Abstract
Intrauterine growth restriction is associated with increased fetal glucocorticoid exposure and an increased risk of adult coronary artery disease. Coronary arteries from sheep exposed to early gestation dexamethasone (Dex) have increased constriction to angiotensin II (ANG II). Prostaglandin E2 (PGE2) helps maintain coronary dilation, but PGE2 production is acutely decreased by Dex administration. We hypothesized early gestation Dex exposure impairs adult coronary PGE2 production with subsequent increases in coronary reactivity. Dex was administered to ewes at 27–28 days gestation (term 145 days). Coronary reactivity was assessed by wire myography in offspring at 4 mo of age (N = 5 to 7). Coronary smooth muscle cells were cultured and prostaglandin production was measured after 90 min incubation with radiolabeled arachidonate. Coronary myocytes from Dex-exposed lambs had a significant decrease in PGE2 production that was reversed with ANG II incubation. Dex-exposed coronary arteries had increased constriction to ANG II and attenuated dilatation to arachidonic acid, with the greatest difference seen after the endothelium was inactivated by rubbing. Preincubation with the cyclooxygenase (COX) inhibitor indomethacin altered control responses and recapitulated the heightened coronary tone seen following Dex exposure. We conclude that impaired coronary smooth muscle COX-mediated PGE2 production contributes to the coronary dysfunction elicited by early gestation Dex. Programmed inhibition of vasodilatory prostanoid production may link an adverse intrauterine environment with adult coronary artery disease.
Keywords: cyclooxygenase, vascular smooth muscle, prostaglandin, fetal programming
while maternal malnutrition and intrauterine growth restriction are independent risk factors for adult cardiovascular disease (10, 19, 33), the pathways and cells types responsible for the inception and propagation of programmed cardiovascular disease have not been fully elucidated. Given their acute physiological effects and potential to elicit epigenetic alterations (13), a number of laboratories have investigated the programming effects of exaggerated intrauterine glucocorticoid exposure. An association between intrauterine steroid exposure and adult cardiovascular disease has been seen in studies showing infants with a low birth weight-to-placenta ratio have the highest risk of hypertension and the lowest activity of placental 11β-hydroxysteroid dehydrogenase (11βHSD) (1). As the enzymatic barrier to transplacental glucocorticoid transfer, 11βHSD typically limits fetal exposure to maternal glucocorticoids, suggesting increased intrauterine glucocorticoid exposure may contribute to adult cardiovascular disease (5, 28).
A direct relationship between intrauterine glucocorticoid exposure and cardiovascular disease has subsequently been demonstrated across animal species. In rats, synthetic glucocorticoid administration during the last week of pregnancy increases offspring blood pressure (2, 18). Similarly, pharmacological inhibition of 11βHSD during pregnancy leads to hypertension in adult offspring (21). Likewise, sheep receiving early gestation dexamethasone (Dex) develop hypertension and coronary artery dysfunction (12, 34). Within this established sheep model, we have now shown that adolescent lambs exposed to Dex in utero have increased coronary constriction to angiotensin II (ANG II) and increased ANG II-induced NAD(P)H oxidase activation (34, 35). Because this programmed coronary dysfunction is independent of changes in ANG II receptor expression (34), we pursued additional pathways that might modulate ANG II-mediated coronary reactivity.
Coronary dysfunction involves both inflammation and altered vascular tone. In atherosclerotic plaques, there is a complex upregulation of ANG II, NAD(P)H oxidase, and cyclooxygenase-2 (COX2) (14, 38, 40). Recent investigations suggest the induction of COX2 expression and subsequent prostaglandin production may provide compensatory coronary artery dilatation in pathological or inflammatory states (8). Nonsteroidal anti-inflammatory agents and glucocorticoids intervene in this process by potently inhibiting prostaglandin production and PGE2 excretion (16).
Beyond global effects on prostanoid production, Dex alters the balance between vasodilatory and vasoconstrictive prostanoids through downregulation of functionally coupled COX2/PGE synthase (17, 23, 26, 27, 29, 43). With recent studies showing the potent vasodilator PGE2 is the major prostanoid produced in the coronary circulation (31, 41), the functional coupling between COX2 and PGE synthase may play a particularly important role in coronary artery physiology. We hypothesized that a suppression of coronary myocyte PGE2 production is an important downstream mechanism of glucocorticoid-mediated fetal programming, resulting in heightened coronary artery tone and increased agonist-induced vasoconstriction.
METHODS
Animal model.
All procedures were performed within the regulations of the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Iowa Animal Care and Use Committee. Time-dated pregnant ewes (mixed Dorset-Suffolk breed) were obtained from Iowa State University (ISU) and housed at the ISU Agricultural Station throughout the study. At 27–28 days gestation, half of the ewes were randomized to receive Dex (0.56 mg/kg) by continuous intravenous infusion over 48 h. Notably, maternal glucocorticoid-dosing guidelines were established by dose-ranging studies in sheep (20), and the early pregnancy exposure we utilized approximates the dose (but not the timing) of Dex used clinically before threatened preterm delivery (24 mg or ∼0.5 mg/kg during the later stage of pregnancy). On a millgram per kilogram basis, the dose we utilized is lower than that used in other preclinical studies that have shown Dex-induced suppression of vascular PGE2 metabolism, including studies in rats (16) and pigeons (11). The timing of the exposure is designed to mimic the effects of maternal-fetal stressors that are operative early in pregnancy. Lambs were born at term (∼145 days gestation) and were allowed to nurse ad libitum before weaning to pasture.
