Abstract
Sepsis and septic shock lead to considerable morbidity and mortality in developed and developing countries. Despite advances in understanding the innate immune events that lead to septic shock, molecular therapies based on these advances have failed to improve sepsis mortality. The clinical failure of laboratory-derived therapies may be, in part, due to the pleiotropic consequences of the acute inflammatory response, which is the focus of this review. A brisk response to infecting organism is essential for pathogen containment and eradication. However, systemic spread of inflammation beyond a single focus leads to organ injury and higher mortality. The primary goal of this article is to discuss recent animal- and human-based scientific advances in understanding the host response to infection and to highlight how these defense mechanisms can be locally beneficial but systemically detrimental. There are other factors that determine the severity of sepsis that are beyond the scope of this review, including the virulence of the pathogen and regulation by Toll-like receptors. Specifically, this review focuses on how the effector mechanisms of platelets, mast cells, neutrophil extracellular traps (NETs), and the endothelium participate in combating local infections yet can induce organ injury during systemic infection.
Keywords: innate immunity, neutrophil extracellular traps, mast cells, platelets, Toll-like receptor
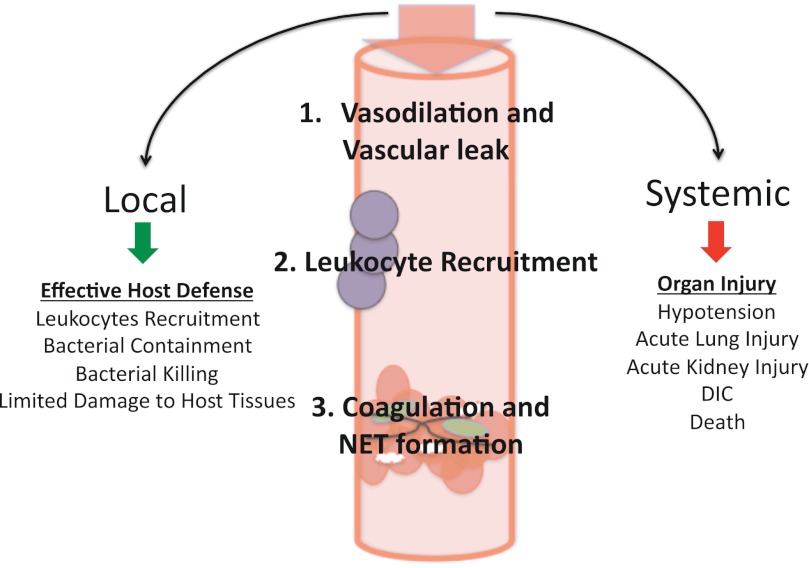
a vigorous response to environmental pathogens is essential for survival. However, sepsis and chronic inflammatory diseases can result from a poorly calibrated immune response. A theme that has evolved over the past 30 years of sepsis research is that locally beneficial host defense mechanisms can become detrimental when activated systemically. Local vasodilation and vascular leak promote the recruitment of neutrophils to sites of infection. However, systemic activation of these same mechanisms leads to diffuse vasodilation, hypovolemia, and septic shock. Local activation of the coagulation cascade slows the hematogenous spread of infection, but diffuse coagulopathy leads to disseminated intravascular coagulation (DIC), a condition associated with increased mortality during septic shock (85). This paradigm, that defense mechanisms are beneficial when activated locally, yet detrimental when activated systemically, is depicted in Fig. 1. The primary objective of this review is to provide an overview of the innate immune sensors that detect pathogens and to outline newly recognized immune mechanisms of platelets, mast cells, neutrophil extracellular traps (NETs), and the endothelium that are beneficial during local immune responses, yet detrimental when systemically activated during severe infection (Table 1).
Fig. 1.
Locally beneficial host defense mechanisms can become detrimental during the systemic spread of infection and inflammation. Local inflammatory mediators, including tumor necrosis factor (TNF) and interleukin (IL)-1β, lead to vasodilation, which recruits leukocytes to sites of infection and sets off a cascade of leukocyte activation, neutrophil extracellular trap (NET) formation, and coagulation. These mechanisms help contain and kill pathogens during localized infection. In contrast, the systemic spread of these same immune mechanisms leads to septic shock, acute organ injury, and potentially death. DIC, disseminated intravascular coagulation.
Table 1.
Beneficial and detrimental actions of platelets, mast cells, NETs, and endothelial cells during infection
| Cell/Mechanism | Beneficial | Detrimental |
|---|---|---|
| Platelets | Phagocytosis (16, 69) | Worsen septic cardiomyopathy (8) |
| Modulate NET formation (21, 26) | Exacerbate lung injury (6) | |
| Facilitate leukocyte diapedesis (14) | Propagate microthrombi formation (42) | |
| Mast Cells | Recruit and activate neutrophils though TNF, IL-6, and DPPI (3, 23, 61, 66) | Lymphocyte apoptosis (58) |
| Increase systemic IL-6 (59) | ||
| NETs | Trap bacteria (18) | Enhance thrombosis (26) |
| Focus antibacterial proteins (51) | Endovascular injury (21) | |
| Endothelial Cells | Directly sense pathogens via TLRs (68) | Propagate disseminated intravascular coagulation (68) |
| Contain local spread of bacteria by local coagulation (41) | Denudation leads to vascular leak (45) |
NET, neutrophil extracellular trap; TNF, tumor necrosis factor; IL-6, interleukin-6; DPPI, dipeptidyl peptidase I; TLRs, Toll-like receptors.
