript. B: specific knockdown was confirmed through immunoblots stained with anti-PODXL or β-actin. C: >90% knockdown is observed in PODXL-knockdown cells (bold gray curve) compared with scramble control cells (bold black curve). Nonspecific adhesion of FITC-conjugated anti-PODXL antibody was assessed via isotype control (thin black curve).
Immunodepletion and knockdown of PODXL abrogate selectin-mediated cell-binding in a blot rolling assay.
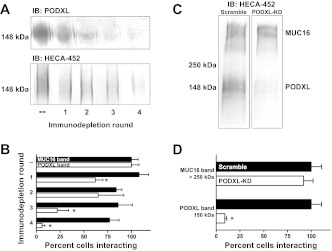
In light of the importance of O-linked mucin-like glycoproteins to metastasis (3), we aimed to directly investigate the importance of PODXL in supporting selectin-dependent adhesion under physiological shear stress. To this end, blot rolling experiments were carried out in which E-selectin expressing CHO (CHO-E) cells were perfused over immunoblots of wild-type or PODXL-depleted cell lysates. Immunoblots were stained with HECA-452, a mAb that binds to sialofucosylated epitopes presented on PODXL such as sLex/a. Depletion of PODXL from lysate was accomplished in two ways: serial immunodepletion of PODXL using an anti-PODXL mAb (Fig. 3, A and B) or shRNA-mediated knockdown of PODXL (Fig. 3, C and D). In each experiment CHO-E binding to the HECA-452-reactive band(s) >250 kDa, which was unchanged by either intervention, was used as control. The upper band(s) correspond to mucin 16 (MUC16), which we (9) have recently identified as an E-/L-selectin ligand expressed by metastatic pancreatic tumor cells.
Fig. 3.
Depletion of PODXL abolishes E-selectin-mediated cell binding in a blot rolling assay. A: anti-PODXL mAb (3D3) was used to immunodeplete PODXL from cell lysate by 4 rounds of sequential immunoprecipitation. Depletion from SW1990 cell lysate was confirmed via immunoblot with anti-PODXL (top) and HECA-452 (bottom). B: effect of PODXL-immunodepletion on E-selectin binding to mucin 16 (MUC16; black bars) and PODXL (white bars) from SW1990 cell lysate was assessed via blot rolling with CHO cells expressing E-selectin (CHO-E). C: HECA-452 reactivity was assessed in SW1990 scramble control (left) and PODXL-KD (right) cells via immunoblot. D: effect of PODXL-KD on CHO-E binding to MUC16 and PODXL from scramble control (black bars) and PODXL-KD (white bars) SW1990 cell lysate was evaluated via blot rolling. CHO-E binding percentages were calculated by comparing the number of cells bound to each band in the PODXL-KD cell blots with those from scramble control cells. Bars depict the average binding from n = 3 experiments; error bars indicate SE. *P < 0.05.
Following four rounds of immunodepletion, the staining intensity of both anti-PODXL and HECA-452 mAbs was virtually eliminated (Fig. 3A). While there is a corresponding decrease in the ability for CHO-E cells to bind to the PODXL band at 150 kDa, the binding to the MUC16 band(s) >250 kDa is statistically indistinguishable through each round of immunodepletion (Fig. 3B). Similarly, the specific knockdown of PODXL results in a dramatic reduction in HECA-452 reactivity at ∼150 kDa (Fig. 3C). This is accompanied by a decrease in the ability for CHO-E cells to bind to immunoblots at 150 kDa. Binding to MUC16, on the other hand, is unchanged by PODXL-knockdown (Fig. 3D).
Sialofucosylated PODXL is a functional E-and L-selectin ligand expressed by metastatic pancreatic tumor cells.