Coronary myocyte culture.
At 4 mo of age, Dex-exposed and control lambs were euthanized with intravenous pentobarbital sodium (50 mg/kg; Abbot Laboratories, Abbott Park, IL). Left anterior descending coronary artery segments were placed in ice-cold PSS with penicillin G (100 U/ml) and streptomycin sulfate (100 μg/ml) and then incubated in minimal essential medium (MEM) with collagenase (1 mg/ml) at 37°C for 60 min. Cells were plated onto collagen-coated dishes, and the cultures were maintained until confluent at 37°C in a humidified atmosphere containing 5% CO2. The incubation media was changed at frequent intervals until second passage cells reached 80% confluence (over 7–9 days). Cells were identified as myocytes given positive staining for α-smooth muscle actin and negative staining for von Willebrand factor.
Radiolabeled arachidonate metabolism.
After overnight serum deprivation (0.1% fetal calf serum), coronary myocytes were equilibrated for 1 h in buffer containing 0.1 μM fatty acid-free bovine serum albumin (Sigma) followed by 90 min incubation in buffer containing 5 μM [3H]arachidonic acid (Perkin-Elmer Life Sciences, Boston, MA). To stimulate prostaglandin production or release, additional incubations were performed in the presence of ANG II (10−10 or 10−7 mol/l). Lipids in the medium were extracted with formic acid followed by addition of ice-cold, water-saturated ethyl acetate. The extracts were dried, and then the lipids were resuspended in acetonitrile and separated by reverse-phase HPLC with a dual pump gradient on a 5-μm 4.6 × 150 mm Discovery C18 column. The retention times of products within the incubation solution were compared with those seen with authentic radiolabeled eicosanoid standards for identification and quantification.
Coronary reactivity.
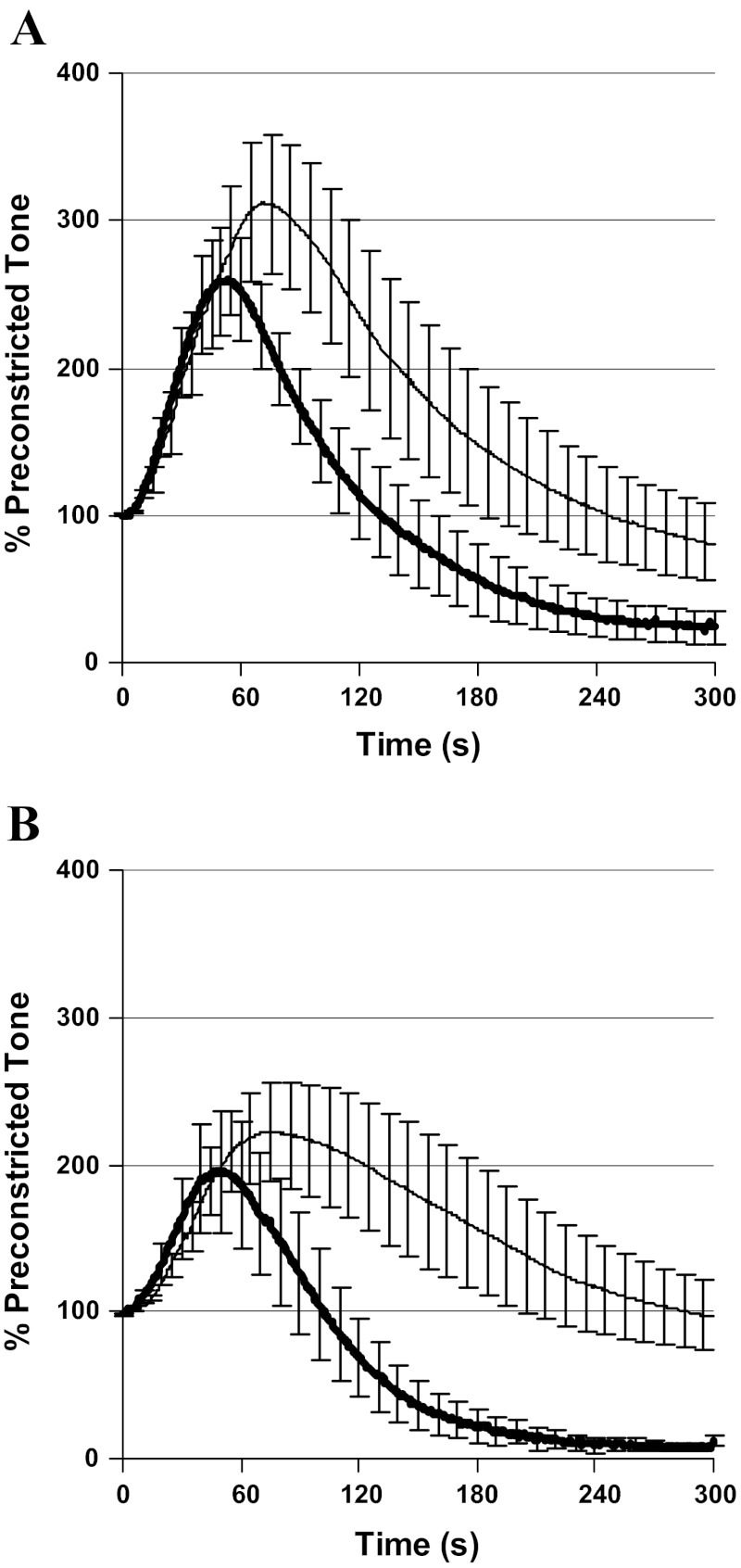