Inciting Events in the Host Response to Infection
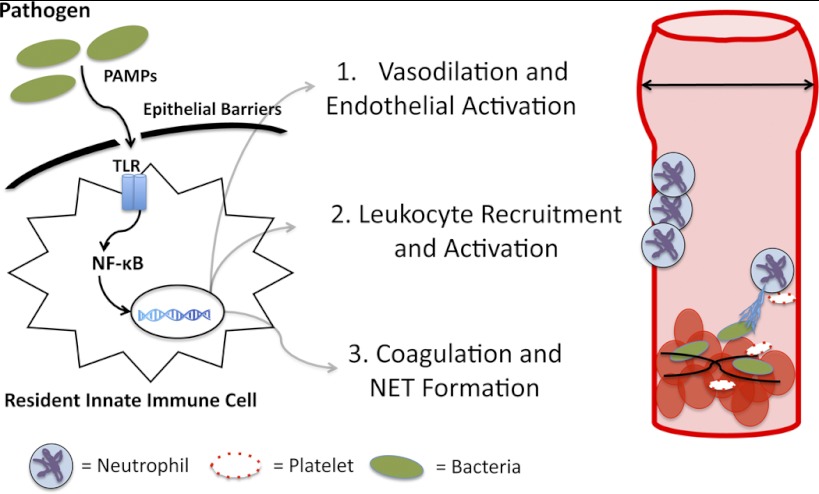
When pathogens breach the mucosal and epithelial barriers that separate the environment from sterile host compartments, pattern recognition receptors, or PRRs, alert the immune system of impending infection. PRR activation leads to a stereotyped transcriptional pattern aimed to recruit and activate leukocytes and contain and kill pathogens (Fig. 2). Five classes of PRRs have been described to date, including Toll-like receptors (TLRs), nucleotide oligomerization domain-like receptors (NLRs), RIG-I-like receptors, C-type lectin receptors, and absence in melanoma 2 (AIM2)-like receptors (11, 12, 22). These receptors are widely expressed on innate and adaptive immune cells as well as on endothelial and epithelial cells. TLRs, a well-characterized subset of PRRs, are germ line encoded and bind molecular motifs specific to microorganisms. A total of 10 human and 13 mouse TLRs have been identified. In humans, TLRs 1, 2, 4, 5, and 6 are expressed on the cell surface and recognize a variety of bacterial, fungal, or protozoal cell surface molecules. In contrast, TLRs 3, 7, 8, and 9 are expressed in the endoplasmic reticulum and endosomal compartments where they recognize microbial nucleic acids, such as unmethylated DNA, and viral single-stranded RNA (71). Together, these receptors recognize the majority of natural pathogens and, when activated, initiate early host defenses against infection.
Fig. 2.
Early cellular and molecular events during infection. Molecular motifs specific to pathogens (PAMPs) are sensed by resident innate immune cells, which express Toll-like receptors (TLRs). TLRs signal via the nuclear factor (NF)-κB signaling cascade, leading to the expression of nitric oxide, which induces vasodilation and increases blood flow to sites of infection. TLR signaling also leads to inflammatory cytokine (TNF, IL-1β, IL-6) and chemokine [monocyte chemotactic protein (MCP)-1, macrophage inflammatory protein (MIP)-1α] production, which recruits monocyte and neutrophil sites of infection. Local cytokine release and the effects of these cytokines on the endothelium increase the procoagulant properties of endothelial cells and induce platelet-neutrophil interactions that lead to the formation of NETs.
TLR4, the most extensively characterized TLR, is essential for defense against lipopolysaccharide (LPS) containing gram-negative bacteria. After gram-negative bacteria invade host tissues, LPS-binding protein recognizes the lipid A moiety of LPS (73), and this complex interacts with CD14, leading to transfer of LPS to TLR4 and MD-2 (40). This complex then signals intracellularly via two adaptor proteins, TRIF and myD88 resulting in the nuclear translocation of AP-1, NF-κB, and IRF3 (66). These transcription factors bind the promoter regions for cytokines [tumor necrosis factor (TNF), interleukin (IL)-1β, IL-6], chemokines (monocyte chemotactic protein-1, macrophage inflammatory protein-1α), reactive oxygen intermediates (ROI), and inducible nitric oxide synthetase (66) (Fig. 2). This transcriptional program helps recruit neutrophils and monocytes to sites of infection and improves the bactericidal capabilities of resident immune cells. Activation of TLR4 is not an exclusively proinflammatory process. In vivo human studies of LPS-induced gene expression in circulating monocytes show that, in addition to inflammatory gene expression, anti-inflammatory genes (IL-10, IL-1Rα) are induced synchronously (21). This bifunctional response rapidly contains and kills pathogens, while limiting collateral damage to adjacent tissue.
The Inflammasome
Inflammasomes are key activators of the host response to infection and tissue damage. Broadly, an inflammasome refers to a macromolecular complex that is required for caspase-1 activation and cleavage of inactive pro-IL-1β into its biologically active form. Four inflammasomes have been discovered thus far. They are named for the PRRs that lead to their assembly, and include the NLRP1, NLRP3, NLRC4, and AIM2 inflammasomes. Both endogenous danger signals, such as double-stranded DNA and uric acid crystals, as well as exogenous pathogen-derived molecules, such as viral RNA or bacterial peptidoglycans, can activate inflammasomes. In addition, the NLRP3 inflammasome is assembled in response to potassium efflux, extracellular ATP, reactive oxygen species (ROS), or lysosomal proteases (22). Thus inflammasomes can either sense pathogens directly or become activated by the intracellular response to infection that may be initiated by other PRRs (44). Multimolecular complexes that form around inflammasome receptors contain a caspase recruitment domain or CARD, which activates the conserved cysteine protease caspase-1. Activated caspase-1 cleaves members of the IL-1 family, including IL-1β and IL-18, into biologically active forms. IL-1β is a potent pyrogenic inflammatory protein that induces the expression of adhesion molecules on endothelial cells, which facilitate neutrophil and monocyte recruitment to sites of infection and injury. In contrast, IL-18 is nonpyrogenic and induces interferon-γ expression by T cells and natural killer cells. Inflammasomes likely have noncaspase-dependent functions as well; however, the molecular mechanisms of these non-IL-1β- and -IL-18-dependent functions have not yet been fully elucidated (36).
