Abstract
Despite advancements in renal replacement therapy, the mortality rate for acute kidney injury (AKI) remains unacceptably high, likely due to remote organ injury. Kidney ischemia-reperfusion injury (IRI) activates cellular and soluble mediators that incite a distinct pulmonary proinflammatory and proapoptotic response. Tumor necrosis factor receptor 1 (TNFR1) has been identified as a prominent death receptor activated in the lungs during ischemic AKI. We hypothesized that circulating TNF-α released from the postischemic kidney induces TNFR1-mediated pulmonary apoptosis, and we aimed to elucidate molecular pathways to programmed cell death. Using an established murine model of kidney IRI, we characterized the time course for increased circulatory and pulmonary TNF-α levels and measured concurrent upregulation of pulmonary TNFR1 expression. We then identified TNFR1-dependent pulmonary apoptosis after ischemic AKI using TNFR1−/− mice. Subsequent TNF-α signaling disruption with Etanercept implicated circulatory TNF-α as a key soluble mediator of pulmonary apoptosis and lung microvascular barrier dysfunction during ischemic AKI. We further elucidated pathways of TNFR1-mediated apoptosis with NF-κB (Complex I) and caspase-8 (Complex II) expression and discovered that TNFR1 proapoptotic signaling induces NF-κB activation. Additionally, inhibition of NF-κB (Complex I) resulted in a proapoptotic phenotype, lung barrier leak, and altered cellular flice inhibitory protein signaling independent of caspase-8 (Complex II) activation. Ischemic AKI activates soluble TNF-α and induces TNFR1-dependent pulmonary apoptosis through augmentation of the prosurvival and proapoptotic TNFR1 signaling pathway. Kidney-lung crosstalk after ischemic AKI represents a complex pathological process, yet focusing on specific biological pathways may yield potential future therapeutic targets.
Keywords: organ crosstalk, acute lung injury, caspase-3, NF-κB, multiple organ failure
acute kidney injury (aki) remains widely prevalent among hospitalized patients, and, despite advancements in renal replacement therapy, AKI in the critically ill independently elicits a 30–60% mortality rate (11, 30). AKI rarely occurs in isolation, however, and multiple clinical studies have identified an association between AKI and distant organ dysfunction (18, 30). AKI and acute lung injury (ALI) constitute the two most commonly implicated organs in multiple organ failure and, even in the absence of volume overload, contribute to a devastating prognosis with mortality rates approaching 80% (23, 26, 34).
Recent experimental evidence has elucidated mediators of kidney-lung crosstalk after AKI, including cellular and soluble factors activated by the postischemic kidney that confer pulmonary injury. AKI and ALI generate a bidirectional, self-propagating cycle of injury; AKI releases reactive oxygen species and mediators of distant organ injury to the lungs, and, in turn, ALI with mechanical ventilation alters renal hemodynamics, thus worsening AKI (21). Although uremic toxins certainly influence pulmonary dysfunction, we have previously shown that kidney ischemia-reperfusion injury (IRI) incites a distinct pulmonary genomic and functional response compared with bilateral nephrectomy alone (8). Kidney IRI induces endothelial cell activation, transcriptional upregulation of proinflammatory and proapoptotic pathways, and disruption of the pulmonary microvascular barrier with caspase-dependent apoptosis (3, 8, 9).
Given the complexity of interorgan crosstalk and the need to elucidate functionally important pathways that could lead to potential therapeutic targets, our laboratory focused specifically on mediators activated by the postischemic kidney, which may induce pulmonary injury. Previous analysis of all ischemia-specific and apoptosis-related lung candidate genes after ischemic AKI revealed that 37% were related to the tumor necrosis factor receptor (TNFR) pathway, and validation by protein expression and immunofluorescent colocalization of the TNFR1 receptor with apoptotic cells established TNFR1 as a key potential mediator of pulmonary apoptosis after ischemic AKI (9).
We hypothesized that TNFR1 triggers ischemic AKI-induced pulmonary apoptosis and aimed to elucidate specific molecular signals leading to programmed cell death. Here, we describe a time course for circulatory TNF-α activation and pulmonary TNFR1 expression and provide evidence for TNFR1-dependent apoptotic signaling in the lungs after kidney IRI. Additionally, by competitive inhibition of circulatory TNF-α with Etanercept, we characterize the critical role of TNF-α-mediated TNFR1 activation in mediating lung apoptosis and microvascular barrier dysfunction after ischemic AKI. In the pursuit of specific mechanisms of TNFR1-mediated apoptosis, we assayed for Complex I (NF-κB) and Complex II (Caspase-8) expression, which mediate prosurvival and proapoptotic pathways, respectively. We identified activation of NF-κB (Complex I) after ischemic AKI-induced pulmonary apoptosis without the induction of caspase-8 (Complex II) expression. Direct inhibition of NF-κB with Bay 11–7082 resulted in an apoptotic phenotype, lung microvascular barrier dysfunction, and altered expression of cellular flice inhibitory protein (C-FLIP) isoforms, suggesting that the balance between NF-κB (Complex I) and caspase-8 (Complex II) signaling may regulate ischemic AKI-induced pulmonary apoptosis and injury.
MATERIALS AND METHODS
Animal care.
All procedures were approved by The Institutional Animal Care and Use Committee at The Methodist Hospital Research Institute and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male 6–8-wk-old mice (C57BL6/J or TNFR1-/), weighing 25–30 g, were obtained from Jackson Laboratory (Bar Harbor, ME), housed under pathogen-free conditions according to NIH guidelines, and acclimated for ≥5 days before procedures. All procedures were performed using strict sterile techniques under general anesthesia with pentobarbital sodium (50 mg/kg ip) and analgesia with buprenorphine (0.03 mg/kg ip given before incision). Assessment of adequate anesthesia and analgesia was obtained by paw and tail pinching.