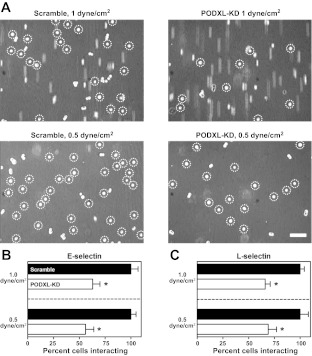
To demonstrate the functional importance of PODXL in tumor cell-selectin binding, we perfused SW1990 scramble control and PODXL-knockdown cells over immobilized E- and L-selectin (10 and 20 μg/ml, respectively) under physiologically relevant levels of shear stress. Figure 4A, top, depicts a representative experiment in which scramble control and PODXL-knockdown cells were allowed to flow over E-selectin for 2 min at a shear stress of 1 dyn/cm2, after which the number of interacting cell singlets was counted. Figure 4A, bottom, shows binding of control and knockdown cells following 2 min at 0.5 dyn/cm2. As expected, the decrease in shear stress is reflected by an increase in the total number of cells bound, an effect that is observed in both control and knockdown cells. In comparing scramble control to PODXL-knockdown cells, we see a marked decrease in E-selectin binding. This decrease is >40% at both shear stresses tested (Fig. 4B).
Fig. 4.
PODXL is a functional selectin ligand expressed by SW1990 metastatic pancreatic tumor cells. Effect of PODXL-KD on E- (A and B) and L-selectin (C) binding was assessed via parallel-plate flow chamber. A: scramble control and PODXL-KD SW1990 cells were flowed overimmobilized E-selectin (10 μg/ml) at shear stresses of 1 and 0.5 dyn/cm2. Micrographs were captured after 2 min, and bound cell singlets are indicated by dashed circles. Scale bar = 100 μm. B and C: scramble control (black bars) or PODXL-KD (white bars) cell singlet binding to E- and L-selectin (10 and 20 μg/ml, respectively) was quantified over n = 3 experiments. Bars represent average binding compared with scramble control cells, error bars indicate SE. *P < 0.05. Control cell binding to E-selectin at 1 and 0.5 dyn/cm2 was 51 ± 4 and 87 ± 7 static cells/mm2, respectively. Control cell binding to L-selectin at 1 and 0.5 dyn/cm2 was 150 ± 15 and 339 ± 43 swift tethering events/mm2, respectively.
To generalize these results, we generated PODXL-KD in Pa03C metastatic pancreatic cancer cells. When these cells were perfused over E-selectin at 0.5 dyn/cm2, there was a statistically significant ∼25% reduction in PODXL-KD cell binding (Fig. 5). The smaller inhibitory effect of PODXL-knockdown on selectin-dependent adhesion observed with Pa03C cells is attributed to the lower PODXL expression levels and weaker sialofucosylation of PODXL relative to SW1990 cells.
Fig. 5.
PODXL is a functional E-selectin ligand expressed by Pa03C metastatic pancreatic tumor cells. Effect of PODXL-KD on E-selectin binding was assessed via parallel-plate flow chamber. A: scramble control and PODXL-KD Pa03C cells were flowed over immobilized E-selectin (10 μg/ml) at a shear stress 0.5 dyn/cm2. Scramble control (black bars) or PODXL-KD (white bars) cell singlet binding to E-selectin was quantified over n = 3 experiments. Bars represent average binding compared with scramble control cells, error bars indicate SE. *P < 0.05. B: micrographs were captured after 2 min and bound cell singlets are indicated by dashed circles. Scale bar = 100 μm. Control cell binding to E-selectin was 53 ± 6 static cells/mm2.
Different from E-selectin, which typically supports stationary adhesion, L-selectin mediates swift tethering events with cells expressing L-selectin ligands. We show PODXL to play a significant, functional role in SW1990 metastatic tumor cell tethering to L-selectin, as the number of interacting cells is decreased by >30% following PODXL depletion. As noted with E-selectin, this effect is observed at both 1 and 0.5 dyn/cm2 (Fig. 4C).
