Abstract
Coupled gating (synchronous openings and closures) of groups of skeletal muscle ryanodine receptors (RyR1), which mimics RyR1-mediated Ca2+ release underlying Ca2+ sparks, was first described by Marx et al. (Marx SO, Ondrias K, Marks AR. Science 281: 818–821, 1998). The nature of the RyR1-RyR1 interactions for coupled gating still needs to be characterized. Consequently, we defined planar lipid bilayer conditions where ∼25% of multichannel reconstitutions contain mixtures of coupled and independently gating RyR1. In ∼10% of the cases, all RyRs (2–10 channels; most frequently 3–4) gated in coupled fashion, allowing for quantification. Our results indicated that coupling required cytosolic solutions containing ATP/Mg2+ and high (50 mM) luminal Ca2+ (Calum) or Sr2+ solutions. Bursts of coupled activity (events) started and ended abruptly, with all channels activating/deactivating within ∼300 μs. Coupled RyR1 were heterogeneous, where highly active RyR1 (“drivers”) seemed open during the entire coupled event (Po = 1), while other RyR1s (“followers”) displayed abundant flickering and smaller amplitude. Drivers mean open time increased with cytosolic Ca2+ (Cacyt) or caffeine, whereas followers flicker frequency was Cacyt independent and more sensitive to inhibition by cytosolic Mg2+. Coupled events were insensitive to varying lumen-to-cytosol Ca2+ fluxes from ∼1 to 8 pA, which does not corroborate coupling of neighboring RyR1 by local Ca2+-induced Ca2+ release. However, coupling requires specific Calum sites, as it was lost when Calum was replaced by luminal Ba2+ or Mg2+. In summary, coupled events reveal complex interactions among heterogeneous RyR1, differentially modulated by cytosolic ATP/Mg2+, Cacyt, and Calum, which under cell-like ionic conditions may parallel synchronous RyR1 gating during Ca2+ sparks.
Keywords: calcium release channels, excitation contraction coupling, calcium signaling
in striated muscle, the ryanodine receptor isoform 1 (RyR1) at the terminal cisternae (TC) of sarcoplasmic reticulum exists in ordered arrays, where each RyR1 channel is closely associated with neighboring RyR1s, providing the opportunity to interact (25, 26, 51, 58). Indeed, RyR1-mediated calcium (Ca2+) sparks are likely generated by brief and synchronous activation/deactivation of multiple channels at an individual sarcoplasmic retiticulum (SR) Ca2+ release site (54, 74, 77). The functional properties of TC-clustered RyR1s in intact cells are difficult to predict, as most of the available analysis and modeling of RyR1s has been performed on single channels isolated in vitro. Several theoretical constraints, including some type of cooperative interactions between RyR1 channels (67, 70), seem to be required to explain the characteristics of Ca2+ sparks and the process of excitation-contraction coupling (Ca2+-induced Ca2+ release gain, termination, reversibility, etc.).
For many years, RyR research has focused on single channels (11, 41, 62, 64, 69). The first robust evidence of cooperative interactions among multiple RyR1 channels was reported by Marks et al. (38, 39), who observed that several (2–6) neighboring RyR1 (or RyR2) channels can be found to literally open and close simultaneously. Subsequently, our preliminary communications (8, 48) and publications by others (34) confirmed the existence of coupled gating and suggested that coupling may be susceptible to modulation by endogenous factors (luminal Ca2+ and cytosolic ATP/Mg2+). A deeper exploration of the mechanisms underlying coupled gating may explain the synchronicity of RyR1 channel function in cells during Ca2+ sparks (5). However, reports on RyR1 coupled gating are scarce, which may raise questions regarding the significance of these events for Ca2+ signaling in cells. We think that the frequency of coupled gating depends on the purification techniques utilized for RyR1 isolation and the experimental conditions utilized to study the channels. In this work, we identify specific isolation and experimental conditions to reconstitute groups of RyR1 channels and observe coupled gating with a relatively high frequency. Although the ionic composition of the solutions used in this study did not mimic the intracellular environment (in terms of the luminal Ca2+ levels and pH), we defined some of the characteristics of modulation of coupled RyR1s by luminal ions, as well as by cytosolic Ca2+, caffeine, and ATP/Mg2+.
MATERIALS AND METHODS
Drugs and chemicals.
CaCl2 standard for Ca2+ calibration was from Word Precision Instruments (Sarasota, FL). Phospholipids were obtained from Avanti (Alabaster, AL). All other drugs and chemicals were from Sigma-Aldrich (St. Louis, MO) or were reagent grade.
SR vesicle preparation.
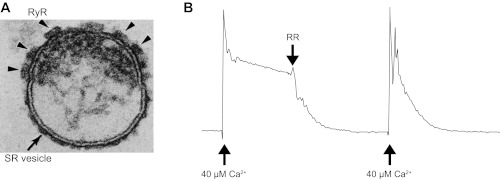
All procedures with animals were designed to minimize pain and suffering and conformed to the guidelines of the National Institutes of Health. Southern Illinois University School of Medicine animal research procedures have Association for Assessment and Accreditation of Laboratory Animal Care accreditation and Public Health Safety Assurances No. 000551 and A3209-01, respectively. The Laboratory Animal Care and Use Committee of Southern Illinois University School of Medicine reviewed and approved the protocols for animal use in our laboratory (196-05-021 and 196-11-010). The best preparations for obtaining coupled channels were microsomes enriched in terminal cisternae fraction (TC microsomes) isolated from predominantly fast-twitch skeletal muscle (back and leg; adult New Zealand white rabbits), as previously described (59). Electron microscopy images of SR microsomes (Fig. 1A), as utilized here, have shown a clear abundance of intact TC membranes with a preserved junctional face membrane containing arrays of RyR1 channels (20, 59). The SR microsomes also have high levels of RyR1-mediated Ca2+ leak (Fig. 1B). All preparations were aliquoted in cryovials (300 μl each) and kept in liquid N2 (safer long-term storage). Every month, a few cryovials were used to generate smaller TC aliquots (15 μl each) that were stored at −80°C for easy access. For experiments, aliquots were quickly thawed in water, kept on ice, and used within 3–5 h.
Fig. 1.
Characteristics of sarcoplasmic reticulum (SR) microsomes utilized for coupled ryanodine receptors isoform 1 (RyR1) gating studies. A: electron micrograph (250 × 200 nm) of a terminal cisternae vesicle of skeletal muscle SR. Cross section shows an isolated terminal cisternae of SR vesicle, typical of the field of SR R4 fractions. Junctional face membrane (the upper membrane) contains the foot structures (arrowheads) that have been identified as the RyR1. Tangential section of the junctional face membrane (not shown) reveals that the foot structures are oriented in a 2-dimensional interconnected quasi-checkerboard array (20–23, 58, 59). B: net Ca2+ loading rate of SR was measured by ultraviolet spectrometry using antipyrylazo III as described previously (3, 13). Fifty micrograms of SR microsomes were added to 1 ml of buffer containing 100 mM K-phosphate, 2 mM MgCl2, 1 mM ATP, and 0.5 mM antipyrylazo III to monitor Ca2+ levels as the increase in absorbance between 710 and 790 nm (3, 13). The total recording time was 8 min. After CaCl2 was increased to 40 μM, the Ca2+ uptake rate calculated in this example was 0.204 μmol Ca2+·min−1·mg protein−1 (control conditions). Addition of the RyR1 blocker ruthenium red (RR; 5 μM), increased the rate of loading to 1.28 μmol Ca2+·min−1·mg protein−1, indicating that most of the leak was mediated by RyR1 channels. In general, the R4 fractions used here had 15–20 pmol [3H]ryanodine binding per mg protein. Loading rates of the R4 fractions were >1 μmol Ca2+ min−1 mg protein−1 in the presence of RR and their loading ratio (absence of RR/presence of RR) was lower than 0.25. The numbers indicate a significant level of intact vesicles (that can uptake Ca2+) and a high density of RyR1 channels (that produce the leak).
RyR channel recordings.
Groups of RyR1s were reconstituted into planar lipid bilayers (5:4:1 mixture of bovine brain phosphatidylethanolamine, phosphatidylserine, and phosphatidylcholine; 45–50 mg/ml in decane) as previously described (9). Briefly, planar lipid bilayers were formed on 80- to 150 μm-diameter circular holes in Teflon septa, separating two 1.1-ml compartments. The trans-compartment was filled with HEPES-Ca2+ solution [250 mM HEPES and 53 mM Ca(OH)2 pH 7.4] and clamped at 0 mV by an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA). The cis-compartment (ground) was filled with HEPES-Tris solution (250 mM and HEPES 120 mM Tris pH 7.4). To promote “intact” SR vesicle fusion with bilayers, 250–500 mM CsCl and 1 mM CaCl2 were added to the cis-solution with SR microsomes (5–10 μg) (9). After currents were observed (>100 pA at 0 mV; mixture of Cs and Cl− currents), the cis-chamber was perfused with HEPES-Tris solution for 10 min at 4 ml/min. Mixtures of BAPTA and dibromoBAPTA were used to buffer free Ca2+ and Mg2+ on the cytosolic surface of the channel. Standard solutions of Ca2+, Mg2+, BAPTA, and diBromoBAPTA were utilized to prepare the mixtures required to obtain specific concentrations of free cytosolic Ca2+ (Ca2+cyt) and free Mg2+ (Mg2+cyt) in Mg2+/ATP mixtures. As previously performed, free Ca2+ and Mg2+ levels in solution were calculated using Winmaxc2.5 (http://www.stanford.edu/∼cpatton/maxc.html). Free Ca2+ and Mg2+ levels in the mixtures were confirmed with fluo-3 fluorescence or mag-fura-2 fluorescence, respectively. In Mg2+-free solutions, Ca2+ levels were also estimated with Ca2+ electrodes. In some experiments, the trans-chamber was also perfused for 10 min at 4 ml/min with HEPES-Mn+ solution (where Mn+ was Ba2+, Mg2+, Sr2+, or Cs+; Ref. 14). As previously described (9), RyR1 channels were identified by slope conductance (∼100 pS), reversal potential (−40 mV; trans-cis), as well as by the response to diagnostic agonists, blockers, and conductance modifiers. RyR1 channel currents, depicted as positive (upward) deflections in results, reflect cation flux (mostly Ca2+) from the trans (luminal)- to the cis (cytosolic)-compartment.