Simultaneous with the left anterior descending coronary harvest for myocyte culture, circumflex coronary arteries were harvested for myography and studied at optimal resting tension (0.7 g-force), as previously described (34). The endothelium of one segment was rubbed with suture, and additional segments were preincubated for 10 min in either indomethacin (10−5 mol/l; nonselective COX inhibitor), NS398 (10−5 mol/l; selective COX2 inhibitor), or PSS buffer alone. The arterial segments were then preconstricted with endothelin-1 (10−7 mol/l) to assess the subsequent vasodilatory responses to 10−6 mol/l arachidonic acid. To verify endothelial inactivation with preserved contractile function, coronary responses to bradykinin (10−7 mol/l) were evaluated. All intact and rubbed vessels initially constricted, but those with a rubbed endothelium lacked subsequent vasodilation (Fig. 1). To further define the role of prostaglandins in programmed coronary reactivity, additional circumflex coronary arteries were collected from a second set of lambs. Initially, contractile responses to 120 mmol/l KCl were recorded, and rings achieving <0.5 g-force were excluded from further analysis. Coronary segments were then reequilibrated and cumulative concentration-response curves were generated for either ANG II (10−11 to 10−7 mol/l) or the thromboxane A2 mimetic U46619 (10−10 to 10−6 mol/l) following a 10-min preincubation with indomethacin (10−5 mol/l), bumetanide (10−5 mol/l), or buffer alone. Bumetanide inhibits Na-K-2Cl cotransporters and increases COX2-dependent prostaglandin production (7, 44). All compounds were acquired from Sigma Chemical (St. Louis, MO) with the exception of endothelin-1 and U46619, both supplied by Alexis (San Diego, CA).
Fig. 1.
Coronary artery segments were preconstricted with U46619 (10−6 mol/l) to assess vasodilatory responses to 10−7 mol/l bradykinin. Results were compared between endothelial intact (thick line) and rubbed arteries (thin line) from control (A, N = 7) and dexamethasone (Dex)-exposed sheep (B, N = 6). Compared with intact arteries, rubbed arteries retained vasoconstriction, but lacked vasodilation.
Immunoblots.
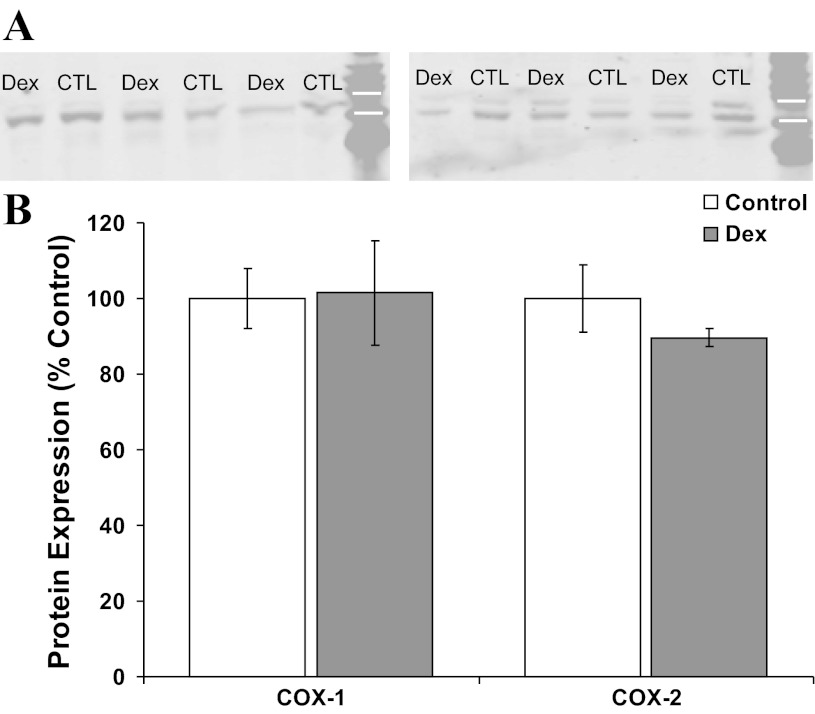
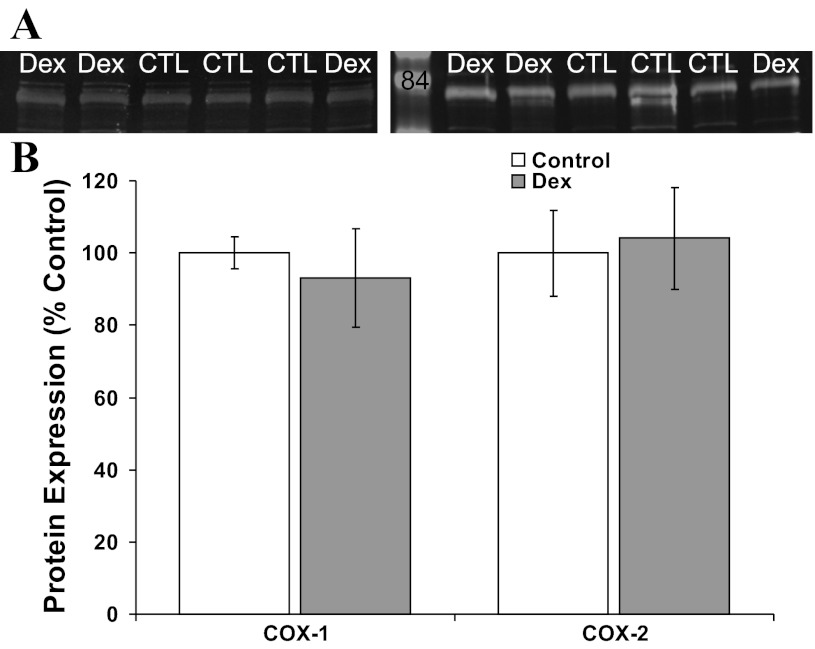