Surgical procedures.
Animals were placed on a heating blanket after anesthesia induction, and core (rectal) temperatures were maintained ∼36°C. Our kidney IRI methods have been described in detail previously (8, 9). Briefly, animals underwent midline laparotomy with bilateral renal pedicle isolation, and, for those assigned to IRI, an atraumatic vascular clamp was applied across both renal pedicles for 60 min. Sham animals underwent identical procedures without vascular clamp application. After 60 min, the clamps (if present) were removed, animals were resuscitated with 1 ml of warm sterile saline IP, and the incision was closed in two layers with 4–0 silk suture. Animals recovered with free access to food and water. In some experiments, a competitive inhibitor of soluble TNF-α, Etanercept (Enbrel; Immunex, Thousand Oaks, CA) 100 μg (0.2 ml, reconstituted in PBS per manufacturer's instructions) was administered IP 15 h and 1 h before surgery. Etanercept dosing was determined based on prior murine models, which demonstrated TNF-α-neutralization (1, 6, 10, 35). In another set of experiments, animals received 20 mg/kg Bay 11–7082 (Enzo Life Sciences, Plymouth Meeting, PA), an irreversible inhibitor of Iκ-Bα phosphorylation that prevents NF-κB activation and translocation into the nucleus, dissolved in 0.5% DMSO administered IP 30 min before IRI or sham laparotomy (29). Animals were killed at 4 or 24 h after the experimental procedure by exsanguination under general anesthesia, and tissues were collected for analysis.
Renal function.
Upon death, inferior vena cava blood samples (∼0.5 ml) were obtained from each animal and centrifuged at 3,000 revolution/min for 15 min for serum. Serum creatinine levels (mg/dl) were measured as a marker of renal function using an Ortho-Clinical Diagnostics VITROS 5,1 Fusion (Ortho-Clinical Diagnostics, Raritan, NJ).
Bronchoalveolar lavage.
Bronchoalveolar lavage (BAL) fluid analysis was performed as a surrogate measurement of pulmonary microvascular permeability as previously described (8, 9, 31). BAL fluid was obtained by slow delivery of 0.75 ml warm (∼37°C) PBS via a tracheotomy. The fluid was withdrawn by gentle suction; the process was repeated twice, and solutions were combined and stored on ice. Recovered BAL fluid underwent BCA total protein assay (Bio-Rad, Hercules, CA) according to the manufacturer's protocol. Optical density readings of samples were converted to micrograms per milliliter using values obtained from standard curves generated with serial dilutions of BSA.
Assessment of lung apoptosis.
Upon death, the right main bronchus was isolated and ligated. The left lung was filled with 0.5% low-melting agarose in 10% formalin at a constant pressure of 25 mmH20, allowing for homogeneous expansion of lung parenchyma. Inflated lungs were then fixed in 10% formalin for 48 h and embedded in paraffin blocks. Sections of paraffin-embedded tissues (5 μm) were obtained, and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assays were performed with an ApopTag Peroxidase In Situ Apoptosis Detection Kit following manufacturer's instructions (Millipore, Billerica, MA). Briefly, after deparaffinization and rehydration, slides were treated with 20 μg/ml proteinase K for 15 min, immersed in equilibration buffer (75 μl/5 cm2) and incubated at room temperature for 30 min. Slides were then treated with 55 μl/5 cm2 of TdT enzyme at 37°C for 1 h, washed with buffer, and incubated with anti-digoxigenin conjugate (65 μl/5 cm2) for 30 min at room temperature. Color was developed with peroxidase substrate (75 μl/5 cm2), methyl green counterstain (0.5% wt:vol) was applied, and coverslips were mounted on slides. Measurements of TUNEL-positive nuclei were performed by counting the average number of positive cells on 10 images/slide (40× magnification) captured by an independent observer blinded to the experiment using a Zeiss microscope, and digital images were saved.
Active (cleaved) caspase-3 immunohistochemistry (IHC) was performed on paraffin-embedded, formalin-fixed mouse lungs. Deparaffinization was performed with xylene, sections were rehydrated with distilled water, and then antigen retrieval was performed with high-pH unmasking solution (Vector Laboratories, Burlingame, CA) by steaming for 20 min followed by cooling at room temperature for another 20 min. Specimens were incubated with cleaved caspase-3 (Asp175) antibody (Cell Signaling Technology, Danvers, MA) in a 1:100 dilution at room temperature for 1 h followed by ImmPRESS anti-rabbit IgG polymer detection kit secondary antibody (Vector Laboratories) for 20 min at room temperature. Staining was completed by 2-min incubation with ImmPACT diaminobenzidene peroxidase substrate at room temperature. Measurements of active caspase-3-positive cells were performed on 10 images/slide (40× magnification) captured by an independent observer blinded to the experiment. Results for both TUNEL and caspase-3 IHC are represented as mean fold change (FC) relative to wild-type (WT) sham.
Measurement of serum TNF-α levels.
Circulatory and whole lung homogenate TNF-α production was analyzed with the ELISA MAX Deluxe Set (BioLegend, San Diego, CA) per manufacturer's instructions. Briefly, lung tissue was homogenized after isolation as previously described, and serum and lung homogenate samples underwent ELISA assay for TNF-α determination (9). Absorbance was measured with a Bio-TEK Synergy HT plate reader (Bio-TEK Instruments, Winooski, VT), and results are expressed in pg/ml.