Except when indicated otherwise, RyR1 recordings (4–8 min in duration at each experimental condition) were carried out at 0 mV, filtered through a low-pass Bessel filter at 1 kHz, digitized at 20 kHz with a Digidata 1320 (Axon Instruments) and stored on DVD for computer analysis. The pClamp9 software (Axon Instruments) was utilized to calculate global open probability [(nPo)global] of channels under all conditions tested. To estimate the degree of coupling, two parameters have been defined (see appendix for details). One of them, the cooperativity ratio (CR), is independent of channel Po and can be calculated based on the current amplitude distribution histograms (31). Here, we calculated the CR in recordings lasting 4 min or more by examining the current levels 0, 1, and 2. Thus we estimated
where Φs are the areas measured for each peak (corresponding to the current levels 0, 1, and 2) in the all-point amplitude distribution histograms and k is the maximal number of channels observed (estimated from 8-min recordings in presence of 5 mM ATP and 1 μM Ca2+). CR <0.25 is considered evidence of coupled gating; see appendix.
The duration and amplitude of coupled events were measured with pClamp9 by using the 50%-threshold method between the baseline and the amplitude of the first level of current (i.e., a coupled event was defined as the period between a transition from zero to some level of multichannel activity and the consecutive transition to the 0 level). For these events, we estimated the mean weighted Ca2+ current (ĪW) as follows:
where Ii is the amplitude of the i event, during which at least one channel remained active (determined with a 50% threshold) and where Di is the respective event duration. ĪW is estimated from all the events observed in 4- to 8-min recordings. From ĪW, we obtained the (nPo)event, which represents the level of RyR1 activity within an event and is derived from
where A1 is the amplitude of the first current level and A2 + k is the average amplitude of openings observed in levels 2 to k. We consider that a group of channels behave in a cooperative manner when the average (nPo)event ± SE obtained during a 4-min recording is >10% higher than the maximal value expected for independent channels. A detailed description of these estimators of cooperativity is presented in the appendix.
Statistical analysis.
Data are presented as means ± SE of n measurements. Statistical comparisons between groups were performed with Student's t-test of paired samples. Differences were considered statistically significant at P < 0.05.
RESULTS
Coupled RyR1 gating requires cytosolic solutions containing Mg2+/ATP.
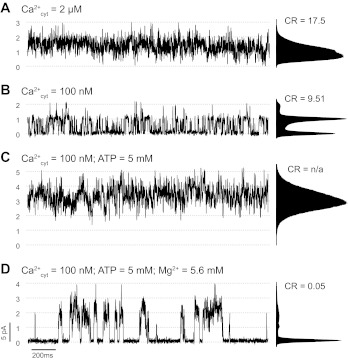
Figure 2 shows a multichannel recording collected after reconstituting RyR1s from TC vesicles with high level of integrity as evaluated with the procedure described in materials and methods and illustrated in Fig. 1. Dotted lines represent multiples of the single-channel full conductance level. In the presence of 2 μM cytosolic free Ca2+, multiple RyR1 channels are active and gate independently (Fig. 2A). Lowering the cytosolic free Ca2+ concentration to 100 nM reduced overall channel activity (Fig. 2B). The CR (an estimator of the degree of coupling) was calculated to be 9.51. As explained in appendix, CR >1 suggests independent and heterogeneous RyR1 activity. This behavior of RyR1 multichannels with no clear evidence of coupled gating is what we have commonly observed in all our experiments collected over the last decade with cytosolic Ca2+ as the only agonist. The estimated CR in a sample of n = 23 experiments performed in the conditions described for Fig. 2B (cytosolic Ca2+ = 100 nM), ranged from 0.13 to ∞. The average CR was 3.63 ± 1.2 (excluding 3 recordings where the calculated CR was ∞). Overall, in only 2 of these 23 experiments CR < 0.25, suggesting RyR1-RyR1 cooperativity.
Fig. 2.
Cytosolic ATP/Mg2+ requirement for coupled RyR1 gating. Multichannel recordings of rabbit skeletal muscle RyR1 at membrane voltage (Vm) = 0 mV. Luminal solution contained 50 mM Ca(OH)2 and 250 mM HEPES (pH 7.4). Cytosolic solution contained 100 mM Tris and 250 mM HEPES (pH 7.4). A and B: RyR1 channel activity with cytosolic Ca2+ levels ([Ca2+]cyt) of 2 and 100 nM, respectively. C: RyR1 activity after the addition of 5 mM ATP to the cytosolic solution. D: addition of 5.6 mM Mg2+ (free [Mg2+] ∼ 1 mM). Channel openings are shown as upward deflections of the current. Dotted lines represent multiples of the full conductance level of a single RyR1. Corresponding all-point amplitude distribution histograms and cooperativity ratio (CR) values are shown next to each trace. CR is 1 of the 2 parameters we used to evaluate the degree of coupling (see appendix). Other parameter that can estimate cooperativity is the average level of RyR1 activity within a multichannel event [(nPo)event], which was 1.478 ± 0.006 (from n = 760 events during the recording). This number is significantly >1.103, which was estimated from the global activity of channels during the recording, considering them identical and independent.
With the same RyR1 reconstitution as in Fig. 2, A and B, addition of ATP to the cytosolic solution dramatically increased channel activity and number of active RyR1s (Fig. 2C). This indicates that in the absence of ATP some RyR1 channels are silent. Subsequent addition of cytosolic Mg2+ reduced the overall channel activity and revealed coupled gating as synchronized periods of channel activity and complete rest (CR = 0.05; Fig. 2D). In the 23 experiments performed in the same conditions of cytosolic Ca2+ and ATP/Mg2+, we calculated CR values averaged 0.062 ± 0.015 (ranging between 0.001 to 0.221). Moreover, the (nPo)event values obtained in these experiments support coupling as the average current during periods of activity (i.e., when at least 1 channel is open) is greater than the statistically expected current from independent channel openings and closures (see appendix). For example, in Fig. 2D, the (nPo)global during a 4-min recording was 0.261, the calculated (nPo)event for independent channels was 1.103, and the experimental (nPo)event was 1.478, i.e., 34% higher. For the n = 23 experiments mentioned above, we found that the (nPo)event values were all significantly higher (39 ± 5%, ranging from 12 to 99%) than the maximum expected for independent channels.
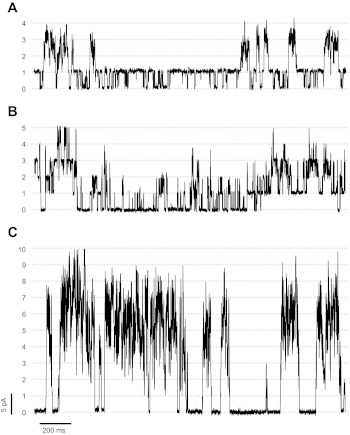
The reconstitutions where all RyR1s are fully coupled represented ∼10% of the observations with multichannels. In an additional 15% of all multichannel reconstitutions in the presence of Mg/ATP, we found coupled RyR1s mixed with independently gating RyR1s, as shown in Fig. 3, A and B. In the example of Fig. 3A, one RyR1 was frequently open and seemed to gate independently from three channels that gated in a coupled manner. In Fig. 3B, two independent sets of two coupled channels each are intermingled with single openings of one or two independently gating RyR1s. Although cooperativity can be visualized in these recordings and the behavior of these mixed channels cannot be simulated assuming independent gating, we cannot apply CR or (nPo)event. Consequently, we only analyzed those experiments where all RyR1 channels (between 2 and ∼10 channels; typically 3 to 4) gated as a single coupled unit during most of the recordings (Fig. 3C).
Fig. 3.
Examples of partially and fully coupled channels. Representative traces of multichannel recordings performed at 0 mV. Channels were exposed to cytosolic Ca2+ (100 nM), ATP (5 mM), and Mg2+ (5.6 mM; free Mg2+ ∼ 1 mM). A: example of partially coupled RyR1s where one independently-gating channel is open most of the time and other three channels sporadically gate in synchrony. B: 2 independently gating channels displaying variable levels of activity are intermingled with periods where 2 pairs of coupled RyR1s gate independent of each other. C: fully coupled channels gating in synchrony (n = 10 levels of current).