Western blot analysis for COX1 and COX2 was performed on second passage coronary smooth muscle cells from the initial set of sheep, as well as left anterior descending coronary artery segments from the second set of sheep, as previously described (34). Primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were raised in rabbits. Nitrocellulose blots (20 μg protein/lane) were incubated with the primary antibody overnight at 4°C (cells) or 2 h at room temperature (arterial segments). Blots were rinsed, washed, and then incubated for 1 h with a 1:10,000 dilution of goat anti-rabbit infra-red labeled secondary antibody (Molecular Probes, Eugene, OR). Expression was quantified using the Odyssey infrared scanner (Li-Cor, Lincoln, NE). Simultaneous analysis was completed for Dex and control-exposed vessels. For the arterial segments, two replicates were performed for COX1 expression and four replicates for COX2 expression. For each replicate, results were normalized by setting the mean densitometry of the control at 100%. Replicates were averaged for each sheep before obtaining the group average.
Data analysis.
Data are presented as means ± SE. Growth parameters, discrete vascular responses, immunoblot band density, and prostaglandin levels were compared using Student's unpaired, two-tailed t-test (with significance at P < 0.05). Cumulative concentration-responses were compared using analysis of variance (ANOVA), factoring for treatment group and the presence or absence of inhibitors. If ANOVA identified significant differences (P < 0.05), pairwise comparisons were made using the Tukey test. All analyses were performed using SigmaStat 3.0 (SPSS, Chicago, IL).
RESULTS
Myocyte culture.
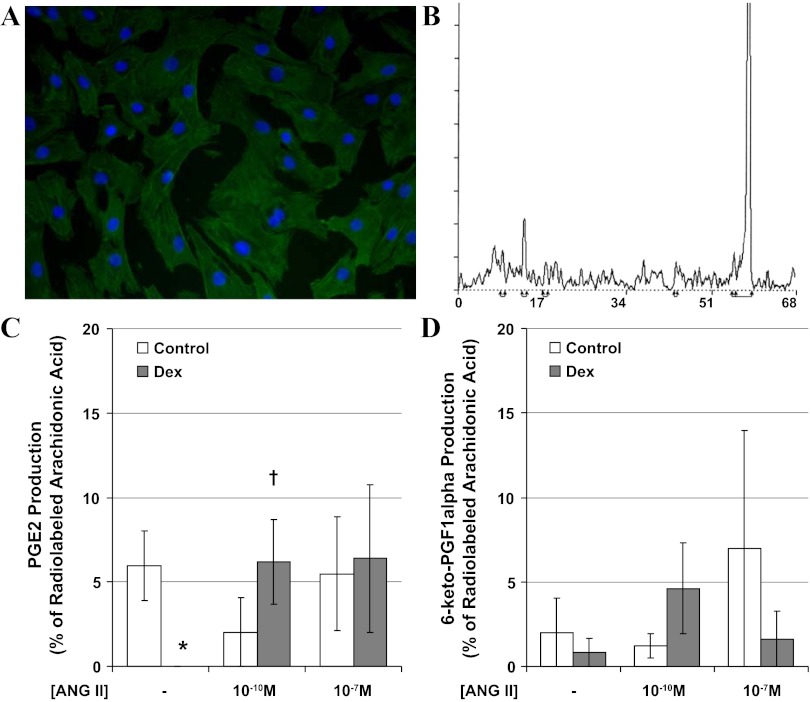
Smooth muscle cell cultures (Fig. 2A) were utilized to evaluate coronary arachidonate metabolism with radiolabeled eicosanoid detection by HPLC (Fig. 2B). While three of four control cultures produced PGE2, no PGE2 was produced by myocytes cultured from Dex-exposed sheep (P < 0.05, Fig. 2C). Incubation with ANG II selectively increased PGE2 production by myocytes from Dex-exposed sheep (P < 0.05, Fig. 2C). Production of the only other eicosanoid identified, 6-keto-PGF1α (stable metabolite of prostacyclin), was not altered by Dex or ANG II (Fig. 2D).
Fig. 2.