DISCUSSION
There is an extensive body of evidence indicating that the specific interactions between circulating tumor cells and vascular selectins, which recognize sialofucosylated oligosaccharides like sLex/a, represent a vital step in the metastatic cascade. sLex is not present in healthy pancreatic tissue, but its expression increases with tumor progression and correlates with metastasis and poor prognosis in humans (1, 2, 35–37, 43). Interfering with selectin-tumor cell binding has been shown to prevent metastasis, as preincubation with a soluble E-selectin prevents the arrest in and colonization of lung tissue by colon carcinoma cells (27). Similarly, L-selectin knockout mice exhibit markedly reduced metastatic burden following injection of tumor cells (4).
The functional importance of E-selectin in metastasis is in the direct binding of tumor cells to activated vascular endothelium, which expresses E-selectin. The role of L-selectin in cancer metastasis, on the other hand, is less developed. It is believed that tumor cells expressing L-selectin ligands can form complexes with platelets and leukocytes. These multicellular aggregates are prone to arrest in the microvasculature of distant organs, allowing for subsequent extravasation and colonization of secondary sites (18, 19). Laubli et al. (25) show that leukocyte L-selectin may also enhance metastasis by interacting with endothelial L-selectin ligands induced proximal to established tumor cell emboli. Interestingly, this work demonstrates that glycosaminoglycans are capable of interfering with L-selectin-dependent binding in vivo, as treatment with heparin 6–18 h following tumor cell injection suppresses metastasis mainly by preventing the interactions between leukocytes and the L-selectin ligands upregulated by endothelial cells adjacent to established tumor cells (25). Therefore, PODXL-expressing tumor cells can facilitate metastasis through both E- and L-selectin-dependent mechanisms.
Our results indicate that the functional selectin ligand PODXL is expressed by a majority of primary pancreatic cancer specimens (69%). In cases with focal expression, PODXL upregulation was typically restricted to the invasive front, and, in ∼34% of all PODXL-positive cases, to foci of incipient invasion. We also show by flow cytometry and immunoblot that a number of metastatic pancreatic cancer cell lines overexpress PODXL (Fig. 1, A–C). These observations, along with others showing that PODXL expression in primary tumors is focused at the leading edge of developing tumors (24), suggest a putative link between PODXL-overexpression and metastasis. This link is strengthened by studies showing that ectopic PODXL overexpression augments the invasive potential of human tumor cells and canine kidney cells (17, 40). PODXL was also shown to be vital in epithelial-to-mesenchymal transition (31), a process often associated with metastasis (48).
Previous studies (3) have shown the removal of O-linked mucin-like glycoproteins from tumor cells resulted in a pronounced decrease in experimental murine metastasis. At the same time, sialofucosylated oligosaccharides such as sLex/a, expressed on PODXL through O-linked glycans (45), are recognized by selectins. Overexpression of these epitopes is correlated with tumor progression and poor overall prognosis (22). Furthermore, treatment of metastatic SW1990 pancreatic cancer cells with an anti-sLea antibody prior to their inoculation to the spleen of nude mice inhibited the development of liver metastasis (16). Despite these observations, the role of PODXL in adhesive behavior of tumor cells has been largely ignored in the literature. While we (45) have previously shown that purified PODXL can interact with E- or L-selectin, ultimately the challenge is to distinguish molecules that can support selectin binding from those that do so under dynamic fluid conditions similar to those encountered in vivo (47).
We assess this functionality through depletion of the putative selectin ligand PODXL from the cell surface via shRNA. The effect of this intervention on cell binding to immobilized selectins under shear flow is subsequently tested via parallel plate flow chamber assays. Our results indicate that knockdown of PODXL in metastatic pancreatic cancer cells markedly suppresses E- and L-selectin binding, thereby demonstrating the functional importance of sialofucosylated PODXL in selectin-dependent adhesion in shear flow.