Activation of inflammasomes during sepsis and trauma serve to amplify inflammatory signaling (23). However, the influence of this amplification on host defense or organ injury depends on the extent and duration of inflammasome activation. Prior studies have shown that deletion of caspase-1 and caspase inhibitors are protective during mouse models of LPS (87). In addition, recent studies show that IL-18 is elevated in lung-injured mice, and inhibition of caspase 1 or depletion of IL-18 decreases lung injury. In patients with sepsis and lung injury, elevations in IL-18 or markers of caspase-1 activation correlate with increased morbidity and mortality (26). In contrast, activation of the inflammasome may be protective during burn injury (65), since blockade of capase-1 during burn injury in mice increased mortality. Thus key questions regarding how activation of the inflammasome impact outcomes, either favorably or unfavorably during sepsis and trauma, remain to be answered.
TLR4: An Initiator of Both Host Defense and Sepsis Pathology
TLR4 signaling plays an essential role in both host defense against gram-negative infection and in the pathogenesis of sepsis. The “double-edged sword” of TLR4 signaling is highlighted by a review of the early research that led to its discovery. In 1965, Heppner and Weiss (37) reported that C3H/HeJ mice were resistant to LPS-induced shock. This resistance was traced to a spontaneous mutation on chromosome 4, foreshadowing the genetic locus of TLR4 (25). Fifteen years later, it was discovered that LPS-resistant mice were more likely to die when infected with LPS-containing bacteria, including Salmonella typhimurium (62), Neisseria meningities (89), and Escherichia coli (33). Unlike their resistance to purified LPS isolated from bacteria, C3H/HeJ mice died from overwhelming infection when inoculated with as few as two S. typhimium bacteria. This is 1/5,000th the lethal dose for congenic LPS-sensitive mice (62). This example illustrates that activation of the innate immune system through TLR4 is essential for the control of local infections, yet detrimental during systemic immune activation (62).
The Race Between Host and Pathogen
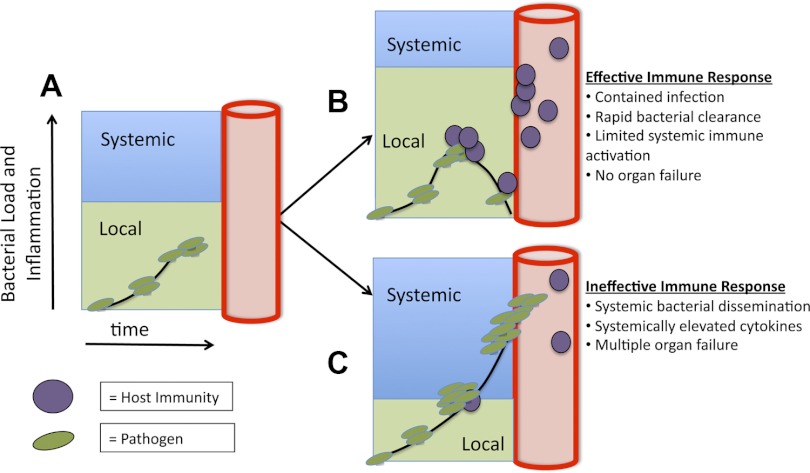
Early infections represent a race between the ability of pathogens to multiply and spread and the hosts' ability to sequester and kill pathogens before they disseminate. This race starts after resident innate immune cells expressing TLRs recognize pathogens, leading to local vasodilation, increased vascular permeability, recruitment of neutrophils and monocytes, and local coagulopathy (63, 72) (Fig. 2). These tightly controlled early events are essential for host protection. They contain and eradicate pathogens, tipping the balance in this race to favor the host. However, if the inoculum is high, the pathogen evades host defenses, or if the host response is slow to gain control over multiplying pathogens, then both the pathogen and the inflammatory response aimed at pathogen containment extend beyond the local environment, and both systemic infection and systemic inflammation ensue (Fig. 3). When the lead in this race changes hands from host to pathogen, many elements of the local immune response, which were once beneficial, become self-defeating. Systemic vasodilation and vascular leak lead to septic shock. Systemic neutrophil and monocyte activation lead to acute lung and kidney injury. Systemic coagulopathy leads to DIC. Evolutionary conservation of these host defense mechanisms has shaped immunity to win frequent local encounters with pathogens at the cost of self-defeating systemic inflammation during systemic infection (10). The following sections outline newly recognized cellular and molecular mechanisms that are necessary for effective host defense and yet contribute to sepsis pathology.
Fig. 3.
Sepsis is a numeric and geographic race between bacterial growth and host defense. A: after the initial inoculum, bacteria or other pathogens begin to propagate within local compartments. B: if the immune response is sufficiently fast, then the spread of pathogens is limited by defense mechanisms, including NET formation, local thromboses, and neutrophil and monocyte recruitment. C: in contrast, if invading pathogens are able to spread outside a single compartment and the inflection point where specific host defense mechanisms shift from benefit to detriment is crossed, then both infection and the inflammatory response to the infection become systemic, resulting in diffuse organ injury and shock.
Platelets
Platelets play an essential role in clot formation and homeostasis. However, emerging data indicate that platelets also play a key role in antibacterial host defense and the pathophysiology of sepsis. Platelets express TLR2, TLR4, and TLR9, allowing them to directly sense pathogens that breach endothelial barriers (7). Activated platelets directly kill pathogens by releasing platelet microbicidal proteins, including kinocidins and thrombocidines, which are stored in platelet granules (93), and by directly phagocytosing pathogens (16).
Platelets also regulate the function of endothelial cells and leukocytes during infection. Stimulation of TLR2 or TLR4 leads to platelet activation, facilitating platelet adherence to endothelial cells in tandem with neutrophils and other leukocytes (14). These interactions are dependent on upregulation of P-selectin on platelets and P-selectin glycoprotein ligand-1 (PSGL-1) on monocytes, lymphocytes, and neutrophils (3). Platelets, which bind more avidly to the endothelium after TLR4 stimulation, can snare neutrophils via P-selectin-PSGL-1 interactions, allowing for secondary interactions between neutrophils and the endothelium via lymphocyte function-associated antigen 1. In addition to enhancing neutrophil recruitment into infected tissues, platelets also enhance the bactericidal activity of neutrophils by increasing levels of TNF (7, 28, 77) and by secreting IL-1β (46) and thromboxane A2 (TXA2), which enhances the respiratory burst activity of neutrophils (56). Antibody blockade of P-selectin decreases neutrophil infiltration and bacterial clearance in a rat model of cecal ligation and puncture (CLP) (64), supporting an in vivo role for platelet P-selectin in host defense. Last, platelets and platelet TLR4 facilitate the formation of microbe-containing NETs. These are discussed in more detail later in this review (see Neutrophils and Extracellular Traps).