Second passage coronary myocytes obtained from adolescent sheep uniformly stained positive for α-smooth muscle actin (A, Dex-exposed cells with DAPI nuclear counterstain). Ninety minutes after incubation with radiolabeled arachidonic acid in the presence and absence of ANG II, lipids were extracted from the media, and eicosanoids were separated by HPLC with peaks identified for 6-keto-PGF1α (retention time 9 min), PGE2 (retention time 13 min), and arachidonic acid (retention time of 57 min) (B, representative run from control cells, y-axis: counts per minute, x-axis: retention time in minutes). The production of PGE2 (C) and 6-keto-PGF1α (D) was compared between control (open bars) and Dex-exposed sheep (solid bars). N = 4 sheep per group, *P < 0.05 vs. control, †P < 0.05 vs. buffer alone.
Myography.
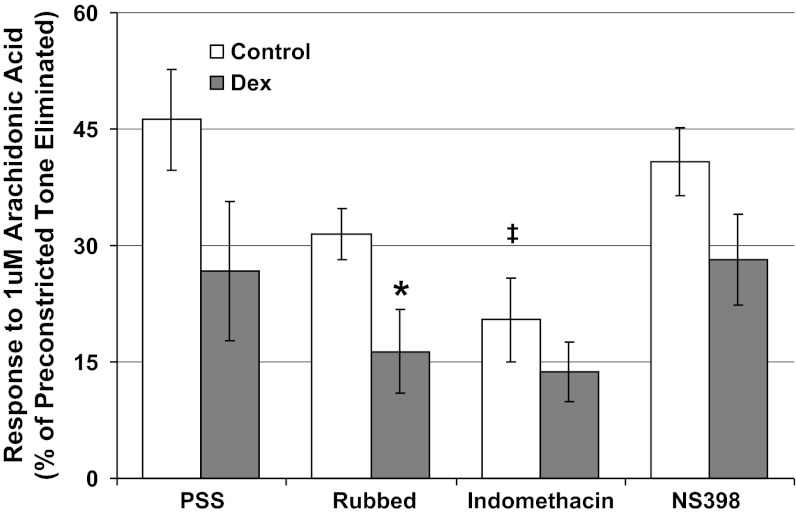
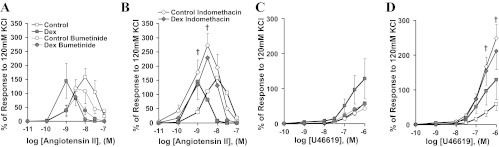
Cumulative addition of arachidonic acid led to coronary vasodilatation that was not significantly decreased by prenatal Dex exposure (Fig. 3, P = 0.10). Rubbed arteries from the same Dex-exposed sheep had significantly decreased arachidonic acid-induced vasodilation (P < 0.05 vs. control), consistent with the impairment in smooth muscle PGE2 production seen in cell culture. Likewise, the nonselective cyclooxygenase inhibitor indomethacin significantly decreased vasodilatation of control (P < 0.01) but not Dex-exposed coronary arteries (P = 0.19), whereas the selective COX2 inhibitor NS398 did not significantly alter coronary responses (Fig. 3). Among the second group of sheep, Dex-exposure enhanced sensitivity to ANG II (Fig. 4A, concentration at which peak response seen: Dex 10−8.8+/−0.1 M, control 10−7.9+/−0.2 M, P < 0.05). For all vessels, cumulative additions of ANG II ultimately led to diminished responses (Fig. 4A). Comparing responses to 10−9 M ANG II (before the loss of tension for any vessel), bumetanide tended to decrease the response of Dex-exposed coronaries (Fig. 4A, P = 0.15) and indomethacin selectively increased the response of control coronary arteries (Fig. 4B, P = 0.02). Similar responses were seen to U46619 with indomethacin selectively increasing the response of control coronaries arteries (Fig. 4D, P = 0.008).
Fig. 3.
Coronary artery segments were preconstricted with endothelin to assess vasodilatory responses to 10−6 mol/l arachidonic acid. Results were compared between endothelial intact arteries in buffer alone (PSS), rubbed arteries with endothelial inactivation, and arteries pretreated with either indomethacin (10−5 mol/l) or the selective COX2 antagonist NS398 (10−5 mol/l). Responses were assessed in Dex-exposed lambs (solid symbols, N = 7) and control lambs (open symbols, N = 7). *P < 0.05 vs. control, ‡P < 0.01 vs. PSS alone.
Fig. 4.
Coronary artery vasoconstriction to cumulative concentrations of ANG II (A and B) or U46619 (C and D) was assessed for control (open symbols, N = 6) and Dex-exposed sheep (shaded symbols, N = 5). Reactivity was assessed during incubation in buffer alone (square symbols), bumetanide (circular symbols), or indomethacin (rhomboid symbols). Compared with responses in buffer alone, indomethacin significantly increased the response of control coronary arteries (†P < 0.05).
Protein expression.
Based on the myocyte and myograph results, we speculated early gestation Dex exposure may permanently decrease coronary smooth muscle cell cyclooxygenase expression. There was no effect of Dex on whole coronary artery COX1 expression by Western blot (Fig. 5). Although Dex decreased coronary COX2 expression to 90 ± 2% of the control mean, this did not approach statistical significance (Fig. 5, P = 0.29). Likewise, early gestation Dex exposure did not significantly alter coronary smooth muscle cell COX1 or COX2 expression (Fig. 6).