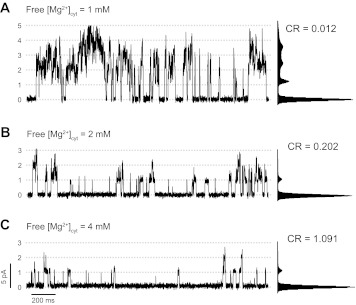
As previously described for independent channels (9, 10, 32, 62), when free cytosolic Mg2+ levels increased from 1 to 4 mM, RyR1 activity, measured as (nPo)global, significantly decreased from 2.34 ± 0.61 to 0.19 ± 0.07 (n = 8 experiments; Fig. 4). CR values increased from 0.04 ± 0.01 to 2.71 ± 1.34, indicating a decrease in coupled gating. Cytosolic Mg2+ also induced a decrease in (nPo)event from 3.254 ± 0.012 to 1.033 ± 0.050, indicating that the remaining events observed in the presence of high cytosolic Mg2+ are mainly individual channel openings. Overall, the frequency of openings (either coupled or individual) decreased as the levels of free Mg2+ increased, suggesting additional effects of Mg2+ on the gating of individual channels. Also, the duration of the events decreased at higher concentrations of Mg2+ (36 ± 2.42 to 9.60 ± 0.71 ms with 1 and 4 mM Mg2+, respectively).
Fig. 4.
Effect of cytosolic Mg2+ on coupled RyR1 gating. A: representative trace taken from a recording of a group of 5 coupled RyR1s in the presence of cytosolic Ca2+ (100 nM), ATP (5 mM) and Mg2+ (5.6 mM; free Mg2+ ∼ 1 mM); Vm = 0 mV. B and C: recordings of the same group of channels after increasing the concentration of cytosolic free Mg2+ to ∼2 and ∼4 mM, respectively. Corresponding amplitude distribution histograms and CR values are shown next to each trace.
Coupled RyR1 gating results from interaction of heterogeneous RyR1s.
In previous reports, we and others (9, 10, 35, 36) showed that RyR1s behave as heterogeneous channels. When attempting to model coupled gating, we found that heterogeneity is also present in coupled channels but this phenomenon is not random. As shown in the example of Fig. 2C, for n = 4 coupled channels, during the coupled event the current levels never reach the baseline (0 level) for a significant period of time (ms). Additionally, there are moderate to very high levels of flickering between channel levels 1 to 4. Modeling of this behavior requires the assumption of heterogeneous channels, with at least one channel having a Po = 1 during the coupled event (driver RyR1) and the others having a much lower Po (follower RyR1s). Coupled RyR1s also seem to have differences in their amplitudes. Openings of the driver channel (from level 0 to 1) have an amplitude of 3.35 ± 0.07 pA, while openings to levels 2 and 3 have lower amplitudes (2.94 ± 0.05 and 2.83 ± 0.06 pA, respectively; n = 15 paired experiments). Thus coupling represents the interaction of channels that differ in their gating properties and current amplitude. The channels involved in a coupled cluster also seem to be heterogeneous in their modulation by endogenous regulators. For example, notice that some flickering channels are silent in the absence of ATP (Fig. 2, compare A and C). Furthermore, increasing Mg2+ levels preferentially inhibits the flickering channels (Fig. 4).
On and off rates of RyR1 coupled events are very fast.
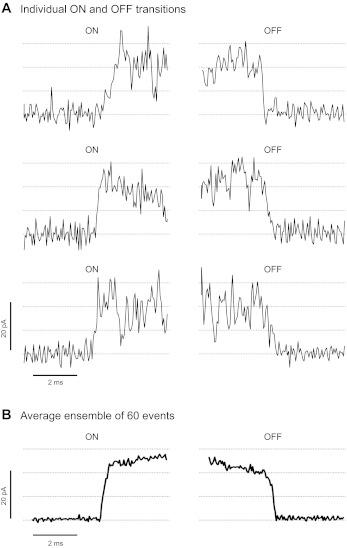
The experiments shown in Fig. 5 were carried out at membrane voltage (Vm) = +20 mV (the rate of acquisition was 50 kHz and cutoff filter frequency of 5 kHz). For 60 consecutive events, the time that the channels within a coupled group take to transition from close to open state (ON RATE) was measured. For the same events, the time required to transition from the open state to the baseline at the end of the coupled event (OFF RATE) was subsequently measured. Figure 5A shows examples of ON and OFF transitions. Figure 5B shows the ensemble currents during the ON and OFF transitions of 60 coupled events. Our data suggest that there are indeed some distinguishable steps during the ON and OFF of some of the coupled events. This may imply that the channels do not open and close in one instantaneous “lock step.” However, the rates of the ON and OFF transitions were very fast and close to our limit of resolution (296 ± 23 and 332 ± 35 μs, respectively; n = 4 experiments analyzed after filtering at 5 kHz). ON rates resemble the fast activation of RyR1 channels in response to rapid Ca2+ pulses (75). However, OFF rates of coupled events are much faster than the rates of decay of single-channel activity measured upon fast Ca2+ removal (75).
Fig. 5.
Kinetics of the ON and OFF transitions of coupled event. Coupled RyR1s were recorded at +20 mV using a cutoff frequency of 5 KHz to increase time resolution. A: examples of ON and OFF transitions of 3 consecutive events. B: average ensemble of 60 ON and OFF transitions. Averages of the duration of the ON and OFF transitions were 330 ± 28 and 370 ± 42 μs (n = 60 events), respectively, and were estimated by fitting the individual transitions to a straight line.
Coupled RyR1 gating requires luminal solutions containing Ca2+ or Sr2+.
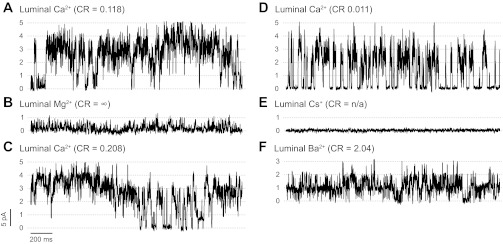
We found that, when used as current carriers, not all alkaline earth cations (M2+) are equivalent for coupled gating. In Fig. 6A, five channels with a good degree of coupling were in the presence of 50 mM luminal Ca2+ (the cytosolic solution contained 100 nM free Ca2+ and ATP/Mg2+). After superfusion of the luminal chamber with a solution containing 50 mM Mg2+, channel activity decreased and coupled gating was not observed (Fig. 6B). Coupling was recovered by switching the luminal solution back to that containing Ca2+ (Fig. 6C). In Fig. 6, D-F, we show another group of RyR1 channels sequentially exposed to luminal Ca2+, Cs+, and Ba2+, respectively. Notice that channel activity ceased in the presence of luminal Cs+, even after 1 mM caffeine was added (Fig. 6E). Subsequent perfusion with luminal Ba2+ restored RyR1 activity, but coupled gating was not observed (Fig. 6F). CR values were 0.096 ± 0.042 with luminal Ca2+ and 4.69 ± 1.79 with luminal Mg2+/Ba2+ (n = 4 experiments). In the presence of luminal Ba2+ (Fig. 6F) or Mg2+ (not shown), the addition of caffeine or increases in cytosolic Ca2+ levels could produce an increase in RyR1 activity but coupling was not detected. Recovery of coupling was observed after switching back the luminal solution to Ca2+ as shown in Fig. 6C. Thus luminal Ca2+ was crucial for observing RyR1 coupled gating. While coupling was never observed in the presence of Mg2+ or Ba2+ as current carriers, we found coupling [i.e., CR < 0.25 and (nPo)event higher than expected for independent channels] using luminal Sr2+ (n = 4 data not shown).
Fig. 6.
Effects of the luminal current carrier on RyR1 coupled gating. A: representative trace taken from a recording of a group of coupled channels performed at Vm = 0 mV in the presence of luminal Ca2+ (50 mM) as the current carrier. B: recordings of the same set of channels after replacing the current carrier by superfusing the luminal chamber with a solution containing 50 mM Mg2+. C: same group of coupled RyR1s were recorded after returning to the original conditions by perfusing the luminal chamber with a solution containing 50 mM Ca2+. A–C: cytosolic solution contained Ca2+ (100 nM), ATP (5 mM), and Mg2+ (5.6 mM; free Mg2+ ∼ 1 mM) and remained unchanged throughout the experiment. D–F: in a different experiment, another group of coupled RyR1s were sequentially exposed to luminal Ca2+ (50 mM), Cs+ (100 mM), and Ba2+ (50 mM), respectively. Cytosolic conditions in D were the same as above. Notice that in E and F caffeine (2 mM) was added to increase channel activity. However, RyR1 remained silent in the presence of luminal Cs+. Only some of the channels started gating again after perfusion with luminal Ba2+ but coupling was not observed.
Cytosolic Ca2+ and caffeine affect coupled RyR1 gating.
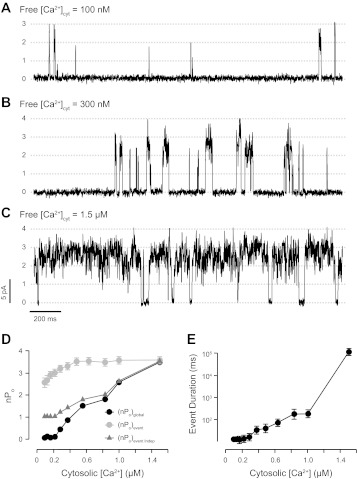
The effect of increasing cytosolic Ca2+ levels ([Ca2+]cyt) on coupled gating was evaluated to compare with previous independent channels studies. RyR1 coupled events are scarce and brief with [Ca2+]cyt = 100 nM (Fig. 7A), and they become longer and predominant upon increasing [Ca2+]cyt to 1.5 μM (Fig. 7C). However, these RyR1s always displayed coupled gating, regardless of the global activity. This is reflected by (nPo)event, which was much higher than the (nPo)event expected for independent channels [called (nPo)event indep; see appendix]. The (nPo)event showed very little, if any, change in response to an increase in [Ca2+]cyt (Fig. 7D, gray circles). This is mainly due to the fact that during a coupled event the frequency of openings and closures between levels 1 and n did not change. In contrast, the estimator of the global activity, (nPo)global, changed from ∼0 to ∼3.3 when we increased [Ca2+]cyt from 100 nM to 1.5 μM (Fig. 7D, black circles). This correlates well with the dramatic increase in the duration and frequency of coupled events with increasing [Ca2+]cyt (Fig. 7E).