Protein expression by immunoblot.
Caspase-8 is the initiator caspase activated by Complex II formation in the cytosol; therefore protein expression of cytosolic caspase-8 (Complex II) was determined with Western immunoblotting. Lung tissue homogenates were fractioned into nuclear and cytosolic portions using the Nuclear/Cytosol Fractionation Kit (BioVision, Mountain View, CA) per manufacturer's instructions. Total protein was determined in these cytosolic fractions using the Bradford assay and then quantitated by Western blot as described in the next paragraph. To ensure that nuclear fractions were not contaminated with cytosolic proteins, caspase-8 was measured and was not found to be present in significant quantities in the nuclear fractions (data not shown).
Equal aliquots of protein (50 μg) were loaded in each well of 12% Tris-glycine gels (Bio-Rad). After electrophoresis for 90 min at 110 V of constant voltage, the gel was blotted onto a nitrocellulose membrane by electrophoretic transfer at 70 V of constant voltage for 1–2 h. The membrane was washed, blocked with a 5% blocking solution, and probed with caspase-8 rabbit monoclonal antibody (1:1,000 dilution in tPBS; Cell Signaling) with β-actin as a control (1:2,000 in tPBS; Santa Cruz Biotechnology, Santa Cruz, CA). The immunoreactive bands were visualized using a secondary antibody conjugated to horseradish peroxidase and a chemiluminescent detection system (Konica Minolta, Ramsey, NJ). Nitrocellulose blots were visualized using a Bio-Rad Gel doc (Bio-Rad) and analyzed by Quantity One 4.6.1 software (Bio-Rad).
TNFR1 gene expression by RT-PCR.
Total RNA was extracted from homogenized frozen whole lung tissue with Trizol (Gibco) and purified with RiboPure Kit (Ambion, Austin, TX) according to the manufacturer's recommendations. The concentrations were determined spectrophotometrically (NanoDrop 1000). RT-PCR was performed on 10 ng of RNA using the iScript One-step RT-PCR kit with SYBR Green (Bio-Rad) and the iQ5 Multicolor Real-time PCR Detection System (Bio-Rad Laboratories) according to the manufacturer's instructions. The primer sets were tested over a temperature gradient for efficiency and specificity and verified over three orders of magnitude of a dilution series of mRNA for linearity. The primers used, designed by PrimerBank, and the RT-PCR conditions were as follows: TNFR1 primers: 5′-CCG GGA GAA GAG GGA TAG CTT-3′ and 5′-TCG GAC AGT CAC TCA CCA AGT-3′, Tm=59.9°C; β-actin primers: 5′-GGCTGTATTCCCCTCCATCG-3′ and 5′-CCAGTTGGTAACAATGCCATGT-3′, Tm=65.5°C. The relative expression levels of each mRNA were calculated using the 2−ΔΔCt method normalizing to β-actin and relative to the control samples. The presence of one product of the correct size was verified by 1.5% agarose gel electrophoresis.
Determination of NF-κB activation.
Complex I formation leads to NF-κB activation and translocation of p65 into the nucleus. The total protein in the nuclear fraction of lung tissue homogenate was measured using the Bradford protein assay and quantitated by Western blot as described above utilizing the NF-κB p65 rabbit monoclonal IgG (1:10,000 dilution in tPBS; Cell Signaling) with β-actin as a control (1:2,000 in tPBS; Santa Cruz Biotechnology).
Statistical analysis.
For comparison within groups, a paired Student's t-test was performed between sham vs. IRI. P values <0.05 were considered significant, with data expressed as means ± SE. To compare creatinine, TUNEL, and cleaved caspase-3 IHC results across groups, data were analyzed by SPSS Statistics 17 software (17.0.0; SPSS, Somers, NY) with the Mann-Whitney U-test and 95% confidence interval (data did not meet criteria for ANOVA). Data with P < 0.05 (2-tailed) were considered statistically significant, with Western blot analyses, TUNEL, and cleaved caspase-3 IHC values expressed as mean FC ± SE and absolute values (AV) listed in the figure legends.
RESULTS
Renal function after experimental ischemic AKI.
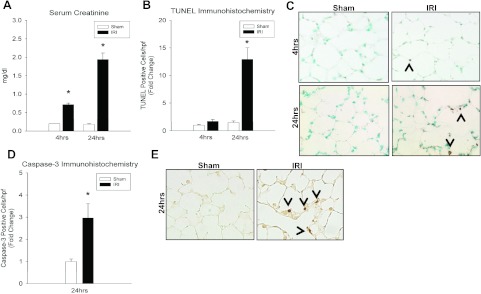
We measured serum creatinine (mg/dl) at 4 and 24 h after sham and kidney IRI to correlate local ischemic kidney damage with distant organ pulmonary effects. Compared with sham, creatinine was increased at 4 h (0.2 ± 0 vs. 0.72 ± 0.1, P = 9.95 × 10−7) and 24 h (0.18 ± 0.02 vs. 1.94 ± 0.17, P = 0.01) after kidney IRI in WT mice (Fig. 1).
Fig. 1.