While knockdown of PODXL does result in a significant decrease in E- and L-selectin binding, residual adhesive interactions are retained after knockdown in pancreatic cancer cells. We have recently identified MUC16, a molecule highly expressed by SW1990 and Pa03C cells, as a functional E- and L-selectin ligand (9). As a result, we hypothesize that much of the residual E- and L-selectin binding following PODXL knockdown may be attributed to MUC16. It is unlikely that there are additional glycoprotein selectin ligands expressed by these cells, as individual depletion of MUC16 (9) or PODXL (Fig. 3) effectively eliminates CHO-E binding at the respective HECA-452-reactive bands in pancreatic tumor cell lysate. In addition to sialofucosylated glycoproteins, it has been shown that glycolipids can contribute to E-selectin binding (6, 8, 38). Glycosylated lipids, then, are also likely contributors to the residual E-selectin binding observed in PODXL-knockdown cells.
Although glycolipids have been shown to lack L-selectin binding activity in colon cancer cells (8), this does not rule out the possibility that glycolipids in pancreatic cancer cells may contribute to the L-selectin interactions we observe here. Additionally, as L-selectin interacts with sulfated, sialylated, and fucosylated Lex epitopes, it is possible that secondary L-selectin ligands that are not HECA-452-reactive are expressed by pancreatic cancer cells. Experiments with HEC-GlcNAc-6-O-sulfotransferase (the enzyme responsible for sulfation of Lex epitopes)-deficient mice have also suggested the existence of alternative L-selectin ligands whose nature has not yet been fleshed out (14). SW1990 and Pa03C cells may also express these secondary ligands, accounting for the remaining L-selectin binding activity following PODXL-knockdown.
We have demonstrated that PODXL expressed by metastatic pancreatic cancer cells possesses functional E- and L-selectin ligand activity. PODXL isolated via immunoblot supports E-selectin-mediated binding under physiologically relevant flow conditions. Importantly, shRNA-mediated knockdown of PODXL significantly reduces tumor cell interaction with immobilized E- and L-selectin. These results offer a novel perspective on both the role of selectins in cancer metastasis and the overexpression of PODXL by metastatic tumor cells. Additionally, these data support PODXL as a viable target for the imaging and targeting of metastatic and circulating tumor cells.
GRANTS
This work was supported by National Cancer Institute Grants R01-CA-101135 and U54- CA-143868.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.R.D., S.-H.C., and K.K. conception and design of research; M.R.D., S.-H.C., M.M.S., S.S., and A.M. performed experiments; M.R.D., S.-H.C., M.M.S., S.S., and A.M. analyzed data; M.R.D., S.-H.C., M.M.S., S.S., A.M., and K.K. interpreted results of experiments; M.R.D. prepared figures; M.R.D. and K.K. drafted manuscript; M.R.D., S.-H.C., M.M.S., A.M., and K.K. edited and revised manuscript; M.R.D., S.-H.C., M.M.S., S.S., A.M., and K.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Affymax for the E-selectin-transfected CHO cells and Cassandra Skittle for critical input to this work.
REFERENCES
- 1. Aubert M, Panicot-Dubois L, Crotte C, Sbarra V, Lombardo D, Sadoulet MO, Mas E. Peritoneal colonization by human pancreatic cancer cells is inhibited by antisense FUT3 sequence. Int J Cancer 88: 558–565, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Aubert M, Panicot L, Crotte C, Gibier P, Lombardo D, Sadoulet MO, Mas E. Restoration of α(1, 2) fucosyltransferase activity decreases adhesive and metastatic properties of human pancreatic cancer cells. Cancer Res 60: 1449, 2000 [PubMed] [Google Scholar]
- 3. Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA 98: 3352–3357, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci USA 99: 2193–2198, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer 71: 612–619, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Burdick MM, Bochner BS, Collins BE, Schnaar RL, Konstantopoulos K. Glycolipids support E-selectin-specific strong cell tethering under flow. Biochem Biophys Res Commun 284: 42–49, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Burdick MM, Konstantopoulos K. Platelet-induced enhancement of LS174T colon carcinoma and THP-1 monocytoid cell adhesion to vascular endothelium under flow. Am J Physiol Cell Physiol 287: C539–C547, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Burdick MM, McCaffery JM, Kim YS, Bochner BS, Konstantopoulos K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am J Physiol Cell Physiol 284: C977–C987, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Chen SH, Dallas MR, Balzer EM, Konstantopoulos K. Mucin 16 is a functional selectin ligand on pancreatic cancer cells. FASEB J 26: 1349–1359, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheung LS, Raman PS, Balzer EM, Wirtz D, Konstantopoulos K. Biophysics of selectin-ligand interactions in inflammation and cancer. Phys Biol 8: 015013, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, Graf T, McNagny KM. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med 194: 13–27, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanley WD, Burdick MM, Konstantopoulos K, Sackstein R. CD44 on LS174T colon carcinoma cells possesses e-selectin ligand activity. Cancer Res 44: 5812–5817, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Hanley WD, Napier SL, Burdick MM, Schnaar RL, Sackstein R, Konstantopoulos K. Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. FASEB J 20: 337–339, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Hemmerich S, Bistrup A, Singer M, Zante AV. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity 15: 237–247, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Horvat R, Hovorka A, Dekan G, Poczewski H, Kerjaschki D. Endothelial cell membranes contain podocalyxin–the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol 102: 484–491, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hosono J, Narita T, Kimura N, Sato M, Nakashio T, Kasai Y, Nonami T, Nakao A, Takagi H, Kannagi R. Involvement of adhesion molecules in metastasis of SW1990, human pancreatic. J Surg Oncol 67: 77–84, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Hsu YH, Lin WL, Hou YT, Pu YS, Shun CT, Chen CL, Wu YY, Chen JY, Chen TH, Jou TS. Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac1 guanine nucleotide exchange factor ARHGEF7. Am J Pathol 176: 3050–3061, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jadhav S, Bochner BS, Konstantopoulos K. Hydrodynamic shear regulates the kinetics and receptor specificity of polymorphonuclear leukocyte-colon carcinoma cell adhesive interactions. J Immunol 167: 5986–5993, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Jadhav S, Konstantopoulos K. Fluid shear- and time-dependent modulation of molecular interactions between PMNs and colon carcinomas. Am J Physiol Cell Physiol 283: C1133–C1143, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Jones S, Zhang X, Parsons DW, Lin JCH, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321: 1801–1806, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin–the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 98: 1591–1596, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J 14: 569–576, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Konstantopoulos K, Kukreti S, McIntire LV. Biomechanics of cell interactions in shear fields. Adv Drug Deliv Rev 33: 141–164, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Larsson A, Johansson ME, Wangefjord S, Gaber A, Nodin B, Kucharzewska P, Welinder C, Belting M, Eberhard J, Johnsson A, Uhlén M, Jirström K. Overexpression of podocalyxin-like protein is an independent factor of poor prognosis in colorectal cancer. Br J Cancer 105: 666–672, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laubli H, Stevenson JL, Varki A, Varki NM, Borsig L. L-selectin facilitation of metastasis involves temporal induction of Fut7-dependent ligands at sites of tumor cell arrest. Cancer Res 66: 1536–1542, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Mannori G, Crottet P, Cecconi O, Hanasaki K, Aruffo A, Nelson RM, Varki A, Bevilacqua MP. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Res 55: 4425, 1995 [PubMed] [Google Scholar]
- 27. Mannori G, Santoro D, Carter L, Corless C, Nelson RM, Bevilacqua MP. Inhibition of colon carcinoma cell lung colony formation by a soluble form of E-selectin. Am J Pathol 151: 233–243, 1997 [PMC free article] [PubMed] [Google Scholar]
- 28. McCarty OJT, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood 96: 1789, 2000 [PubMed] [Google Scholar]
- 29. McEver RP. Selectin-carbohydrate interactions during inflammation and metastasis. Glycoconj J 14: 585–591, 1997 [DOI] [PubMed] [Google Scholar]