Fig. 5.
Western blot analysis for cyclooxygenase (COX) expression in coronary arteries from Dex-exposed and control (CTL) sheep. Representative immunoblots probed with antibodies against COX1 (A, molecular mass 72 kDa) and COX2 (B, molecular mass 72 kDa) identify bands between the 75- and 50-kDa molecular mass markers (white bars). There were no significant differences in COX expression between control (open bars, N = 6) and Dex-exposed sheep (shaded bars, N = 6) (B).
Fig. 6.
Western blot analysis for COX expression by coronary smooth muscle cells cultured from Dex and control (CTL) sheep. A: representative immunoblots probed with antibodies against COX1 (left) and COX2 (right) identify bands just below the labeled 85-kDa molecular mass marker (molecular mass markers at 105 and 50 kDa are seen at the top and bottom of the blot). There were no significant differences in COX expression between control (open bars, N = 6) and Dex-exposed sheep (shaded bars, N = 6) (B).
DISCUSSION
Exaggerated fetal glucocorticoid exposure induces adult-onset hypertension in sheep (12, 34). Hypertension and many of its antecedents are risk factors for the development of coronary artery dysfunction, and coronary heart disease remains the leading cause of death in America (CDC/National Center for Health Statistics). Utilizing this established sheep model, we have previously demonstrated primary alterations in coronary reactivity including enhanced contractility to ANG II and U46619 (35, 36). The major finding of this study is that adolescent lambs exposed to Dex in utero have impaired coronary vasodilation to arachidonic acid in association with impaired coronary myocyte PGE2 production, and this impairment in vasodilatory prostaglandin production contributes to the heightened coronary ANG II reactivity. By utilizing two separate cohorts of sheep for whole coronary artery and coronary artery smooth muscle cell-specific investigations, we have indentified converging evidence of consistent and physiologically relevant alterations in coronary artery physiology.
We previously demonstrated early gestation Dex increases reactive oxygen species production through an ANG II-dependent mechanism. We now confirm heightened ANG II coronary reactivity in this model and have identified an important contribution from programmed alterations in myocyte prostaglandin metabolism. Further studies will be necessary to define the temporal relationship between ANG II-induced reactive oxygen species and PGE2 production. The apparent shift in ANG II responsiveness to lower concentrations suggests the presence of increased ANG II sensitivity. Because we measured PGE2 production over a fixed time interval, the observed increase in PGE2 production may be a manifestation of reduced pathway enzyme Km. Notably, recent investigations have shown reactive oxygen species and PGE2 stimulate each others production within porcine coronary arteries (31, 41).
The suppression of basal coronary myocyte PGE2 production months after intrauterine Dex exposure is a novel finding. As supported by the vascular reactivity data and the known effects of PGE2 on coronary tone, this suppression in PGE2 production appears to have important physiological effects. There are important limitations to isolated myocyte experiments that we attempted to minimize through consistent cell culture approach for Dex and control sheep and the use of complementary whole vessel phenotyping. While we identified PGE2 and prostacyclin as the major eicosanoids produced during the in vitro incubation, it is possible other eicosanoids were produced in lower abundance and were not detected by our assay system. In vivo investigations in both sheep and atherosclerotic-prone species will be needed to further define the functional and pathological significance of these alterations. Further highlighting the benefits of cell-type specific research, a statistically significant reduction in coronary artery vasodilatory response to arachidonic acid was seen in endothelium denuded but not intact coronary arteries. Retrospective power analysis of the intact coronary artery data identified a power of only 0.5 to detect a statistically significant difference of 19.6% with a SD of 17.2%. To have a power of 0.8 to detect a difference of that magnitude, we would have needed to increase our sample size from 7 to 14 sheep. We were instead able to limit the number of vertebrate animals utilized and clarify the cell-type and pathways of interest by capitalizing on length of the sheep coronary artery to perform simultaneous experiments on denuded or otherwise inhibited arterial segments that demonstrated reduced within-group variability.
The anti-inflammatory effects of exogenous glucocorticoids include acute suppression of arachidonate metabolism. We speculated that Dex exposure during a critical window of fetal development might elicit persistent alterations. Clinical studies and animal models have supported this type of longitudinal approach when studying the effects of early life exposures. As a prototypic example, parental-child interactions induce epigenetic alterations of the neuronal glucocorticoid receptor promotor, altering gene expression and adult outcomes (25, 42). Both COX1 and COX2 may play a role in coronary physiology and the inability of NS398 to normalize coronary responses suggests COX1 may be more important than COX2. We initially explored the effects of Dex exposure on COX expression.
Although we did not find a significant difference in COX expression by Western blot, mRNA analysis could identify a shift in the rate of COX enzyme turnover and thus catalytic activity. Further functional and molecular studies focused on COX1 and COX2 regulation are needed, as well as assessment of PGE synthase and the activity of pathways competing for arachidonate or PGH2. These studies could include assessment of the subcellular localization of COX1 and COX2, as well as assessing the potential for presumptive COX2 agonists, such as bumetanide, to improve coronary physiology in Dex-exposed sheep. Furthermore, glucocorticoids are known to suppress arachidonic acid availability by downregulating phospholipase activity (9), and further investigations should incorporate this upstream site of regulation. Notably, ANG II stimulates cPLA2 activity in vascular smooth muscle cells (15), and this could explain the ANG II-mediated reversal of the programmed suppression in PGE2 production.