Although molecular interactions between platelets, neutrophils, and endothelial cells improve host defense mechanisms during local infections, they may also worsen lung injury (6), decrease microvascular blood flow (74), and induce myocardial dysfunction during sepsis (8). Platelet activation during CLP in mice leads to upregulation of CD40L and P-selectin on platelets, which induces platelet-neutrophil aggregation. Activated platelets can promote the deleterious accumulation of neutrophils in the pulmonary capillaries during sepsis (68). Platelets do this directly, through cell-cell contact, or indirectly through shedding of sCD40L and production of TXA2, which increases the expression of neutrophil Mac-1 (CD11b/CD18) and increases the adhesion of neutrophils to the endothelium (6, 68, 74, 94). These platelet-neutrophil interactions contribute to lung injury in mouse models of sepsis. For example, antibody depletion of platelets, antibody blockade of P-selectin (94), inhibition of TXA2 receptors (94), or genetic deletion of CD40L reduce measures of lung injury, including lung myeloperoxidase (MPO) content, bronchoalveolar lavage neutrophils, and the wet-to-dry ratio (6) and improves survival (94). Platelets may also lead to impairment of microvascular perfusion during sepsis (74). LPS and inflammatory cytokines (e.g., TNF) increase platelet P-selectin and von Willebrand factor on endothelial cells, which promotes platelet adhesion to endothelial cells (47). LPS also increases the expression of tissue factor (TF) on endothelial cells and monocytes, (9) which is a key initiating step in the coagulation cascade. Together, these LPS-induced changes promote platelet aggregation and microthrombi formation through activation of the coagulation cascade in the capillaries of septic mice (74). Lastly, platelet-derived microparticles, which are vesicles generated from the plasma membrane of platelets during activation or apoptosis, increase in the circulation during sepsis (55). These microparticles are a source of the chemokine RANTES, which can exacerbate endothelial damage by recruiting leukocytes to the vascular endothelium (51) and can also decrease vascular and myocardial reactivity by releasing nitric oxide (8). Platelet-derived microparticles perpetuate intravascular coagulation by releasing platelet-activating factor and by externalizing the coagulation cascade initiating phosphatidylserine (55). These interactions among platelets, leukocytes, and the endothelium illustrate the web of inflammation and coagulation that is beneficial during localized infections, yet detrimental when systemically activated in vulnerable vascular territories such as the pulmonary capillary endothelium.
Mast Cells
Two landmark studies using mast cell-deficient W/Wv mice illustrated that mast cells regulate survival from septic peritonitis and K. pneumoniae lung infection in mice (27, 50). These studies found that W/Wv mice recruit fewer neutrophils to sites of infection, have lower local levels of TNF, and are more likely to die than wild-type (WT) controls after infection (50) (27). Consequently, the authors concluded that the survival benefit of mast cells is due to their secretion of TNF, which can recruit neutrophils to sites of infection (50). A subsequent study, however, reported that mast cell activation improves host defense in TNF-deficient mice, indicating that other mast cell products also protect the host from infection (52). One of these products is mast cell tryptase, which promotes neutrophil recruitment to the site of infection via an undefined mechanism (88). Additionally, mast cell IL-6 promotes the local control of infection by enhancing neutrophil bacterial killing (79). These local levels of IL-6 are regulated by mast cell dipeptidyl peptidase I (88). Collectively, these data show that mast cells are critical recruiters of neutrophils to sites of infection. In addition, once neutrophils arrive at sites of infection, mast cell-derived cytokines (TNF, IL-6) enhance the microbicidal capacity of recruited neutrophils. A fundamental observation in all of these studies is that mast cell responses occur during the first hours after infection and that these early responses influence survival of septic mice days later. Thus, early mast cell responses are central regulators of sepsis survival.
In contrast, more recent studies show that systemic mast cell activation can worsen sepsis mortality (69, 75). Mast cells close to the nidus of infection rapidly degranulate to recruit neutrophils and monocytes to infected sites (50). Later in the course of infection, mast cells distant to the initial site of infection also degranulate (76). These systemically activated mast cells release IL-6 and potentially other mediators that worsen survival during infection (76). Mast cell stabilization with cromolyn or doxantrazole decreases systemic IL-6 and TNF levels during endotoxemia (76) and improves survival during CLP (69). Although the mechanisms mediated by systemic mast cell activation that worsen survival during sepsis are not entirely clear, evidence suggests that systemically activated mast cells promote lymphocyte apoptosis (69), which has been shown to be detrimental during infection (38, 39). Thus, mast cell degranulation benefits the host during local responses to infection, yet exacerbates systemic inflammation and lymphocyte apoptosis during systemic infection. These examples further illustrate that locally beneficial responses can be detrimental when widely activated during sepsis.
Neutrophils and Extracellular Traps
Neutrophils are key early responders to infection. These short-lived cells contain peroxidases, proteases, and antimicrobial peptides (LL-37, bacterial permeability increasing enzyme, MPO) that directly kill pathogens (4, 19). During infection, circulating neutrophils follow chemokine gradients into the capillary beds closest to invading microorganisms and diapedese out of capillaries into infected tissues. Neutrophils kill microorganisms through two well-described mechanisms [phagocytosis in which microbes are engulfed and killed by proteases and ROS in phagolysosomes (29) and degranulation during which neutrophils release granules filled with antimicrobial proteins and proteases into the surrounding environment to kill nearby pathogens (15)]. A third mechanism (the formation of neutrophil extracellular traps or NETs) has recently been described (17, 83).