Fig. 7.
Effect of cytosolic Ca2+ on coupled RyR1 gating. Activity of a group of coupled RyR1s was measured at Vm = 0 mV in the presence of cytosolic ATP (5 mM), Mg2+ (5.5 mM ; free Mg2+ ∼ 1 mM), and luminal Ca2+ (50 mM) as the current carrier. Concentration of Ca2+ at the cytosolic surface of the channels was increased by consecutive additions of CaCl2 (tested Ca2+ levels ranged between 100 nM and 1.5 μM). In this example, representative recordings taken at [Ca2+]cyt = 100 nM, 300 nM, and 1.5 μM are shown in A, B, and C, respectively. CR values were 0.05, 0.07, and 0.09 for A, B, and C, respectively. D: global open probability, (nPo)global, during 4-min recordings (black circles) and (nPo)event (grey circles) ± SE. (of n > 300 events) as a function of the cytosolic Ca2+ level. Expected values of average (nPo)event for independent channels are shown as grey triangles (see appendix for details). E: mean duration of coupled events ± SE as a function of cytosolic Ca2+.
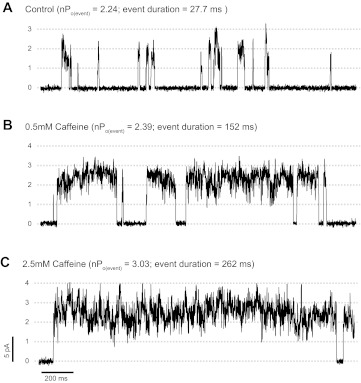
Figure 8 shows the effects of caffeine, an agonist that increases RyR sensitivity to cytosolic Ca2+ (18, 57, 71). Here, we found that although the duration of the openings increases upon addition of 0.5 mM caffeine, the (nPo)event remains relatively constant (2.24 vs. 2.39, control vs. 0.5 mM caffeine, respectively), indicative that caffeine did not affect the kinetic behavior of the flickering channels. Results from n = 5 experiments show that while event duration and frequency increased by 2.6 ± 0.6 and 6.2 ± 2.4 times, (nPo)event only changed by 2 ± 1%. As shown in Fig. 8, at higher concentrations (2.5 mM), caffeine increased (nPo)event to 3.03 ± 0.01. For n = 5, (nPo)event increased by 35 ± 12% relative to control. However, the main effect of 2.5 mM caffeine on channel activity (increase in global activity by 77 ± 26 times) is generated by the increased duration and frequency of openings (by 10.3 ± 2.9 and 15.2 ± 6.5 times, respectively).
Fig. 8.
Effect of cytosolic caffeine on coupled RyR1 gating. A: representative trace taken from a multichannel recording of a group of coupled RyR1s in the presence of cytosolic Ca2+ (100 nM) and ATP/Mg2+. B and C: multichannel recordings performed after addition of caffeine to the cytosolic chamber (0.5 and 2.5 mM, respectively). (nPo)event values and duration of coupled events in each condition are indicated. CR values for A, B, and C were 0.001, 0.002, and 0.003, respectively.
SR membrane voltage does not affect RyR1 coupled gating.
If Ca2+ flux through one RyR1 channel open pore is important for activation/inactivation of neighboring channels in coupled gating, then (nPo)event should depend on the amplitude of the Ca2+ flux. The data shown in Fig. 9 suggest that this is not the case. Here, using luminal Ca2+ as the charge carrier, the SR Vm was changed between 0 and +40 mV, which increased the lumen-to-cytosol Ca2+ current from ∼3 pA to more than 8 pA per channel (Fig. 9, A and B). These changes in Vm did not significantly change (nPo)event, indicating that the flickering within the coupled events was not sensitive to changes in lumen-to-cytosol Ca2+ flux within the tested range. This lack of voltage dependence of (nPo)event was also found in n = 11 coupled channel experiments where the voltage was changed between −20 to +40 mV (i.e., from Ca2+ currents of ∼1 to 8 pA) as shown in Fig. 9C. In a few experiments, the duration of the coupled events decreased with voltage, as in the example shown in Fig. 9A. However, in the majority of experiments, voltage did not significantly affect the mean event duration (Fig. 9B). Overall, there was no significant effect of increased lumen-to-cytosol Ca2+ flux on the duration of coupled events (averages of 11 experiments are summarized in Fig. 9D). Not shown, the number of events or the (nPo)global did not significantly change with voltage. As a whole, our data suggest that coupled channels do not sense changes of Ca2+ levels in their local (cytosolic) environment induced by lumen-to-cytosol Ca2+ flux.
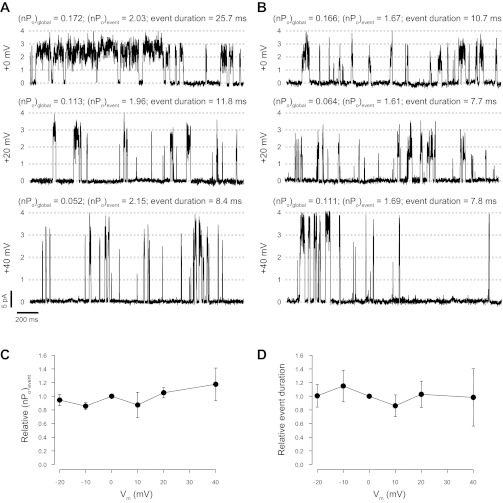
Fig. 9.
Effect of membrane potential changes on coupled RyR1 gating. A and B: 2 examples of groups of coupled RyR1s recorded at Vm = 0, +20, and +40 mV (top to bottom, respectively). In both experiments, 50 mM luminal Ca2+ was used as the current carrier and the cytosolic solution contained Ca2+ (100 nM) and ATP/Mg2+ (5/5.6 mM, respectively). (nPo)global, (nPo)event, and duration of coupled events are indicated. In the example shown in A, representative of 2/11 experiments, the duration of coupled events decreases as a function of increasing Vm. However, in the remaining 9 the experiments Vm did not affect the duration of the coupled events, as shown in B. C: relative (nPo)event values as a function of Vm normalized against values of the same group of channels measured at Vm = 0 mV (average value of n = 11 experiments). D: relative coupled event duration vs. Vm (normalized against paired values at Vm = 0 mV; n = 11). CR values in A were 0.003, 0.004, and 0.001 for the top, middle, and bottom, respectively. In B, CR values were 0.020, 0.012, and 0.017 (top, middle, and bottom, respectively).
We have collected fewer data (n = 3) at Vm < −60 mV, as most bilayers containing coupled RyR1s are disrupted by these extremely negative voltages. In two cases, we found a significant decrease in (nPo)event and an increase in CR (CR > 1), but this could not be fully attributed to the decrease in Ca2+ flux as coupling did not recover immediately after switching the membrane voltage back to 0 mV (results not shown). This may be a sign of direct effects of the voltage itself on RyR1s. Indeed, inactivation at these negative voltages was previously reported (36).
DISCUSSION
We found that coupled gating (synchronous activation/deactivation) of skeletal muscle RyR1s can be consistently observed when channels are reconstituted from TC microsomes that maintained a high level of integrity after isolation. We have determined that coupled RyR1 gating requires the presence of cytosolic Mg2+/ATP as well as luminal Ca2+. Coupled gating results from the interaction of heterogeneous RyR1 channels and is susceptible to modulation by cytosolic Ca2+, Mg2+, and caffeine.
Experimental conditions required to observe coupled gating.
Convincing evidence for functional coupled gating was found in ∼25% of the multichannel reconstitutions from highly purified “intact” TC microsomes, likely to have abundance of structurally coupled RyR1s (59) (Fig. 1). It is possible that our reconstitution method, based on spontaneous vesicle fusion, favors incorporation of intact globular TC microsomes with the capacity to swell/shrink upon osmotic challenge (44, 60). In this regard, the existing reports (34, 39) on coupled RyR1 gating seem to have utilized similar methods for reconstitution. Coupling of purified RyR1s was only found in fractions with a coefficient of sedimentation ≥60S, which are enriched in RyR1 channels that remain associated as dimers or larger groups (39). Coupling was not observed after reconstitution of purified RyR1s from the 30S fraction, which mainly contains isolated channel molecules (39). The extensive use of the 30S fraction to study RyR1s may have contributed to the failure to observe coupling in the past.
Here, the presence of ATP/Mg2+ in the cytosolic solution was required to observe coupled gating consistently. Both ATP and Mg2+ are known regulators of the RyR1 channel (18, 41, 62). When added in the absence of ATP, free Mg2+ (1 mM) is a strong inhibitor of RyR1 channel activity and no evidence of coupled gating is found. When added alone, ATP (5 mM) is a strong agonist of RyR1 and coupling is masked by the high level of activity. The simultaneous action of ATP (the agonist) and Mg2+ (the antagonist) seems to provide the moderate levels of activity suitable to reveal RyR1-RyR1 communication for coupled gating. Two previous reports (34, 52) describing coupled RyR1 gating also performed the experiments in the presence of ATP. One additional report (39) used caffeine-activated RyR1 with no ATP added. As free ATP (not ATP-Mg) is the species that appears to activate RyR1 (42, 65, 68), we have also measured CR values in experiments with 0.5–1 mM ATP (close to the free ATP levels calculated in the mixture of ATP/Mg2+ used in our experiments). CR < 0.25 or visual evidence of coupled channels was found only in 5 of nearly 100 experiments. Thus ATP alone was not as effective as ATP/Mg2+ in allowing observation of coupled gating.