Effect of kidney ischemia-reperfusion injury (IRI) on renal function and lung apoptosis. A: serum creatinine (mg/dl) was increased in wild-type (WT) mice at both 4 h (0.2 ± 0 vs. 0.72 ± 0.1*, P = 10 × 10−7) and 24 h (0.18 ± 0.02 vs. 1.94 ± 0.17*, P = 0.007) after kidney IRI vs. sham. B: kidney IRI produced more TUNEL-positive cells at 24 h (fold change, FC = 1.46 ± 0.31 vs. 12.9 ± 2.1*; absolute value, AV = 0.19 ± 0.04 vs. 1.68 ± 0.27*; P = 0.008) but not at 4 h (FC = 1.0 ± 0.15 vs. 1.69 ± 0.36; AV = 0.13 ± 0.02 vs. 0.22 ± 0.05; P = 0.13) compared with sham controls. C: representative lung micrographs of TUNEL staining for WT mice at 4 and 24 h after IRI vs. sham demonstrating increased apoptosis in IRI-treated mice at 24 h. D: WT mice demonstrated significantly increased cleaved caspase-3-positive cells at 24 h (FC = 1 ± 0.11 vs. 2.96 ± 0.64*; AV = 0.55 ± 0.06 vs. 1.63 ± 0.35*; P = 0.02) after kidney IRI compared with sham. E: representative lung micrographs of caspase-3 immunohistochemistry demonstrating increased caspase-3-positive cells at 24 h after kidney IRI. n ≥ 5/group, *P < 0.05 for IRI vs. sham.
Effect of ischemic AKI on lung apoptosis.
TUNEL assays and cleaved (active) caspase-3 IHC staining techniques were used to assess cellular apoptosis and caspase-3 activation after ischemic AKI (Fig. 1). Kidney IRI did not produce a change in the number of TUNEL-positive cells at 4 h compared with sham (FC = 1.0 ± 0.15 vs. 1.69 ± 0.36, P = 0.13) controls. However, there were more TUNEL-positive cells after IRI at 24 h (FC = 1.46 ± 0.31 vs. 12.9 ± 2.1, P = 0.008) compared with sham. Of note, there was no difference between sham groups at either 4 or 24 h after kidney IRI.
Cleaved (active) caspase-3, the effector caspase in the proapoptotic caspase cascade, was measured at 24 h to corroborate with the onset of TUNEL positivity (Fig. 1). Ischemic AKI increased lung cleaved caspase-3-positive cells (FC = 1 ± 0.11 vs. 2.96 ± 0.64, P = 0.02) compared with sham, indicating that induction of apoptosis after ischemic AKI correlates with the proapoptotic pulmonary phenotype seen by TUNEL staining.
Effect of ischemic AKI on TNF-α and TNFR1 expression.
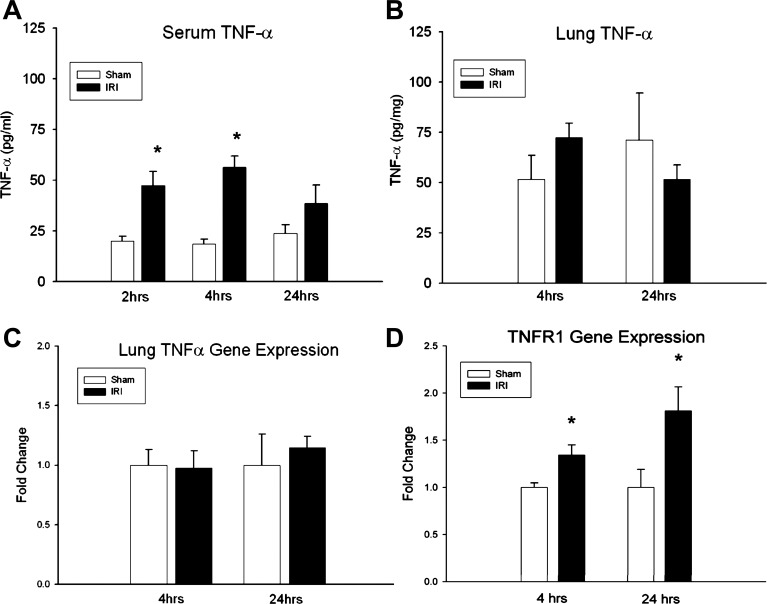
Cytokine TNF-α regulates many biological processes after injury and inflammation, and, because our previous work demonstrated prominent TNFR signaling pathway involvement in the pulmonary transcriptional response after ischemic AKI (9), we measured serum and pulmonary TNF-α levels and pulmonary TNFR1 expression after kidney IRI. Serum TNF-α levels were measured by ELISA at 2, 4, and 24 h following sham or kidney IRI in WT animals (Fig. 2). Serum TNF-α levels (pg/mg) increased early after kidney IRI at 2 h (20.1 ± 2.3 vs. 47.5 ± 6.9, P = 0.04) and 4 h (18.5 ± 2.5 vs. 56.4 ± 5.4, P = 9.16 × 10−5), but no difference in circulatory TNF-α expression was identified at 24 h (23.7 ± 4.3 vs. 38.6 ± 9.0, P = 0.16) compared with sham. Lung tissue homogenate TNF-α levels did not increase at 4 h (51.6 ± 12.0 vs. 72.4 ± 7.2, P = 0.16) or 24 h (71.1 ± 23.5 vs. 51.6 ± 7.3, P = 0.47) after IRI. ELISA results were confirmed by RT-PCR analysis, demonstrating no increase in lung TNF-α expression at either 4 (1 ± 0.1 vs. 0.98 ± 0.1, P = 0.5) or 24 (1 ± 0.3 vs. 1.1 ± 0.09, P = 0.6) h of ischemic AKI.
Fig. 2.