NETS are web-like extracellular structures that form when neutrophils expel genomic DNA impregnated with antimicrobial proteins, including histones, MPO, neutrophil elastase, and cathepsin G (67). NET formation, called NETosis, is a cell death pathway distinct from apoptosis and necrosis (18). During NETosis, signaling via NADPH oxidase and the RAf-MEK-ERK pathway (34) leads to dissolution of the nuclear envelop and cytoplasmic granules, allowing chromatin to mix with granular antimicrobial proteins. Subsequently, this admixture of granules, DNA, and histones is actively expelled from neutrophils into the surrounding extracellular environment. The lattice-like structures of NETs snare microbes and concentrate antimicrobial proteins in the vicinity of these pathogens. NETs also constrain the diffusion of cytotoxic antimicrobial proteins, which can injure host tissues when systemically released (67). Neutrophils form NETs in response to multiple stimuli, including IL-8, LPS, phorbaol 12-myristate,13-acetate, and activated platelets and endothelial cells (18). Importantly, NETs have been observed both in vivo and during in vitro flow conditions that model physiological blood flow and have been shown to bind and kill a wide array of micro-organisms, including gram-positive bacteria (Staphylococcus aureus, Shigella flexneri) (17), gram-negative bacteria (E. coli) (24), fungi (Candida albicans) (53, 84), and protozoa (Leishmania amazonensis) (82).
In vivo studies of NET formation show that NETs play a role in both host defense and organ injury (24) (31). Recently, it was shown that platelet TLR4 expression is essential for NET formation during endotoxic shock (24). In this study, LPS injection led to sequestration of neutrophils in pulmonary capillaries and hepatic sinusoids. However, NET formation required additional signals from activated platelets. When neutrophil TLR4 was deleted, NET formation still occurred. In contrast, deletion of TLR4 from platelets dramatically reduced the formation of NETs. In vivo imaging of fluorescent E. coli showed that NETs can trap bacteria in liver sinusoids. Depletion of platelets in this model abrogated NET formation and E. coli trapping. These data suggest that platelets may serve as a circulating barometer of systemic bacteremia (via TLR4) and that, during severe infection, the activation state of platelets may determine the extent of NET formation.
In addition to trapping circulating bacteria during infection, widespread NET formation may lead to organ injury during sepsis. During LPS-induced inflammation in mice, NETs form in the liver, occlude hepatic sinusoids, and lead to hepatocellar injury (24). Depletion of platelets or neutrophils abrogated this effect. Although it is unclear if NETs are protective against viral infections, NETs have been identified in areas of endothelial and epithelial damage during influenza pneumonia in mice (60). These findings, in addition to in vitro coculture experiments showing that NETs damage endothelial cells, suggest that NET formation contributes to endothelial and epithelial damage during lung injury (60). Although the exact mechanism for endothelial and epithelial damage is unclear, it may be due to exposure of host tissues to cytotoxic histones, peroxidases, and proteases. NETs may also contribute to the pathology of sepsis by linking septic inflammation to microvascular thrombosis (31). When NETs are perfused with blood in vitro, platelet adhesion, activation, and aggregation ensue, closely followed by fibrin deposition and thrombus formation. Additionally, markers of NET formation have been found in large animal models of deep venous thrombosis (31). In summary, NETs benefit the host during local infection by trapping and killing pathogens. However, during systemic infection, NETs can damage the endothelium and cause diffuse thromboses, leading to DIC and acute lung injury, both of which increase mortality during sepsis.
The Endothelium
Historically, the endothelium has been viewed as a passive conduit that carriers oxygen-rich blood and nutrients to vital organs. However, it is now recognized that the endothelium is a regionally diverse immunologic organ that orchestrates key components of the immune response to infection. Stimulation of endothelial cells with TLR agonists (LPS, virus) leads the upregulation of E-selectin, P-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 (1, 2) as well as cytokine (IFN-α, INF-γ, IL-6) and chemokine (CCL2, CCL3, CCL5) production (80). These alterations in the adhesive properties of the endothelium lead to increased rolling, adherence, and migration of leukocytes into infected tissues. The adhesion of leukocytes to endothelial cells sets up an inflammatory loop, mediated by TNF and IL-1β, which induces endothelial cells to increase chemokine expression and in turn recruit more monocytes and neutrophils into infected tissues. LPS-mediated stimulation of endothelial cells also shifts the hemostatic balance of endothelial cells from anticoagulant to procoagulant through decreased expression of thrombomodulin, tissue-type plasminogen activator, and heparin as well as increased expression of TF and plasminogen activator inhibitor 1 (PAI-1) (13, 57). LPS stimulation of endothelial cells also leads to endothelial cell apoptosis, which exposes prothrombotic subendothelial proteins to clotting factors in the blood and leads to cytoskeletal alterations that decrease endothelial barrier function and allow intravascular fluid to leak into the extravascular space. These alterations, which are largely TLR mediated, can be either beneficial or detrimental, depending on the location and duration of endothelial activation.
Animal models of infection show that LPS- and TNF-mediated changes in the endothelium are important for host defense. Although tissue resident innate immune cells (Fig. 1) have been credited with initiating the early immune response, the endothelium can also directly sense and respond to infection. Mice in which TLR4 expression is restricted to endothelial cells are capable of recruiting neutrophils to sites of infection (5). In addition to sensing bacteria, the endothelium regulates the balance between pro- and anticoagulant properties of the blood. When bacterial products activate the endothelium, the balance shifts from anti- to procoagulant, and this shift limits the endovascular spread of infection (54). This was illustrated in a rat model of ascending urinary tract infection. Heparin treatment before the induction of an E. coli urinary tract infection disseminated the bacteria to the liver, spleen, and heart. Untreated animals effectively contained the infection in the urinary tract, suggesting that local coagulation limits the spread of infection (54). In addition, mice that lack two key endothelial procoagulant proteins, TF and PAI-1, have decreased survival during intraperitoneal infection (48) and higher hepatic bacterial burdens, indicating that, under the right circumstances, a procoagulant shift in the endothelium can help limit the endovascular spread of infection.