- 30. McNagny KM, Pettersson I, Rossi F, Flamme I, Shevchenko A, Mann M, Graf T. Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J Cell Biol 138: 1395–1407, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meng X, Ezzati P, Wilkins J. Requirement of podocalyxin in TGF-beta induced epithelial mesenchymal transition. PLos One 6: e18715, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miettinen A, Solin ML, Reivinen J, Juvonen E, Väisänen R, Holthöfer H. Podocalyxin in rat platelets and megakaryocytes. Am J Pathol 154: 813–822, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Napier SL, Healy ZR, Schnaar RL, Konstantopoulos K. Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: the roles of CD44v and alternative sialofucosylated selectin ligands. J Biol Chem 282: 3433–3441, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Ney JT, Zhou H, Sipos B, Büttner R, Chen X, Klöppel G, Gütgemann I. Podocalyxin-like protein 1 expression is useful to differentiate pancreatic ductal adenocarcinomas from adenocarcinomas of the biliary and gastrointestinal tracts. Hum Pathol 38: 359–364, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Park HU, Kim JW, Kim GE, Bae HI, Crawley SC, Yang SC, Gum JR, Batra SK, Rousseau K, Swallow DM, Sleisenger MH, Kim YS. Aberrant expression of MUC3 and MUC4 membrane-associated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas 26: e48–e54, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Pérez-Garay M, Arteta B, Pagès L, de Llorens R, de Bolòs C, Vidal-Vanaclocha F, Peracaula R. alpha2,3-sialyltransferase ST3Gal III modulates pancreatic cancer cell motility and adhesion in vitro and enhances its metastatic potential in vivo. PLos One 5: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Satomura Y, Sawabu N, Takemori Y, Ohta H, Watanabe H, Okai T, Watanabe K, Matsuno H, Konishi F. Expression of various sialylated carbohydrate antigens in malignant and nonmalignant pancreatic tissues. Pancreas 6: 448–458, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Shirure VS, Henson Ka Schnaar RL, Nimrichter L, Burdick MM. Gangliosides expressed on breast cancer cells are E-selectin ligands. Biochem Biophys Res Commun 406: 423–429, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng 7: 151–185, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Sizemore S, Cicek M, Sizemore N, Ng KP, Casey G. Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with ezrin. Cancer Res 67: 6183–6191, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Somasiri A, Nielsen JS, Makretsov N, McCoy ML, Prentice L, Gilks CB, Chia SK, Gelmon Ka Kershaw DB, Huntsman DG, McNagny KM, Roskelley CD. Overexpression of the anti-adhesin podocalyxin is an independent predictor of breast cancer progression. Cancer Res 64: 5068–5073, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Stone J, Wagner D. P-selectin mediates adhesion of platelets to neuroblastoma and small cell lung cancer. J Clin Invest 92: 804, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takahashi S, Oda T, Hasebe T, Sasaki S, Kinoshita T, Konishi M, Ueda T, Nakahashi C, Ochiai T, Ochiai A. Overexpression of sialyl Lewis x antigen is associated with formation of extratumoral venous invasion and predicts postoperative development of massive hepatic metastasis in cases with pancreatic ductal adenocarcinoma. Pathobiology 69: 127–135, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Takeda T, Go WY, Orlando Ra, Farquhar MG. Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell 11: 3219–3232, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thomas SN, Schnaar RL, Konstantopoulos K. Podocalyxin-like protein is an E-/L-selectin ligand on colon carcinoma cells: comparative biochemical properties of selectin ligands in host and tumor cells. Am J Physiol Cell Physiol 296: C505–C0513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E-and L-selectin in shear flow. J Biol Chem 283: 15647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Varki A. Selectin ligands: will the real ones please stand up? J Clin Invest 99: 158–162, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol 11: 1487–1495, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Yasuoka H, Tsujimoto M, Inagaki M, Kodama R, Tsuji H, Iwahashi Y, Mabuchi Y, Ino K, Sanke T, Nakamura Y. Clinicopathological significance of podocalyxin and phosphorylated ezrin in uterine endometrioid adenocarcinoma. J Clin Pathol 65: 399–402, 2012 [DOI] [PubMed] [Google Scholar]