Effect of ischemic acute kidney injury (AKI) on TNF-α levels and pulmonary TNF receptor (TNFR) 1 expression. A: serum TNF-α levels measured by ELISA (pg/mg) increased after kidney IRI at 2 h (20.1 ± 2.3 vs. 47.5 ± 6.9*, P = 0.04) and at 4 h (18.5 ± 2.5 vs. 56.4 ± 5.4*, P = 9.16 × 10−5), but not at 24 h (23.7 ± 4.3 vs. 38.6 ± 9.0, P = 0.16) compared with sham. B: lung tissue TNF-α levels did not increase after kidney IRI at either 4 h (51.6 ± 12.0 vs. 72.4 ± 7.2, P = 0.16) or 24 h (71.1 ± 23.5 vs. 51.6 ± 7.3, P = 0.47) compared with sham controls. C: RT-PCR confirmed no increase in lung TNF-α expression at either 4 (1 ± 0.1 vs. 0.98 ± 0.1, P = 0.5) or 24 (1 ± 0.3 vs. 1.1 ± 0.09, P = 0.6) h of ischemic AKI. n ≥ 5/group, D: relative fold change for WT lung TNFR1 gene expression by quantitative RT-PCR demonstrated increased expression at 4 h (FC = 1 ± 0.05 vs. 1.34 ± 0.11*; AV = 0.48 ± 0.02 vs. 0.65 ± 0.05*; P = 0.03) and 24 h (FC = 1 ± 0.19 vs. 1.81 ± 0.25*; AV = 0.41 ± 0.8 vs. 0.75 ± 0.1*; P = 0.03) compared with sham. *P < 0.05 vs. sham.
TNF-α signals primarily through TNFR1, and we measured TNFR1 gene expression in whole lung tissue homogenate at 4 and 24 h to correlate the proapoptotic pulmonary phenotype with TNFR1 receptor activation (Fig. 2). Kidney IRI produced increased lung TNFR1 gene expression by relative FC at 4 h (1 ± 0.05 vs. 1.34 ± 0.11, P = 0.03) and at 24 h (1 ± 0.19 vs. 1.81 ± 0.25, P = 0.03) compared with sham.
TNFR1-dependent pulmonary apoptosis after ischemic AKI.
TNFR1 activation by TNF-α initiates both proapoptotic (Complex II) and antiapoptotic/proinflammatory (Complex I) pathways (28). Because WT animals exhibit increased pulmonary TNFR1 transcription after kidney IRI, we examined the pulmonary response in TNFR1−/− animals after AKI.
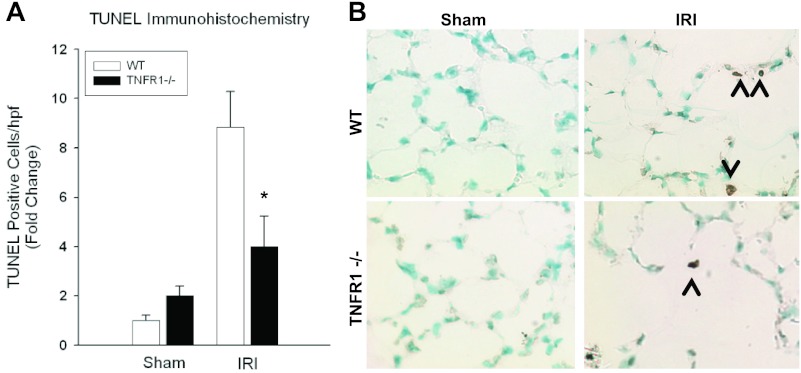
Pulmonary apoptosis after ischemic AKI was measured with TUNEL staining (Fig. 3). Kidney produced fewer TUNEL-positive cells at 24 h in TNFR1−/− mice compared with WT mice (FC = 4 ± 1.23 vs. 8.84 ± 1.44, P = 0.047); however, there was no difference between TNFR1−/− and WT mice following sham laparotomy (FC = 2 ± 0.39 vs. 1 ± 0.2, P = 0.056).
Fig. 3.
Effect of TNFR1−/− on pulmonary apoptosis after kidney IRI. A: kidney IRI produced fewer lung TUNEL-positive cells at 24 h for TNFR1−/− mice compared with WT mice (FC = 4 ± 1.23 vs. 8.84 ± 1.44*; AV = 0.76 ± 0.23 vs. 1.68 ± 0.27*; P = 0.047), but no difference was seen during sham laparotomy (FC = 2 ± 0.39 vs. 1 ± 0.2, AV = 0.38 ± 0.07 vs. 0.19 ± 0.04; P = 0.056). B: representative lung micrographs of TUNEL staining demonstrating decreased lung apoptosis after IRI in TNFR1−/− mice at 24 h.
Renal function was worse after 24 h of kidney IRI for both WT (0.18 ± 0.02 vs. 1.94 ± 0.17, P = 0.007) and TNFR1−/− mice (0.22 ± 0.02 vs. 1.22 ± 0.38, P = 0.009) compared with sham. Of note, TNFR1−/− mice provided no local protection from ischemic AKI, with a similar extent of kidney injury in TNFR1−/− and WT mice after IRI (1.94 ± 0.17 vs. 1.22 ± 0.38, P = 0.17). There was no difference between WT and TNFR1−/− sham groups (0.18 ± 0.02 vs. 0.22 ± 0.02, P = 0.18).
Lung TNFR1 signaling after ischemic AKI.