Although the phenotypic changes of the activated endothelium may limit the spread of regional infection, during severe systemic inflammation, upregulation of endothelial adhesion molecules, disruption of the endothelial barrier, and loss of the anticoagulant properties of the endothelium can worsen organ injury and mortality. This was experimentally illustrated in a transgenic mouse model in which a degradation-resistant form I-κBα was overexpressed in the vascular endothelium, resulting in sequestration of NF-κB and effective blunting of the TLR4 signaling cascade (92). The endothelium of these mice expressed lower levels of vascular adhesion molecules after LPS stimulation. In addition, these mice had improved survival after LPS injection, decreased tissue infiltration of neutrophils, and improved endothelial barrier function. Notably, tissue infiltration of bacteria did not differ between mice with an attenuated endothelial response and wild-type mice. In an influenza virus model of lethal pneumonia, vascular endothelial cells were shown to be a key source of chemokines (CCL2, CCL5) that recruited the monocytes and neutrophils responsible for lung injury (80). In this model, modulating the inflammatory response of the endothelium with sphingosine 1-phosphate agonists improved mortality by downregulating vascular endothelial cell chemokine secretion (80). Thus, the endothelium can both directly sense pathogens and signal to innate immune cells to migrate to sites of infection, yet this same response can be detrimental in vulnerable vascular territories such as the pulmonary vascular endothelium.
Overwhelming endothelial cell activation also leads to endothelial cell apoptosis with subsequent denudation and sloughing of the endothelium. This process results in increased vascular permeability, exacerbates the inflammatory response, and worsens intravascular coagulation. Human studies show that increases in circulating endothelial cells due to widespread endothelial cell apoptosis is associated with increased mortality during sepsis (59). Several recent studies highlight the importance of the damaged endothelium as a key lesion during sepsis and as a potential target for therapy (78). In mouse models of endotoxin-mediated shock, CLP or H5N1 influenza, the delivery of a soluble Slit ligand tightened the vascular endothelium and improved survival. Improved survival was mediated by signaling through the endothelial-specific receptor Robo4, which improved VE-cadherin mediated adhesion between damaged endothelial cells (45). In summary, the endothelium is an active sensor of infection and can recruit cells to sites of infection while restraining the endovascular spread of infection via intravascular coagulation. Nonetheless, widespread activation of these same defense mechanisms can lead to endovascular injury with resultant increases in vascular permeability and a shift toward procoagulant properties of the endothelium. These mechanisms can worsen organ injury and survival during widespread systemic activation due to severe sepsis.
Sterile Inflammation
Endogenous danger-associated molecular patterns (DAMPs), often released from necrotic or apoptotic tissues, can lead to a syndrome of sterile inflammation in postsurgical or trauma patients, which is very similar to the immunological and physiological derangements seen during septic inflammation. Indeed, TLRs and inflammasome-associated receptors can sense endogenous danger signals and lead to an inflammatory response similar to bacterial sepsis termed the systemic inflammatory response syndrome. For example, the release of mitochondrial-derived DNA acts as a clinically relevant DAMP during trauma in humans (95). Mechanistically, mitochondrial DNA is sensed by TLR9, which also sense bacterial DNA. Mitochondria are evolutionary endosymbionts, derived from ancient bacteria, and this relationship between bacteria and mitochondria likely explains the overlap in the recognition of both nucleic acids by TLR9 (35). Additional mediators of sterile or infectious inflammation include histones H3 and H4. These nuclear proteins, which are key structural components of chromatin, are released during sterile injury and during E. coli infection (91). Injection of H3 or H4 into mice leads to neutrophil activation, endothelial injury, and death (91). In addition, extracellular histones lead to inflammatory injury via TLR2 and TLR4 activation during animal models of sterile inflammation and chemically induced cellular injury (90). These examples of endogenous danger signals form a link in the pathophysiological similarities between immune activation during infection and immune activation during trauma or surgery.
Shifting The Targets For Future Sepsis Therapy
An inadequate molecular understanding of the key sepsis lesions has limited the development of therapies for sepsis. Activated protein C, the one clinical victory for molecular sepsis therapy, was recently withdrawn from the market due to lack of efficacy in the PROWESS-SHOCK trial (70). In addition, attempts at antibody blockade of single cytokines during sepsis were unsuccessful, perhaps because early sepsis mediators, including TNF and IL-1β, have peaked by the time therapy was initiated. To more effectively treat patients with sepsis, a focus on late mediators of septic injury or on delayed physiological consequences, including vascular leak and end organ damage, may be a more effective approach. Several recent studies highlight the endothelium as a potential target. Additionally, enhancing anti-inflammatory, anti-bacterial, and repair pathways may have value (32, 42, 61). Mitigating postseptic immunosuppression by immunostimulation may be an additional target for therapy. Lastly, reducing the development of sepsis in high-risk patients, such as those who are immunosuppressed or undergoing high-risk surgical procedures, may prevent septic death.
Conclusions
Platelets, mast cells, NETs, and the endothelium, through their own specific immune mechanisms, can be beneficial during localized infection yet detrimental during systemic inflammation (Table 1). This list of host defense mechanisms is not comprehensive. There are other pathways that clearly contribute to the pathogenesis of sepsis, including components of the complement cascade (43, 86), elements of the coagulation pathway, and classic proinflammatory cytokines (41, 81). An effective host response requires a delicate balance between these different elements of host defense. The inflection points where these immune mechanisms tip from beneficial to detrimental are not likely to be synchronous, which may complicate the design of effective therapy. A global staging system based on systemic biological and clinical markers would be a significant step forward in sepsis research. However, rigorous efforts to stage sepsis have been complicated by the heterogeneity of septic patients, who differ in their predisposition to infection, the type and virulence of pathogens, and the sites of infection (49). Comorbidities, including liver diseases, cancer, advanced age, and environmental variables such as alcohol, cigarette exposure, and socioeconomic factors can also alter host defense mechanisms (20, 30, 58). Eventually, a multimodal therapeutic approach based on inhibiting selected host defense mechanisms when they become detrimental and focusing on cell and molecular therapies that repair damaged organ systems may be the best path forward in treating severe sepsis and septic shock.
GRANTS
M. A. Matthay was supported by National Institutes of Health Grants HL-051856, HL-051854, and AI-053194.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.J.S. prepared figures; E.J.S. drafted manuscript; E.J.S., M.A.M., and P.J.W. edited and revised manuscript; E.J.S., M.A.M., and P.J.W. approved final version of manuscript.