In this work, coupled gating was found when luminal Ca2+ or Sr2+ was the current carrier, but coupling was impaired when luminal Ca2+ was replaced by Mg2+ or Ba2+. In contrast, a previous report (39) described coupling in the presence of luminal Ca2+ or Ba2+. These discrepancies may be related to different experimental conditions, including cytosolic and luminal solutions used by Marx et al. (39) with 2.6 and 3.5 times larger ionic strength, respectively (due to the addition of 250 mM KCl). Still, our data suggest that the probability of finding coupled gating in channels exposed to luminal Ba2+ (or Mg2+) solutions is lower than that with Ca2+ (or Sr2+). Our data also suggest that coupled gating may not be observed when Mg2+/ATP or other agonists (e.g., caffeine) are omitted from cytosolic solutions, which has been the norm in many of our previous studies (3, 9, 10) as well as in most reports in the literature (11, 18, 62, 69, 71).
We used a very high luminal Ca2+ concentration (50 mM) to ensure bilayer stability and to be able to perform long experiments with multiple perfusions on the same group of channels. This approach limits the interpretation of our results, as we did not explore the physiological regulation of coupling by luminal Ca2+. This is a problem that we found in most reports of coupled gating (27, 39, 47, 52). Indeed, studies of independent and coupled RyR1s bathed with solutions containing “cell-like” conditions of pH, ATP/Mg2+, and Ca2+ (both cytosolic and luminal) are lacking. It is known that fully or partially activated independent RyR1s are sensitive to changes in cytosolic and luminal environment (4, 10, 18, 33, 62). Thus it is expected that coupled RyR1s would be highly susceptible to the combining effects of varying the concentrations of these elements within the ranges present in resting cells. In summary, RyR1 coupling still needs to be consistently studied and this will require setting optimal conditions to observe the phenomenon with a relatively high frequency, including the systematic use of preparations (either microsomes or purified RyR1 receptors) enriched in arrays of interacting channels.
Dissecting the coupled RyR1 gating event.
Coupled RyR1 channels activated very fast (∼300 μs) as a group. Structural evidence indicates that neighboring RyR1s are very close to each other, even after the isolation of SR microsomes (25, 26, 51, 58). This would allow for communication between neighboring RyR1s via Ca2+ (or Sr2+) diffusion within microseconds. RyR1 activation by a sudden increase in cytosolic Ca2+ is very fast (28, 29, 75), making feasible a local calcium-induced calcium release (CICR) process that would activate neighboring RyR1s for coupled gating as hypothesized in our preliminary communications (8, 48) and in a report by Laver et al. (34). However, the time resolution of bilayers (200 μs under favorable conditions of voltage and bilayer noise) is not enough to discern if the initial trigger of the coupled event is from the diffusion of Ca2+ (which could take <10 μs) or physical interactions between RyR1 molecules.
When k RyR1s gate coupled, there are many gating transitions between the levels 1 and k, but no transitions to the baseline (level 0) with the exception of those that start and end the coupled event. This observation indicates that one channel (which we call “driver”) is open during the whole event and its gating is locked in a mode where long openings dominate; i.e., “high Po mode” (76). The mean duration of the driver's openings is longer than that of single RyR1 channels, which alternatively gate in low and high Po modes (2) and most openings are very short (up to a few ms; Refs. 9, 34, 61, 66, 73). As a consequence, the duration of coupled events is longer, usually ranging from a few to a hundred milliseconds. However, most of the other channels (“followers”) in a coupled event display abundant short openings, even in the presence of agonists, suggesting they have a different gating mode. Additionally, drivers and followers differed in their current amplitude by ∼13%.
The heterogeneous gating of RyR1s may reflect an asymmetric process of coupling where one channel that activates to a long sojourn starts an interaction with the neighboring channels, triggering their opening to a gating mode of abundant flickering events. This communication between drivers and followers could be mediated by asymmetric RyR1-RyR1 physical interactions or a local CICR process, where individual RyR1s could alternatively act as drivers or followers. However, the differences between drivers and followers may also be explained by the existence of populations of RyR1s displaying high activity and low activity (LA) that we defined in previous reports (9, 10). We found that, in the conditions used here to study coupling, independently gating LA channels are inactive (10). LA inactivity resulted from lower affinity to activating Ca2+ and ATP and higher affinity to inhibitory Mg2+ (9, 10). LA RyR1s that are inactive on their own could be good “followers” for coupled gating if a neighboring and active high activity channel (“driver”) triggers their gating.
The requirement for luminal Ca2+ (or Sr2+) to observe coupled gating suggests that the Ca2+ flowing through a driver activates neighboring followers by interacting with cytosolic activating Ca2+ binding sites (local CICR). However, there are a number of observations suggesting that coupled gating is not sustained by a local CICR alone. In principle, Ca2+ alone would not reactivate LA channels, since in single channels we found that their inactivity in the presence of Mg2+/ATP is not reversed by increasing Ca2+ (9, 10). Another result against the idea of a local CICR is the lack of effect of cytosolic Ca2+ on the (nPo)event (i.e., the flickering of the “followers”). Changes in cytosolic Ca2+ would be expected to affect (nPo)event, as an increase in the cytosolic Ca2+ levels increases RyR1 open times and decreases RyR1 closed times (9, 63). Indeed, changes in cytosolic Ca2+ from 0.1 to 1 μM significantly increased the duration of the coupled events despite the fact that no changes in the flickering of the followers was observed.
Another observation that does not support local CICR as the mechanism for coupled gating is that the duration of coupled events and the (nPo)event were not affected by changes in membrane voltage (i.e., drivers and followers did not sense activating or inhibitory effects of changing lumen-to-cytosol Ca2+ flux). This lack of sensitivity to feeding-through Ca2+ is not entirely surprising for drivers (which are open during the whole event) as single RyRs may only sense cytosolic Ca2+ when they are closed (14). Still, the flickering followers, which open/close many times during the event, should be able to sense the increase in local Ca2+ induced by lumen-to-cytosol flux because cytosolic Ca2+ affects RyR1 activity in a biphasic manner with a narrow peak of activation (6, 9, 35, 45). However, changes in SR membrane voltage between values producing ∼1 and 8 pA Ca2+ fluxes had no effects on (nPo)event.
The lack of sensitivity of (nPo)event to low levels of caffeine, cytosolic Ca2+, or lumen-to-cytosol Ca2+ flux indicates that the flickering of followers may not represent the normal transitions between the closed and open states observed in single channels but transitions from open to block states. In this regard, we were able to mimic the followers behavior (including a decrease in the current amplitude of single RyRs) by exposing the channels to polycationic peptides, including protamine (12, 49). We also found that the openings induced by polycationic peptides display flickering that is insensitive to changes in cytosolic Ca2+ levels or caffeine (12). This would suggest that peptide-peptide interactions (possibly via electrostatic interactions) of positively charged regions in a driver with regions in adjacent followers could produce their activation.
Studies (46, 55) with skeletal muscle fibers suggest that a process of SR Ca2+ flux-induced RyR1 inactivation affects tonic and transient components of depolarization-induced Ca2+ release as well as spark rise time. Our data do not support the idea of RyR1 inactivation mediated by an increase in local cytosolic Ca2+ reaching inhibitory levels after a group of adjacent channels activates. Indeed, (nPo)event was insensitive to changes in the magnitude of RyR1-mediated Ca2+ currents between ∼1 pA and ∼8 pA per channel (this is ∼3–20 times higher than the RyR1 unitary currents estimated in cells; Ref. 43). Moreover, the slope of the mean current in the coupled event as a function of time was flat [i.e., (nPo)event is constant from the sudden start to the abrupt end of the event].
Flash-photolysis studies (75) with single RyRs found that after cytosolic Ca2+ is suddenly removed by uncaging a Ca2+ chelator, RyRs take, on average, ∼10 ms to deactivate. These data suggest that if Ca2+ flowing through a channel was activating its neighbors, then when the first channel closes (and the flux stops), an instantaneous deactivation of neighboring channels should not be expected. Desynchronized Ca2+ unbinding from open channels (19) would have also predicted that the OFF rates of coupled events mediated by local CICR should be variable and, on average, much slower (few milliseconds) than their ON rates. However, our data show that coupled RyR1s deactivated together and the OFF rates of coupled events are as fast as the ON rates (microseconds). These results also suggest that the forces involved in deactivation of coupled channels may not be related to cytosolic, local Ca2+ depletion.
In summary, the analysis of coupled events suggests that the process of coupling is complex and involves functionally heterogeneous channels. The dependence of luminal Ca2+ would suggest involvement of luminal Ca2+ sites and/or lumen-to-cytosol Ca2+ fluxes in coupled gating. Yet, a number of expectations from a simple CICR between neighboring RyR1s are not fulfilled by the experimental results. Indeed, there is evidence that coupled gating could also be found (possibly with lower probability than in our conditions) in the absence of luminal Ca2+ (39, 47), i.e., in the absence of CICR.
Physiological relevance of coupled gating–conclusions.