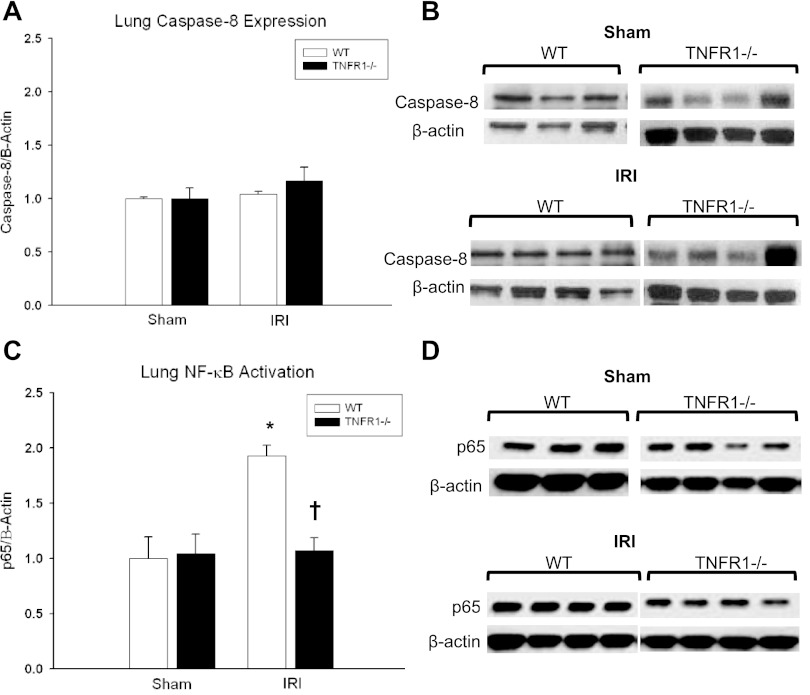
Lung homogenates were fractionated and protein expression of cytosolic caspase-8 (Complex II) and nuclear NF-κB (Complex I) was determined with Western blot (Fig. 4). No difference in caspase-8 (Complex II) expression was demonstrated at 24 h for WT (1 ± 0.03 vs. 1.04 ± 0.06, P = 0.21) or TNFR1−/− (1 ± 0.13 vs. 1.2 ± 0.32, P = 0.37) groups after kidney IRI compared with sham. However, WT mice exhibited an increase in nuclear p65 (activated) NF-κB expression (1 ± 0.2 vs. 1.9 ± 0.1, P = 0.005) at 24 h after kidney IRI. TNFR1−/− mice demonstrated decreased NF-κB expression compared with WT mice (1.9 ± 0.1 vs. 1.07 ± 0.11†, P = 0.047), suggesting that Complex I activation is favored by TNFR1-mediated signaling after kidney IRI.
Fig. 4.
Lung TNFR1 signaling after ischemic AKI. A and B: no difference in caspase-8 expression (Complex II) was demonstrated after kidney IRI in WT vs. TNFR1−/− animals (FC = 1.04 ± 0.06 vs. 1.2 ± 0.32, P = 0.37). C and D: lung NF-κB p65 (Complex I) expression was decreased in TNFR1−/− animals compared with WT (FC = 1.9 ± 0.1 vs. 1.07 ± 0.11†, P = 0.047) after ischemic AKI. Compared with sham, WT animals had an increased in lung NF-κB expression after ischemic AKI (FC = 1 ± 0.2 vs. 1.9 ± 0.1*; AV = 0.26 ± 0.05 vs. 0.51 ± 0.03*; P = 0.005). *P < 0.05 for IRI vs. sham, †P < 0.05 for WT vs. TNFR1−/−.
Inhibition of TNF-α-TNFR1 binding attenuates pulmonary apoptosis and lung injury after kidney IRI.
Animals receiving Etanercept, a competitive inhibitor of soluble TNF-α, or vehicle underwent either 60 min of bilateral renal pedicle ischemia or sham laparotomy as described, and assays for renal function, pulmonary apoptosis, lung barrier leak, and Complex I vs. Complex II formation were performed.
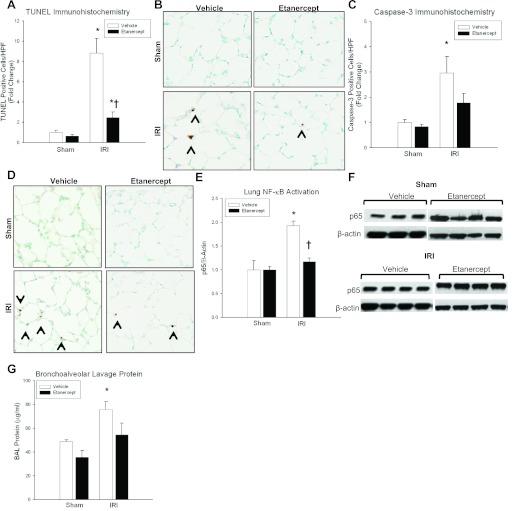
Kidney IRI produced TUNEL-positive cells at 24 h in mice treated with Etanercept (FC = 0.63 ± 0.16 vs. 2.47 ± 0.53, P = 0.008) although the extent of TUNEL positivity was less than vehicle-treated mice (FC = 2.47 ± 0.53 vs. 8.84 ± 1.44, P = 0.009) (Fig. 5). The increase in cleaved caspase-3 expression seen in vehicle mice (FC = 1 ± 0.11 vs. 2.96 ± 0.64, P = 0.02) was not evident in Etanercept mice, with no difference compared with sham (FC = 0.84 ± 0.10 vs. 1.78 ± 0.37, P = 0.12). Similar to vehicle animals, Etanercept groups demonstrated an increase in creatinine after 24 h of ischemic AKI (FC = 0.20 ± 0.03 vs. 2.12 ± 0.17, P = 0.008).