REFERENCES
- 1. Aird WC. The hematologic system as a marker of organ dysfunction in sepsis. Mayo Clin Proc 78: 869–881, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101: 3765–3777, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Ait-Oufella H, Maury E, Lehoux S, Guidet B, Offenstadt G. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med 36: 1286–1298, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha FQ. The role of neutrophils in severe sepsis. Shock 30, Suppl 1: 3–9, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Andonegui G, Zhou H, Bullard D, Kelly MM, Mullaly SC, McDonald B, Long EM, Robbins SM, Kubes P. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J Clin Invest 119: 1921–1930, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asaduzzaman M, Lavasani S, Rahman M, Zhang S, Braun OO, Jeppsson B, Thorlacius H. Platelets support pulmonary recruitment of neutrophils in abdominal sepsis. Crit Care Med 37: 1389–1396, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, Ni H, Lazarus AH, Freedman J, Semple JW. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 107: 637–641, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Azevedo LC, Janiszewski M, Pontieri V, Pedro Mde A, Bassi E, Tucci PJ, Laurindo FR. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit Care 11: R120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergmeier W, Chauhan AK, Wagner DD. Glycoprotein Ibalpha and von Willebrand factor in primary platelet adhesion and thrombus formation: lessons from mutant mice. Thromb Haemost 99: 264–270, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430: 257–263, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Beutler B. Toll-like receptors and their place in immunology. Where does the immune response to infection begin? Nat Rev Immunol 4: 498, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Beutler B, Poltorak A. Sepsis and evolution of the innate immune response. Crit Care Med 29: S2–S7, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci USA 84: 9238–9242, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, Hayashi C, Genco CA, Iafrati M, Freedman JE. Stimulation of Toll-like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3-kinase. Circ Res 104: 346–354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borregaard N. Neutrophils, from marrow to microbes. Immunity 33: 657–670, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Boukour S, Cramer EM. Platelet interaction with bacteria. Platelets 16: 215–217, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5: 577–582, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet 368: 157–169, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, Cohen MJ. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med 183: 1660–1665, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature 437: 1032–1037, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10: 826–837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med 37: 291–304, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 13: 463–469, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Coutinho A, Forni L, Melchers F, Watanabe T. Genetic defect in responsiveness to the B cell mitogen lipopolysaccharide. Eur J Immunol 7: 325–328, 1977 [DOI] [PubMed] [Google Scholar]
- 26. Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, Choi AM. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 185: 1225–1234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381: 75–77, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Elzey BD, Schmidt NW, Crist SA, Kresowik TP, Harty JT, Nieswandt B, Ratliff TL. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood 111: 3684–3691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ermert D, Zychlinsky A, Urban C. Fungal and bacterial killing by neutrophils. Methods Mol Biol 470: 293–312, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study (Abstract). Crit Care 13: R18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 107: 15880–15885, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179: 1855–1863, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Svanborg EC. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun 46: 839–844, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol 7: 75–77, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Hauser CJ, Sursal T, Rodriguez EK, Appleton PT, Zhang Q, Itagaki K. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma 24: 534–538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henry T, Monack DM. Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell Microbiol 9: 2543–2551, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Heppner G, Weiss DW. High susceptibility of strain a mice to endotoxin and endotoxin-red blood cell mixtures. J Bacteriol 90: 696–703, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol 1: 496–501, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 6: 813–822, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Juan TS, Hailman E, Kelley MJ, Busse LA, Davy E, Empig CJ, Narhi LO, Wright SD, Lichenstein HS. Identification of a lipopolysaccharide binding domain in CD14 between amino acids 57 and 64. J Biol Chem 270: 5219–5224, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Koebernick H, Grode L, David JR, Rohde W, Rolph MS, Mittrucker HW, Kaufmann SH. Macrophage migration inhibitory factor (MIF) plays a pivotal role in immunity against Salmonella typhimurium. Proc Natl Acad Sci USA 99: 13681–13686, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 302: L1003–L1013, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laarman AJ, Bardoel BW, Ruyken M, Fernie J, Milder FJ, van Strijp JA, Rooijakkers SH. Pseudomonas aeruginosa alkaline protease blocks complement activation via the classical and lectin pathways. J Immunol 188: 386–393, 2012 [DOI] [PubMed] [Google Scholar]
- 44. Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol 11: 213–220, 2011 [DOI] [PubMed] [Google Scholar]
- 45. London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, Day CW, Barnard DL, Zimmerman GA, Krasnow MA, Li DY. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med 2: 19–23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Loppnow H, Bil R, Hirt S, Schonbeck U, Herzberg M, Werdan K, Rietschel ET, Brandt E, Flad HD. Platelet-derived interleukin-1 induces cytokine production, but not proliferation of human vascular smooth muscle cells. Blood 91: 134–141, 1998 [PubMed] [Google Scholar]
- 47. Lou J, Donati YR, Juillard P, Giroud C, Vesin C, Mili N, Grau GE. Platelets play an important role in TNF-induced microvascular endothelial cell pathology. Am J Pathol 151: 1397–1405, 1997 [PMC free article] [PubMed] [Google Scholar]