Coupled gating may help explain the efficacy of the process of control of RyR1 function by dihydropyridine receptors (DHPRs) in skeletal muscle fibers. During excitation (and upon t-tubular depolarization), a conformational change in the DHPRs located in the t-tubule triggers the activation of the RyR1s present in terminal cisternae (53). In the resting fiber, DHPRs prevent RyR1-mediated Ca2+ leak (78). Although electron microscopy studies showed that DHPRs may not physically communicate with all RyR1s (24, 26), our results and previous reports (39) indicate that RyR1s are coupled through physical interactions. This RyR1-RyR1 coupling in an array would allow large groups (if not the whole array) to activate/deactivate at once despite the fact that not all the channels are directly controlled by the DHPRs. Our results also indicate that, after activation, coupled RyR1s would be unable to sense the feedback regulation by the local increase in cytosolic Ca2+ generated during their burst of synchronous activity (coupled event). These characteristics of coupled RyR1s may explain, at least in part, the recent observation that RyR1s under DHPR control do not seem to be activated by local CICR (17). In summary, RyR1-RyR1 interactions would explain the mechanism of DHPR-triggered activation of RyR1 channels that are not directly facing T-tubule voltage sensors as well as their deactivation under conditions of high Ca2+.
On average, coupled RyR1s are more active than independently gating channels. Indeed, under resting cytosolic conditions single openings are very short, whereas coupled events have durations ranging from those of sparks (∼20 ms) to those of embers (hundred of ms to s) (5, 77). Moreover, coupled channels are more sensitive to activation by cytosolic Ca2+ and caffeine compared with previous findings in single channels (9, 10, 13, 50). Coupled RyR1 gating would provide a simple explanation to the synchrony of the SR Ca2+ release observed in sparks (5, 77). The Ca2+-insensitive group behavior after the initiation of a coupled event could also provide an alternative explanation for the termination of sparks without requiring SR Ca2+ depletion or RyR1 inactivation (5, 18, 54, 55, 77). Thus, under resting conditions, coupled RyR1s may be leakier than uncoupled RyR1s and may be responsible for the generation of Ca2+ sparks and leak. This suggests that the increased RyR1-mediated Ca2+ leak observed in diseases may not relate to the switch from coupled to uncoupled RyR1s as previously suggested (39, 40). It is clear that weakening of DHPR/RyR1 interaction would play a preponderant role for increased leak (16). Our results suggest that changes in the RyR1 environment that occur during physiological and pathological processes (1, 72) could affect coupled RyR1 gating and RyR1-mediated Ca2+ leak under resting conditions. For example, in conditions of fatigue, where ATP levels and pH decrease while Mg2+ levels increase (1, 7), coupled RyR1-mediated Ca2+ leak would be significantly decreased.
In summary, our data indicate that coupled gating results from interactions between neighboring RyR1 channels that are stabilized by luminal Ca2+ and cytosolic ATP/Mg2+. There are important differences in the behavior of coupled RyR1s compared with what was previously described for single RyR1s, suggesting that there may be differences in their regulation and pharmacology. As this study was oriented to define mechanistic aspects of coupled gating, we did not test the regulation of coupled RyR1s in an ionic environment with pH and luminal Ca2+ levels matching those of a resting cell. Consequently, an increased knowledge on the coupled gating mechanism, specially its physiological regulation, will be crucial to understand RyR1 function in health as well as in genetically inheritable diseases (such as malignant hyperthermia and central core disease) where abnormalities of RyR1 channel behavior are observed.
ACKNOWLEDGMENTS
We thank M. Fill for support and critical input as well as C. A. Villalba-Galea and M. Reyes for helpful contribution to the development of the Montecarlo simulation software. We also thank J. Bryan, Office System Specialist, Southern Illinois University School of Medicine, for proofreading the manuscript.
APPENDIX
In all our multiple-channel studies, the global level of activity of k observed channels was estimated from 4- to 6-min recordings by calculating their macroscopic open probability, (nPo)global, with pClamp9 software using the half-amplitude threshold of the different levels or the amplitude distribution histograms as previously described (9). Mean open times within a burst of activity can be estimated in limited situations, when individual channels with simple kinetics and identical gating properties operate independently from each other (15, 31). Estimation of this parameter is invalid in our experimental system as the kinetics of individual RyR1s is very complex, the channels are heterogeneous and they do not gate independently. Consequently, we have only used (nPo)global as an estimate of channel activity to compare the function of a group of RyR1s in different conditions. However, this method does not evaluate the presence/absence of cooperative interactions between multiple channels.
Cooperativity between channels is usually tested with a simple probability-based method that evaluates if the openings of two or more channels are binomially distributed (30, 31, 37). Here, we used one of these parameters, the cooperativity ratio; CR (31). Let us consider that we have N identical channels and that we estimated the individual open probability (Po), which represents the probability of finding each one of these single channels open. For these N functionally identical channels gating independently with the same Po, we can estimate the probability of finding n channels (from 0 to N) open at any given time [Φ(n)] from the binomial distribution
| A1 |
where ∑ Φ(n) = 1.
Experimentally, Φ(n) for each level is obtained by Gaussian fitting of the all-point amplitude distribution histogram of a 4-min (or longer) recording of multichannel experiments. The average Po of each channel can be estimated from the ratio of global activity of the channels during the recording [(nPo)global] divided by k, the maximum number of active channels observed in the bilayer. Notice, however, that from Eq. A1 is apparent that the ratio
| A2 |
will be independent of Po as the terms containing this parameter are cancelled out. From this ratio, an index of cooperativity (CR) that is valid at all Po can be generated. When considering the 0, 1, and 2 current levels,
| A3 |
Krouse and Wine (31) defined
| A4 |
so that when all the present channels are identical and independent, CR = 1. CR > 1 when the channels are independent but have different Po values. CR < 1 when the channels are gating in a coupled (cooperative) fashion. Note that if the channels open and close in perfect lock-step, then the CR, as presented, will be zero for k = 2 channels but it will an indetermination for k > 2. However, as coupled channels display flickering events between levels 1 and k, this is not the case experimentally. In general, for 2- to 4-min recordings, values of CR < 0.25 are statistically different from expected values for independently gating channels (31).
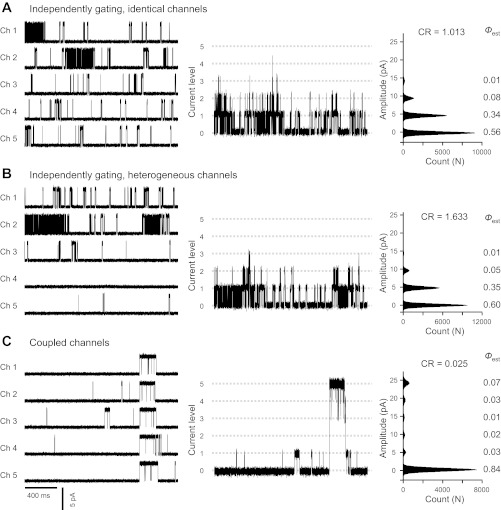
We have tested some of the formulations shown above using channel simulations. For example, simulations of five RyR1 channel recordings were generated with an algorithm developed by Dr. A. Escobar for testing CR (Fig. A1). Figure A1A, left, shows 2 s of a 1-min simulation of the behavior of channels 1, 2, 3, 4, and 5 (all 5 simulated channels have identical gating and Po = 0.1). Figure A1A, center, shows the sum of all five traces which represents the ensemble current resulting for these five identical and independent channels. In Fig. A1A, right, we show the current-amplitude histogram from the sum of a 1-min simulation of all five records. As expected for identical channels, CR = 1.01. In Fig. A1B, we present the results for heterogeneous channels. Here, the Pos were 0.25, 0.15, 0.06, 0.03, and 0.01 for the five channels involved and the resulting CR value was 1.63. In Fig. A1C, the channels were made identical (Po = 0.1) but they were simulated to start and end bursts of activities at identical times, typical of the coupled channels. Under these assumptions, CR = 0.025. The probability (p) of two independent (either identical or heterogeneous) channels having CR < 0.5 after analyzing >5,000 individual channel events is P < 0.0001 (31). In our experiments, we analyzed 4- to 8-min segments of multichannel activity (i.e., a much higher number of individual events) and we consider that CR < 0.25 is conclusive evidence that some or all the channels are gating cooperatively. We have noticed that, in real experiments, the maximum number of channels observed (k) could underestimate the total number of channels (M). However, this would produce moderate effects in the CR values as the ratio (k − 1)/2k varies from ¼(k = 2 channels) to ½ (M = ∞ number of channels). For the examples shown in Fig. A1, underestimation of channels from k = 5 to M = ∞, would imply an underestimation of CR from 1.01 to 1.26 (independent), from 1.63 to 2.04 (heterogeneous), and from 0.025 to 0.031 (coupled channels). Thus defining CR < 0.25 as cooperativity from a 4-min recording (or longer if events are scarce) will minimize errors due to underestimation of the total number of channels. The application of this approach to real data is shown in Figs. 2, 4, and 9 and in the text. Notice that in conditions that favor coupled gating (presence of cytosolic ATP/Mg2+, caffeine, or luminal Ca2+) CR was ≤ 0.2 while uncoupled channels usually had CR ≥ 1. These data demonstrate how the CR approach can be used to evaluate the coupled gating phenomenon.
Fig. A1.