There are clearly diverse effects of COX-derived prostanoids on coronary physiology. Although enhanced COX2 expression may theoretically predispose to early atherogenesis through leukocyte activation (22) and one study demonstrated a protective effect of highly selective COX2 inhibitors in atheroma-prone mice (6), three other rodent studies have shown highly selective COX2 inhibitors either had no effect on atherogenesis or accelerated lesion progression (30, 32, 37). In humans, an atheroprotective role of vascular COX2 has been hypothesized, based on reduction of COX-derived vasodilatory prostaglandin biosynthesis after COX-inhibition in healthy subjects (24). The important role prostaglandins play in coronary health and disease has been further highlighted following the dissemination of clinical data showing increased cardiovascular morbidity among patients receiving selective COX2 inhibitor therapy (3, 4, 39).
Perspectives and Significance
There is strong theoretical and experimental support for important links between programmed alterations in glucocorticoid homeostasis and prostaglandin metabolism in the development of coronary artery dysfunction. Our glucocorticoid exposure model was designed to mimic the end result of a variety of maternal-fetal stressors, and the physiological alterations we have identified may likewise follow prenatal conditions associated with an increase in transplacental glucocorticoid exposure, including intrauterine growth restriction. Our findings highlight a potentially important role for altered PG production in the programming of coronary artery dysfunction, and our data may have implications for a more generalized effect of glucocorticoids on the vasculature. They suggest the presence of a long-term suppression in basal PGE2 production with an exaggerated sensitivity to ANG II. With evolving pathology, ANG II may activate NAD(P)H oxidase, with resultant inflammation and vasoconstriction. Beyond the direct effects of PGE2 on vascular reactivity, functional coupling between arachidonic acid metabolism and reactive oxygen species production may induce synergistic effects that could ultimately lead to adult-onset coronary artery disease.
GRANTS
This study was supported by National Institutes of Health Grants ES-012268, HD-041922, HD-050359, and HL-62483.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.D.R., F.S.L., and J.L.S. conception and design of research; R.D.R. and K.A.V. performed experiments; R.D.R. analyzed data; R.D.R., K.A.V., F.S.L., and J.L.S. interpreted results of experiments; R.D.R. prepared figures; R.D.R. drafted manuscript; R.D.R., K.A.V., F.S.L., and J.L.S. edited and revised manuscript; R.D.R., K.A.V., F.S.L., and J.L.S. approved final version of manuscript.
REFERENCES
- 1. Barker DJP. Mothers, Babies and Health in Later Life. Edinburgh, UK: Churchill Livingstone, 1998. [Google Scholar]
- 2. Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CRW. Glucocortoid exposure in utero: new model for adult hypertension. Lancet 341: 339–341, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ. for The Study Group VIGOR Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 343: 1520–1528, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Adenomatous polyp prevention on Vioxx (APPROVe) trial investigators. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352: 1092–1102, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Brown RW, Chapman KE, Kotelevtsev Y, Yau JL, Lindsay RS, Brett L, Leckie C, Murad P, Lyons V, Mullins JJ, Edwards CR, Seckl JR. Cloning and production of antisera to human placental 11 beta-hydroxysteroid dehydrogenase type 2. Biochem J 313: 1007–1017, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burleigh ME, Babaev VR, Oates JA, Harris RC, Guatam S, Riendeau D, Marnett LJ, Morrow JD, Fazio S, Linton MF. Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation 105: 1816–1823, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Cheng HF, Wang JL, Zhang MZ, McKanna JA, Harris RC. Role of p38 in the regulation of renal cortical cyclooxygenase-2 expression by extracellular chloride. J Clin Invest 106: 681–688, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cipollone F, Rocca B, Patrono C. Cyclooxygensase-2 expression and inhibition in atherothrombosis. Arterioscler Thromb Vasc Biol 24: 246–255, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cueille C, Frayon S, de Vernejoul MC, Garel JM. Dexamethasone decreases phospholipase C beta1 isozyme expression in human vascular smooth muscle cells. J Steroid Biochem Mol Biol 86: 173–178, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Prevention of cardiovascular disease: birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation 94: 3246–3250, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Deitemeyer D, Yunker RL, Ashraf M, Subbiah MT. Effect of glucocorticoid administration early in life on aortic prostaglandin synthesis and morphology in atherosclerosis-susceptible pigeons. Exp Clin Endocrinol 85: 147–154, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci 94: 149–155, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol 288: R34–R38, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Dzau VJ. Cell biology and genetics of angiotensin in cardiovascular disease. J Hypertens 12: S3–S10, 1994 [PubMed] [Google Scholar]
- 15. Freeman EJ, Ruehr ML, Dorman RV. ANG II-induced translocation of cytosolic PLA2 to the nucleus in vascular smooth muscle cells. Am J Physiol Cell Physiol 274: C282–C288, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Handa M, Kondo K, Suzuki H, Saruta T. Dexamethasone hypertension in rats: role of prostaglandins and pressor sensitivity to norepinephrine. Hypertension 6: 236–241, 1984 [PubMed] [Google Scholar]