Fig. 5.
Effect of TNF-α inhibition after kidney IRI. A: kidney IRI produced more lung TUNEL-positive cells at 24 h for vehicle-treated compared with Etanercept-treated groups (FC = 8.84 ± 1.44 vs. 2.47 ± 0.53†, AV = 1.68 ± 0.27 vs. 0.47 ± 0.1†, P = 0.009); however, there was no difference in TUNEL positivity after sham laparotomy (FC = 1 ± 0.21 vs. 0.63 ± 0.16, AV = 0.19 ± 0.04 vs. 0.12 ± 0.03, P = 0.205). B: representative lung micrographs of TUNEL staining for vehicle and Etanercept groups at 24 h. C: kidney IRI produced no difference in caspase-3-positive cells between vehicle-treated and Etanercept-treated mice (FC = 2.96 ± 0.64 vs. 1.78 ± 0.37, AV = 1.63 ± 0.35 vs. 0.98 ± 0.2, P = 0.142). There was also no difference between vehicle-treated and Etanercept-treated mice after sham laparotomy (FC = 1 ± 0.11 vs. 0.84 ± 0.1, AV = 0.55 ± 0.06 vs. 0 ± 0.05, P = 0.341). D: representative lung micrographs of caspase-3 immunohistochemistry for vehicle and Etanercept groups at 24 h. E and F: NF-κB p65 expression was decreased in Etanercept-treated mice compared with vehicle-treated mice following kidney IRI (FC = 1.9 ± 0.1 vs. 1.17 ± 0.08†, AV = 0.56 ± 0.04 vs. 0.51 ± 0.03†, P = 0.008), but there was no difference after sham laparotomy (FC = 1 ± 0.07 vs. 1 ± 0.2, AV = 0.48 ± 0.03 vs. 0.26 ± 0.05, P = 0.49). G: bronchoalveolar lavage (BAL) protein was decreased in Etanercept-treated mice during kidney IRI (75.8 ± 6.6 vs. 54.6 ± 8.4*, P = 0.01) compared with vehicle-treated groups. *P < 0.05 for IRI vs. sham; †P < 0.05 for WT vs. Etanercept.
Focusing on molecular pathways of TNFR1-mediated apoptosis, we aimed to determine whether TNF-α-TNFR1 signaling promoted Complex I or Complex II formation after ischemic AKI. Lung tissue homogenate from Etanercept-treated animals was analyzed for nuclear Complex I (NF-κB) and cytosolic Complex II (Caspase-8) activation. Lung NF-κB expression was decreased in Etanercept-treated mice compared with vehicle-treated mice during kidney IRI (1.17 ± 0.08 vs. 1.9 ± 0.1, P = 0.008), suggesting that pulmonary apoptosis after ischemic AKI is associated with Complex I activation initiated by TNF-α-TNFR1 signaling. Kidney IRI produced no change in caspase-8 (Complex II) expression in Etanercept-treated mice compared with vehicle (1.42 ± 0.03 vs. 1.57 ± 0.16, P = 0.41).
To establish a potential direct role for TNF-α/TNFR1 signaling-induced pulmonary apoptosis and lung microvascular barrier dysfunction, we measured BAL protein levels (μg/ml) in vehicle-treated and Etanercept-treated mice 24 h after ischemic AKI. Vehicle-treated mice demonstrated an increase in BAL protein after IRI compared with sham (48.7 ± 1.7 vs. 75.8 ± 6.6, P = 0.01), whereas Etanercept-treated mice had BAL protein levels after IRI, which did not differ from sham controls (35.7 ± 5.6 vs. 54.6 ± 8.4, P = 0.08). These results suggest that TNFR1-dependent pulmonary apoptosis is an important mediator of lung microvascular barrier injury during ischemic AKI.
NF-κB inhibition augments lung apoptosis.
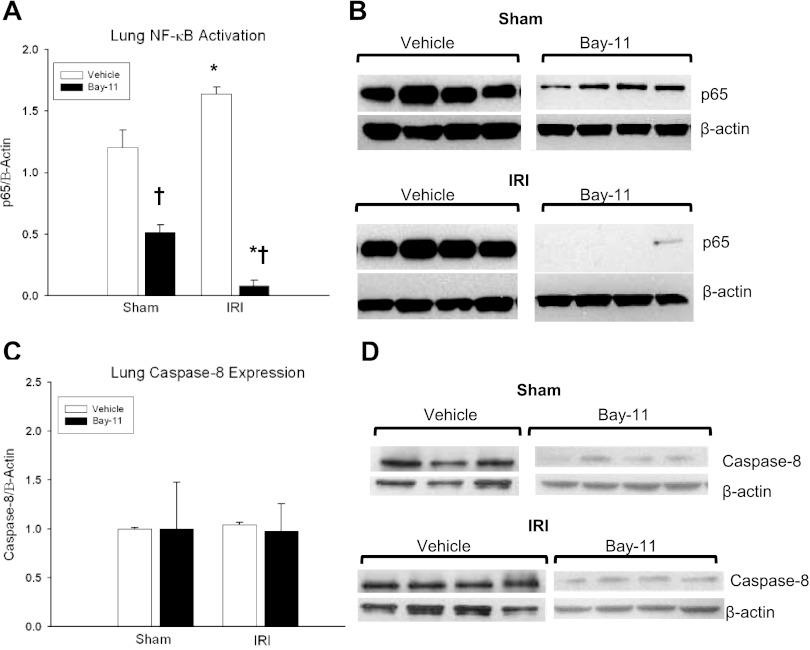
WT mice were treated with Bay 11–7082 (Bay 11), an irreversible inhibitor of NF-κB activation, or vehicle to correlate Complex I activation with the proapoptotic pulmonary response. Efficacy of NF-κB inhibition with Bay-11 was confirmed with p65 Western immunoblotting, which demonstrated a decrease in nuclear NF-κB expression in Bay-11 groups compared with vehicle (1.63 ± 0.06 vs. 0.08 ± 0.05, P = 2.8 × 10−4) during ischemic AKI (Fig. 6). Caspase-8 (Complex II) activation remained equivocal, with no change in caspase-8 expression after 24 h of kidney IRI in Bay-11 groups (1.04 ± 0.06 vs. 0.98 ± 0.28, P = 0.96) compared with vehicle.