Simulation of independent and coupled RyR1 channels. Simulation of channel behavior was performed using a Markovian scheme of RyR1 gating (19, 56). First simulation (A) assumed 5 independently gating channels (Ch1 to Ch5) with identical Po = 0.10. Second simulation (B) assumed 5 independent and heterogeneous channels with Pos = 0.25 (Ch1), 0.15 (Ch2), 0.06 (Ch3), 0.03 (Ch4), and 0.01 (Ch5). Third simulation (C) assumed that Ch1 to Ch5 were identical (Po = 0.1) and coupled. Left: for each channel (Ch1 to Ch5), 2-s currents taken from 1-min simulations. Center: ensemble multichannel currents generated by summing recordings of Ch1 to Ch5. Right: all-point amplitude distribution histograms obtained from one min of ensemble multichannel currents. Probability (Φ) of finding 0, 1, 2, 3, 4, and 5 channels open simultaneously was measured from Gaussian fitting of the amplitude distribution histograms. Estimated CR values are shown next to each histogram.
To further evaluate the degree of coupling we have defined a parameter that estimates the average open probability value of the RyR1s during a coupled event. We called this parameter the mean (nPo)event, and it represents the mean Ca2+ current generated during the event by the activity of all RyR1s.
To experimentally determine the (nPo)event, the Axopatch pClamp 9 software is used to analyze the recording placing a 50% threshold between the zero level (baseline) and first level of conductance (one open channel). pClamp9 then determines the number of events, mean Ca2+ current per event (I) and dwell time (D). These data are used to calculate the weighted average current (ĪW).
| A5 |
from ĪW we obtained the (nPo)event, which represents the level of RyR1 activity within an event, and is derived from
| A6 |
where A1 is the amplitude of the first current level (∼3.5 pA at 0 mV) and A2 + k is the average amplitude of openings observed in levels 2 to k.
For k = 3 channels, the (nPo)event can be calculated where A1 = current amplitude for a full opening to level 1 and A2 + 3 = average amplitude of openings from level 1 to level 3.
| A7 |
The variance (σ2) of weighted amplitude can be determined by using the average weighted amplitude (ĪW) and the I and D of the N individual events
| A8 |
The SE for the (nPo)event can then be determined
| A9 |
(nPo)event is not ratiometric (as CR) and seems to be a more direct parameter to test the level of RyR1 activity within a coupled event as mean (nPo)event ± SE. We can then estimate if this value is statistically different from the expected maximal value for independent channels [(nPo)event independ] by considering them homogeneous at each level of global activity. For independent channels, it would be expected that the mean (nPo)event independent ∼ (nPo)global/[1 − Φ(0)], which will follow the global activity of the channels. For example, probability analysis of 3 independently gating RyRs with identical individual open probabilities, Po, of ∼0.05, can be done by using the binomial distribution (Eq. A1). We estimated that 85.7% of the time all channels will be closed. The probabilities of observing one, two and three channels open are 13, 0.7, and 0.01%, respectively. The expected value of
suggests that most of the events would be single-channel openings. Indeed, a (nPo)event ∼ 1.05 was also obtained from a 4-min simulation of three independent RyR1s with Po = 0.05. Notice that for independent RyRs, (nPo)event will continuously increase with increased Po up to a maximum (nPo)event = 3 when all channels are fully active (all individual channels have Po = 1). In contrast, for three fully coupled RyR1s, (nPo)event = 3 at any global Po. This is because “coupled channels” gate simultaneously; i.e., at any given time within the coupled event the channels are all open (Po =1) and they all open or close as one. As indicated, for k = 3 independent and identical channels with individual Po ∼0.05, we would expect (nPo)event independent ∼1.05. A higher (nPo)event ∼1.08 would be possible if we underestimated k = 3 channels instead of M = ∞ channels [with equivalent (nPo)global of 0.15]. In bilayers, three coupled RyR1s could have a (nPo)event of 1.80 ± 0.02. This estimate is significantly different from the maximal expected (nPo)event independent of 1.08 with P < 0.00001. However, when Po ∼0.5 (the global nPo ∼1.5), the expected (nPo)event independent could range from 1.71 (k = 3 channels observed) to 1.93 (if M = ∞ channel were present). As the experimental (nPo)event would remain ∼1.80, these conditions would not be capable to discern between independent and coupled channels. Thus, when the global activity of the channels is relatively low, the differences in (nPo)event between “independent” and “coupled” are much larger than the error in the determination of coupling and provide confidence in the parameter. Large differences between coupled and independent behavior would also prevent misclassification due to underestimation of the number of channels involved. In summary, the level of reliability of these parameters is not different than that of parameters estimated from single RyR1 channels (Po, mean open time). For coupled channels, large changes in CR (e.g., from 0.02 to 0.2) or in (nPo)event (e.g., from 3.5 to 1.5 for k = 4 channels) can be used with confidence to evaluate changes in the degree of coupling, while small changes would have dubious meaning.
GRANTS
This work was supported by the National Institute of General Medical Sciences Grant R01-GM-078665 (to J. A. Copello).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.P., P.L.D.-S., J.T.N., A.L.E., S.F., and J.A.C. conception and design of research; M.P., P.L.D.-S., J.T.N., S.F., and J.A.C. performed experiments; M.P., P.L.D.-S., J.T.N., A.L.E., and J.A.C. analyzed data; M.P., P.L.D.-S., J.T.N., A.L.E., S.F., and J.A.C. interpreted results of experiments; M.P., P.L.D.-S., J.T.N., and J.A.C. prepared figures; M.P., P.L.D.-S., J.T.N., and J.A.C. drafted manuscript; M.P., P.L.D.-S., J.T.N., A.L.E., S.F., and J.A.C. edited and revised manuscript; M.P., P.L.D.-S., J.T.N., A.L.E., S.F., and J.A.C. approved final version of manuscript.
REFERENCES
- 1. Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol 104: 296–305, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Armisen R, Sierralta J, Velez P, Naranjo D, Suarez-Isla B. A modal gating in neuronal and skeletal muscle ryanodine-sensitive Ca2+ release channels. Am J Physiol Cell Physiol 271: C144–C153, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Barg S, Copello JA, Fleischer S. Different interactions of cardiac and skeletal muscle ryanodine receptors with FK-506 binding protein isoforms. Am J Physiol Cell Physiol 272: C1726–C1733, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Capes EM, Loaiza R, Valdivia HH. Ryanodine receptors. Skelet Muscle 1: 18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng H, Lederer WJ. Calcium sparks. Physiol Rev 88: 1491–1545, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Chu A, Fill M, Stefani E, Entman ML. Cytoplasmic Ca2+ does not inhibit the cardiac muscle sarcoplasmic reticulum ryanodine receptor Ca2+ channel, although Ca2+-induced Ca2+ inactivation of Ca2+ release is observed in native vesicles. J Membr Biol 135: 49–59, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Cooke R. Modulation of the actomyosin interaction during fatigue of skeletal muscle. Muscle Nerve 36: 756–777, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Copello J, Porta M, Diaz-Sylvester P, Nani A, Escobar A, Fleischer S, Fill M. Coordinated gating of multiple ryanodine receptors (RyRs). Biophys J 84: 17A–17A, 2003 [Google Scholar]
- 9. Copello JA, Barg S, Onoue H, Fleischer S. Heterogeneity of Ca2+ gating of skeletal muscle and cardiac ryanodine receptors. Biophys J 73: 141–156, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Copello JA, Barg S, Sonnleitner A, Porta M, Diaz-Sylvester P, Fill M, Schindler H, Fleischer S. Differential activation by Ca2+, ATP and caffeine of cardiac and skeletal muscle ryanodine receptors after block by Mg2+. J Membr Biol 187: 51–64, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Coronado R, Morrissette J, Sukhareva M, Vaughan DM. Structure and function of ryanodine receptors. Am J Physiol Cell Physiol 266: C1485–C1504, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Diaz-Sylvester PL, Copello JA. Voltage-dependent modulation of cardiac ryanodine receptors (RyR2) by protamine. PLos One 4: e8315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diaz-Sylvester PL, Porta M, Copello JA. Halothane modulation of skeletal muscle ryanodine receptors: dependence on Ca2+, Mg2+, and ATP. Am J Physiol Cell Physiol 294: C1103–C1112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diaz-Sylvester PL, Porta M, Copello JA. Modulation of cardiac ryanodine receptor channels by alkaline Earth cations. PLos One 6: e26693, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ding S, Sachs F. Evidence for non-independent gating of P2X2 receptors expressed in Xenopus oocytes. BMC Neurosci 3: 17, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eltit JM, Li H, Ward CW, Molinski T, Pessah IN, Allen PD, Lopez JR. Orthograde dihydropyridine receptor signal regulates ryanodine receptor passive leak. Proc Natl Acad Sci USA 108: 7046–7051, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Figueroa L, Shkryl VM, Zhou J, Manno C, Momotake A, Brum G, Blatter LA, Ellis-Davies GC, Rios E. Synthetic localized calcium transients directly probe signalling mechanisms in skeletal muscle. J Physiol 590: 1389–1411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev 82: 893–922, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Fill M, Zahradnikova A, Villalba-Galea CA, Zahradnik I, Escobar AL, Gyorke S. Ryanodine receptor adaptation. J Gen Physiol 116: 873–882, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fleischer S. Personal recollections on the discovery of the ryanodine receptors of muscle. Biochem Biophys Res Commun 369: 195–207, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Fleischer S, Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem 18: 333–364, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Fleischer S, Ogunbunmi EM, Dixon MC, Fleer EA. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci USA 82: 7256–7259, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fleischer S, Tonomura Yu, United States-Japan Cooperative Science Program Structure and Function of Sarcoplasmic Reticulum. Proceedings of a Symposium on Structure and Function of Sacroplasmic [sic] Reticulum, held at Kansai Seminar House, Ikuno, Dojo-Cho, Kita-Ku, Kobe, Japan, November 1982. New York: Academic, 1985 [Google Scholar]