- 17. Kubuju DA, Herschman HR. Dexamethasone inhibits mitogen induction of the TIS10 prostaglandin synthase/cyclooxygenase gene. J Biol Chem 267: 7991–7994, 1992 [PubMed] [Google Scholar]
- 18. Langley-Evans SC, Phillips GJ, Gardner DS, Jackson AA. Role of glucocorticoids in programming of maternal diet-induced hypertension in the rat. J Nutr Biochem 7: 173–178, 1996 [Google Scholar]
- 19. Leon DA, Lithell HO, Vâgerö D, Koupilová I, Mohsen R, Berglund L, Lithell UB, McKeigue PM. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15,000 Swedish men and women born 1915–1929. Br Med J 317: 241–245, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol 45: 515–523, 1969 [DOI] [PubMed] [Google Scholar]
- 21. Lindsay RS, Lindsay RM, Edwards CRW, Seckl JR. Inhibition of 11α-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension 27: 1200–1204, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Maier JA, Barenghi L, Bradamante S, Pagani F. Modulators of oxidized LDL-induced hyperadhesiveness in human endothelial cells. Biochem Biophys Res Commun 204: 673–677, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Masferrer JL, Seibert K, Zweifel BS, Needleman P. Endogenous glucocorticoids regulate an inducible cyclooxygenase enzyme. Proc Natl Acad Sci USA 89: 3917–3921, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA 96: 272–277, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12: 342–348, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitchell J, Belvisi M, Akarasereenont P, Robbins RA, Kwon OJ, Croxtall J, Barnes PJ, Vane JR. Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: regulation by Dexamethasone. Br J Pharmacol 113: 1008–1014, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, Kojima F, Ikeda T, Fueki M, Ueno A, Oh S, Kudo I. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem 275: 32783–32792, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Murphy BE, Clark SJ, Donald IR, Pinsky M, Vedady D. Conversion of maternal cortisol to cortisone during placental transfer to the human fetus. Am J Obstet Gynecol 118: 538–541, 1974 [DOI] [PubMed] [Google Scholar]
- 29. Newton R, Seybold J, Kuitert LME, Bergmann M, Barnes PJ. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J Biol Chem 273: 32312–32321, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Olesen M, Kwong E, Maztli A, Kontny F, Seljeflot I, Arnesen H, Lyngdorf L, Falk E. No effect of cyclooxygenase inhibition on plaque size in atherosclerosis-prone mice. Scand Cardiovasc J 36: 362–367, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Oltman CL, Kane NL, Miller FJ, Jr, Spector AA, Weintraub NL, Dellsperger KC. Reactive oxygen species mediate arachidonic acid-induced dilation in porcine coronary microvessels. Am J Physiol Heart Circ Physiol 285: H2309–H2315, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Pratico D, Tillmann C, Zhang ZB, Li H, FitzGerald GA. Acceleration of atherogenesis by COX-1-dependent prostanoid formation in low density lipoprotein receptor knockout mice. Proc Natl Acad Sci USA 98: 3358–3363, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, Colditz GA, Willett WC, Hennekens CH. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. Br Med J 315: 396–400, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roghair RD, Lamb FS, Miller FJ, Jr, Scholz TD, Segar JL. Early gestation dexamethasone programs enhanced postnatal ovine coronary artery vascular reactivity. Am J Physiol Regul Integr Comp Physiol 288: R46–R53, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Roghair RD, Miller FJ, Jr, Scholz TD, Lamb FS, Segar JL. Coronary constriction to angiotensin II is enhanced by endothelial superoxide production in sheep programmed by dexamethasone. Pediatr Res 63: 370–374, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roghair RD, Segar JL, Sharma RV, Zimmerman MC, Jagadeesha DK, Segar EM, Scholz TD, Lamb FS. Newborn lamb coronary artery reactivity is programmed by early gestation dexamethasone before the onset of systemic hypertension. Am J Physiol Regul Integr Comp Physiol 289: R1169–R1176, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rott D, Zhu J, Burnett MS, Zhou YF, Zalles-Ganley A, Ogunmakinwa J, Epstein SE. Effects of MF-tricyclic, a selective cyclooxygenase-2 inhibitor, on atherosclerosis progression and susceptibility to cytomegalovirus replication in apolipoprotein-E knockout mice. J Am Coll Cardiol 41: 1812–1819, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Schonbeck U, Sukhova GK, Graber P, Coulter S, Libby P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am J Pathol 155: 1281–1291, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Solomon DH, Schneeweiss S, Glynn RJ, Kiyota Y, Levin R, Mogun H, Avorn J. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation 109: 2068–2073, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol 285: H2255–H2263, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci 7: 847–854, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Yamada M, Niki H, Yamashita M, Mue S, Ohuchi K. Prostaglandin E2 production dependent upon cyclooxygenase-1 and cyclooxygenase-2 and its contradictory modulation by auranofin in rat peritoneal macrophages. J Pharmacol Exp Ther 281: 1005–1012, 1997 [PubMed] [Google Scholar]
- 44. Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem 275: 37922–37929, 2000 [DOI] [PubMed] [Google Scholar]