- 48. Luo D, Szaba FM, Kummer LW, Plow EF, Mackman N, Gailani D, Smiley ST. Protective roles for fibrin, tissue factor, plasminogen activator inhibitor-1, and thrombin activatable fibrinolysis inhibitor, but not factor XI, during defense against the gram-negative bacterium Yersinia enterocolitica. J Immunol 187: 1866–1876, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lvovschi V, Arnaud L, Parizot C, Freund Y, Juillien G, Ghillani-Dalbin P, Bouberima M, Larsen M, Riou B, Gorochov G, Hausfater P. Cytokine profiles in sepsis have limited relevance for stratifying patients in the emergency department: a prospective observational study. PLoS One 6: e28870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 381: 77–80, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Mastronardi ML, Mostefai HA, Meziani F, Martinez MC, Asfar P, Andriantsitohaina R. Circulating microparticles from septic shock patients exert differential tissue expression of enzymes related to inflammation and oxidative stress. Crit Care Med 39: 1739–1748, 2011 [DOI] [PubMed] [Google Scholar]
- 52. Maurer M, Echtenacher B, Hultner L, Kollias G, Mannel DN, Langley KE, Galli SJ. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med 188: 2343–2348, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, Heesemann J, Ebel F. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect 12: 928–936, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Melican K, Boekel J, Mansson LE, Sandoval RM, Tanner GA, Kallskog O, Palm F, Molitoris BA, Richter-Dahlfors A. Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis Cell Microbiol 10: 1987–1998, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Meziani F, Delabranche X, Asfar P, Toti F. Bench-to-bedside review: circulating microparticles–a new player in sepsis? Crit Care 14: 236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miedzobrodzki J, Panz T, Plonka PM, Zajac K, Dracz J, Pytel K, Mateuszuk L, Chlopicki S. Platelets augment respiratory burst in neutrophils activated by selected species of gram-positive or gram-negative bacteria. Folia Histochem Cytobiol 46: 383–388, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Moore KL, Andreoli SP, Esmon NL, Esmon CT, Bang NU. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest 79: 124–130, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 31: 869–877, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Mutunga M, Fulton B, Bullock R, Batchelor A, Gascoigne A, Gillespie JI, Baudouin SV. Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med 163: 195–200, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 179: 199–210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15: 42–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. O'Brien AD, Rosenstreich DL, Scher I, Campbell GH, MacDermott RP, Formal SB. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol 124: 20–24, 1980 [PubMed] [Google Scholar]
- 63. Opal SM, Esmon CT. Bench-to-bedside review: functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit Care 7: 23–38, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Opal SM, Sypek JP, Keith JC, Jr, Schaub RG, Palardy JE, Parejo NA. Evaluation of the safety of recombinant P-selectin glycoprotein ligand-immunoglobulin G fusion protein in experimental models of localized and systemic infection. Shock 15: 285–290, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Osuka A, Hanschen M, Stoecklein V, Lederer JA. A protective role for inflammasome activation following injury. Shock 37: 47–55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113: 153–162, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol 30: 513–521, 2009 [DOI] [PubMed] [Google Scholar]
- 68. Rahman M, Zhang S, Chew M, Ersson A, Jeppsson B, Thorlacius H. Platelet-derived CD40L (CD154) mediates neutrophil upregulation of Mac-1 and recruitment in septic lung injury. Ann Surg 250: 783–790, 2009 [DOI] [PubMed] [Google Scholar]
- 69. Ramos L, Pena G, Cai B, Deitch EA, Ulloa L. Mast cell stabilization improves survival by preventing apoptosis in sepsis. J Immunol 185: 709–716, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med 366: 2055–2064, 2012 [DOI] [PubMed] [Google Scholar]
- 71. Salomao R, Martins PS, Brunialti MK, Fernandes Mda L, Martos LS, Mendes ME, Gomes NE, Rigato O. TLR signaling pathway in patients with sepsis. Shock 30, Suppl 1: 73–77, 2008 [DOI] [PubMed] [Google Scholar]
- 72. Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol 83: 536–545, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ. Structure and function of lipopolysaccharide binding protein. Science 249: 1429–1431, 1990 [DOI] [PubMed] [Google Scholar]
- 74. Secor D, Li F, Ellis CG, Sharpe MD, Gross PL, Wilson JX, Tyml K. Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Med 36: 1928–1934, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Seeley EJ, Sutherland RE, Kim SS, Wolters PJ. Systemic mast cell degranulation increases mortality during polymicrobial septic peritonitis in mice. J Leukoc Biol 90: 591–597, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Seeley EJ, Sutherland RE, Kim SS, Wolters PJ. Systemic mast cell degranulation increases mortality during polymicrobial septic peritonitis in mice. J Leukoc Biol 90: 591–597, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 11: 264–274, 2011 [DOI] [PubMed] [Google Scholar]
- 78. Su G, Atakilit A, Li JT, Wu N, Bhattacharya M, Zhu J, Shieh JE, Li E, Chen R, Sun S, Su CP, Sheppard D. Absence of integrin alphavbeta3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am J Respir Crit Care Med 185: 58–66, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol 181: 5598–5605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146: 980–991, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330: 662–664, 1987 [DOI] [PubMed] [Google Scholar]
- 82. Urban C, Zychlinsky A. Netting bacteria in sepsis. Nat Med 13: 403–404, 2007 [DOI] [PubMed] [Google Scholar]
- 83. Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5: e1000639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 8: 668–676, 2006 [DOI] [PubMed] [Google Scholar]
- 85. Voves C, Wuillemin WA, Zeerleder S. International Society on Thrombosis and Haemostasis score for overt disseminated intravascular coagulation predicts organ dysfunction and fatality in sepsis patients. Blood Coagul Fibrinolysis 17: 445–451, 2006 [DOI] [PubMed] [Google Scholar]
- 86. Ward PA, Gao H. Sepsis, complement and the dysregulated inflammatory response. J Cell Mol Med 13: 4154–4160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Weber P, Wang P, Maddens S, Wang P, Wu R, Miksa M, Dong W, Mortimore M, Golec JM, Charlton P. VX-166: a novel potent small molecule caspase inhibitor as a potential therapy for sepsis (Abstract). Crit Care 13: R146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem 276: 18551–18556, 2001 [DOI] [PubMed] [Google Scholar]
- 89. Woods JP, Frelinger JA, Warrack G, Cannon JG. Mouse genetic locus Lps influences susceptibility to Neisseria meningitidis infection. Infect Immun 56: 1950–1955, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol 187: 2626–2631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med 15: 1318–1321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ye X, Ding J, Zhou X, Chen G, Liu SF. Divergent roles of endothelial NF-kappaB in multiple organ injury and bacterial clearance in mouse models of sepsis. J Exp Med 205: 1303–1315, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yeaman MR. Platelets in defense against bacterial pathogens. Cell Mol Life Sci 67: 525–544, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest 116: 3211–3219, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]