- 24. Franzini-Armstrong C. Functional implications of RyR-dHPR relationships in skeletal and cardiac muscles. Biol Res 37: 507–512, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Franzini-Armstrong C. Ultrastructural studies on feet/ryanodine receptors. In: Ryanodine Receptors, edited by Sorrentino V. Boca Raton, FL: CRC, 1995, p.1–16 [Google Scholar]
- 26. Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca2+ release units and couplons in skeletal and cardiac muscles. Biophys J 77: 1528–1539, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaburjakova J, Gaburjakova M. Identification of changes in the functional profile of the cardiac ryanodine receptor caused by the coupled gating phenomenon. J Membr Biol 234: 159–169, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Gyorke S, Fill M. Ryanodine receptor adaptation: control mechanism of Ca2+-induced Ca2+ release in heart. Science 260: 807–809, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Gyorke S, Velez P, Suarez-Isla B, Fill M. Activation of single cardiac and skeletal ryanodine receptor channels by flash photolysis of caged Ca2+. Biophys J 66: 1879–1886, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klein S, Timmer J, Honerkamp J. Analysis of multichannel patch clamp recordings by hidden Markov models. Biometrics 53: 870–884, 1997 [PubMed] [Google Scholar]
- 31. Krouse ME, Wine JJ. Evidence that CFTR channels can regulate the open duration of other CFTR channels: cooperativity. J Membr Biol 182: 223–232, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Laver DR, Baynes TM, Dulhunty AF. Magnesium inhibition of ryanodine-receptor calcium channels: evidence for two independent mechanisms. J Membr Biol 156: 213–229, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Laver DR, Eager KR, Taoube L, Lamb GD. Effects of cytoplasmic and luminal pH on Ca(2+) release channels from rabbit skeletal muscle. Biophys J 78: 1835–1851, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laver DR, O'Neill ER, Lamb GD. Luminal Ca2+-regulated Mg2+ inhibition of skeletal RyRs reconstituted as isolated channels or coupled clusters. J Gen Physiol 124: 741–758, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laver DR, Roden LD, Ahern GP, Eager KR, Junankar PR, Dulhunty AF. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membr Biol 147: 7–22, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Ma J. Desensitization of the skeletal muscle ryanodine receptor: evidence for heterogeneity of calcium release channels. Biophys J 68: 893–899, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manivannan K, Ramanan SV, Mathias RT, Brink PR. Multichannel recordings from membranes which contain gap junctions. Biophys J 61: 216–227, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors). Circ Res 88: 1151–1158, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Marx SO, Ondrias K, Marks AR. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science 281: 818–821, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol 56: 485–508, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Meissner G, Darling E, Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry 25: 236–244, 1986 [DOI] [PubMed] [Google Scholar]
- 43. Mejia-Alvarez R, Kettlun C, Rios E, Stern M, Fill M. Unitary Ca2+ current through cardiac ryanodine receptor channels under quasi-physiological ionic conditions. J Gen Physiol 113: 177–186, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller C. Ion Channel Reconstitution. New York: Plenum Press, 1986 [Google Scholar]
- 45. O'Brien J, Valdivia HH, Block BA. Physiological differences between the alpha and beta ryanodine receptors of fish skeletal muscle. Biophys J 68: 471–482, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Olivera JF, Pizarro G. A reappraisal of the Ca2+ dependence of fast inactivation of Ca2+ release in frog skeletal muscle. J Muscle Res Cell Motil 31: 81–92, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Ondrias K, Mojzisova A. Coupled gating between individual cardiac ryanodine calcium release channels. Gen Physiol Biophys 21: 73–84, 2002 [PubMed] [Google Scholar]
- 48. Porta M, Diaz-Sylvester PL, Nani A, Fill M, Fleischer S, Copello JA. Modulation of coordinated gating of ryanodine receptor (RyR) channels in planar lipid bilayers. Biophys J 86: 241A–241A, 2004 [Google Scholar]
- 49. Porta M, Diaz-Sylvester PL, Nani A, Ramos-Franco J, Copello JA. Ryanoids and imperatoxin affect the modulation of cardiac ryanodine receptors by dihydropyridine receptor peptide A. Biochim Biophys Acta 1778: 2469–2479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Porta M, Zima AV, Nani A, Diaz-Sylvester PL, Copello JA, Ramos-Franco J, Blatter LA, Fill M. Single ryanodine receptor channel basis of caffeine's action on Ca2+ sparks. Biophys J 100: 931–938, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Protasi F. Structural interaction between RYRs and DHPRs in calcium release units of cardiac and skeletal muscle cells. Front Biosci 7: d650–658, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C, Huang F, Gaburjakova M, Gaburjakova J, Rosemblit N, Warren MS, He KL, Yi GH, Wang J, Burkhoff D, Vassort G, Marks AR. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol 160: 919–928, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 325: 717–720, 1987 [DOI] [PubMed] [Google Scholar]
- 54. Rios E, Stern MD. Calcium in close quarters: microdomain feedback in excitation-contraction coupling and other cell biological phenomena. Annu Rev Biophys Biomol Struct 26: 47–82, 1997 [DOI] [PubMed] [Google Scholar]
- 55. Rios E, Zhou J, Brum G, Launikonis BS, Stern MD. Calcium-dependent inactivation terminates calcium release in skeletal muscle of amphibians. J Gen Physiol 131: 335–348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rosales RA, Fill M, Escobar AL. Calcium regulation of single ryanodine receptor channel gating analyzed using HMM/MCMC statistical methods. J Gen Physiol 123: 533–553, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rousseau E, Ladine J, Liu QY, Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophys 267: 75–86, 1988 [DOI] [PubMed] [Google Scholar]
- 58. Saito A, Inui M, Radermacher M, Frank J, Fleischer S. Ultrastructure of the calcium release channel of sarcoplasmic reticulum. J Cell Biol 107: 211–219, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saito A, Seiler S, Chu A, Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol 99: 875–885, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schindler H. Planar lipid-protein membranes: strategies of formation and of detecting dependencies of ion transport functions on membrane conditions. Methods Enzymol 171: 225–253, 1989 [DOI] [PubMed] [Google Scholar]
- 61. Sitsapesan R, McGarry SJ, Williams AJ. Cyclic ADP-ribose competes with ATP for the adenine nucleotide binding site on the cardiac ryanodine receptor Ca(2+)-release channel. Circ Res 75: 596–600, 1994 [DOI] [PubMed] [Google Scholar]
- 62. Sitsapesan R, Williams A. The Structure and Function of Ryanodine Receptors. London, UK: Imperial College Press, 1999 [Google Scholar]
- 63. Sitsapesan R, Williams AJ. The gating of the sheep skeletal sarcoplasmic reticulum Ca2+-release channel is regulated by luminal Ca2+. J Membr Biol 146: 133–144, 1995 [DOI] [PubMed] [Google Scholar]
- 64. Smith JS, Coronado R, Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature 316: 446–449, 1985 [DOI] [PubMed] [Google Scholar]
- 65. Smith JS, Coronado R, Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J Gen Physiol 88: 573–588, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith JS, Imagawa T, Ma J, Fill M, Campbell KP, Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol 92: 1–26, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sobie EA, Dilly KW, dos Santos Cruz J, Lederer WJ, Jafri MS. Termination of cardiac Ca(2+) sparks: an investigative mathematical model of calcium-induced calcium release. Biophys J 83: 59–78, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sonnleitner A, Fleischer S, Schindler H. Gating of the skeletal calcium release channel by ATP is inhibited by protein phosphatase 1 but not by Mg2+. Cell Calcium 21: 283–290, 1997 [DOI] [PubMed] [Google Scholar]
- 69. Sorrentino V. Ryanodine Receptors. Boca Raton, FL: CRC, 1996 [Google Scholar]
- 70. Stern MD, Cheng H. Putting out the fire: what terminates calcium-induced calcium release in cardiac muscle? Cell Calcium 35: 591–601, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev 49: 53–98, 1997 [PubMed] [Google Scholar]
- 72. Trapani V, Farruggia G, Marraccini C, Iotti S, Cittadini A, Wolf FI. Intracellular magnesium detection: imaging a brighter future. Analyst 135: 1855–1866, 2010 [DOI] [PubMed] [Google Scholar]
- 73. Tripathy A, Xu L, Mann G, Meissner G. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor). Biophys J 69: 106–119, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tsugorka A, Rios E, Blatter LA. Imaging elementary events of calcium release in skeletal muscle cells. Science 269: 1723–1726, 1995 [DOI] [PubMed] [Google Scholar]
- 75. Velez P, Gyorke S, Escobar AL, Vergara J, Fill M. Adaptation of single cardiac ryanodine receptor channels. Biophys J 72: 691–697, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zahradnikova A, Zahradnik I. Description of modal gating of the cardiac calcium release channel in planar lipid membranes. Biophys J 69: 1780–1788, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhou J, Brum G, Gonzalez A, Launikonis BS, Stern MD, Rios E. Ca2+ sparks and embers of mammalian muscle. Properties of the sources. J Gen Physiol 122: 95–114, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhou J, Yi J, Royer L, Launikonis BS, Gonzalez A, Garcia J, Rios E. A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle. Am J Physiol Cell Physiol 290: C539–C553, 2006 [DOI] [PubMed] [Google Scholar]