Fig. 6.
Effect of NF-κB inhibition on TNFR1 signaling after kidney IRI. A and B: Bay-11 served as an effective NF-κB inhibitor, decreasing NF-κB expression after 24 h of kidney IRI compared with sham (AV = 1.63 ± 0.06 vs. 0.08 ± 0.05*; P = 2.75 × 10−4). NF-κB expression was also decreased in sham animals treated with Bay-11 compared with vehicle (AV = 1.2 ± 0.14 vs. 0.5 ± 0.06†, P = 9.5 × 10−5). C and D: no difference in caspase-8 expression (Complex II) was demonstrated at 24 h in vehicle (FC = 1 ± 0.03 vs. 1.04 ± 0.06; AV = 1.37 ± 0.02 vs. 1.43 ± 0.03; P = 0.21) or Bay-11 mice (FC = 1 ± 0.47 vs. 0.98 ± 0.28; AV = 0.08 ± 0.04 vs. 0.08 ± 0.02; P = 0.97) after kidney IRI compared with sham. n ≥ 4/group. *P < 0.05 for IRI vs. sham, †P < 0.05 for vehicle vs. Bay-11.
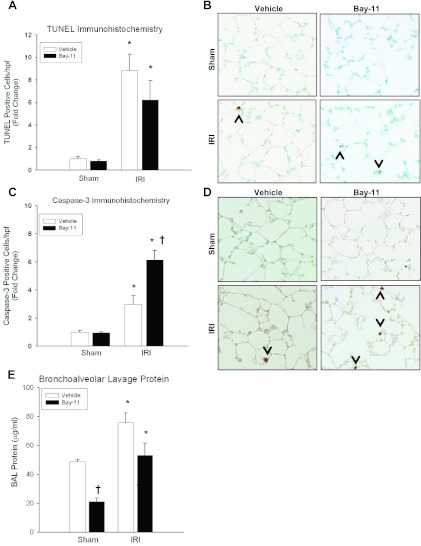
Whereas inhibition of TNF-α/TNFR1 signaling with Etanercept decreased lung apoptosis and NF-κB expression during ischemic AKI, direct NF-κB (Complex I) inhibition produced an apoptotic phenotype (Fig. 7). Similar to vehicle controls, Bay-11-treated groups demonstrated a significant increase in TUNEL-positive lung cells during 24 h of kidney IRI (FC = 0.79 ± 0.12 vs. 6.2 ± 1.7, P = 0.009). Additionally, mice receiving Bay-11 expressed more cleaved caspase-3-positive cells after 24 h of ischemic AKI compared with vehicle (FC = 6.13 ± 0.70 vs. 2.96 ± 0.64, P = 0.03). Renal function was significantly worse after 24 h of kidney IRI for both vehicle-treated (0.18 ± 0.02 vs. 1.94 ± 0.17, P = 0.007) and Bay-11-treated mice (0.14 ± 0.02 vs. 1.94 ± 0.07, P = 0.007) compared with sham.
Fig. 7.
Effect of NF-κB inhibition on pulmonary apoptosis and injury. A: kidney IRI induced TUNEL positivity in lungs of both vehicle-treated and Bay 11-treated mice (FC = 8.84 ± 1.44 vs. 6.2 ± 1.7, AV = 1.68 ± 0.27 vs. 1.18 ± 0.33, P = 0.402), both of which were increased compared with sham. B: representative lung micrographs of TUNEL staining at 24 h exhibit increased apoptosis in both vehicle and Bay-11 groups. C: kidney IRI induced more caspase-3 positivity in Bay-11-treated groups compared with vehicle-treated groups (FC = 2.96 ± 0.64 vs. 6.13 ± 0.70†, AV = 1.63 ± 0.35 vs. 3.37 ± 0.38, P = 0.03), both of which were increased compared with sham. D: representative lung micrographs of cleaved caspase-3 immunohistochemistry show increased cleaved caspase-3-positive cells in both vehicle and Bay-11 groups after IRI. E: BAL protein was measured at 24 h in vehicle-treated and Bay-11-treated mice following sham or ischemic AKI. Similar to vehicle-treated mice (48.7 ± 1.7 vs. 75.8 ± 6.6*, P = 0.01), Bay-11-treated mice demonstrated increased BAL protein leak during kidney IRI compared with sham (21.1 ± 2.4 vs. 53.1 ± 8.4*, P = 0.006). Whereas there was no difference in BAL protein leak following IRI between vehicle-treated and Bay-11-treated mice (75.8 ± 6.6 vs. 53.1 ± 8.4, P = 0.09), Bay-11 mice had decreased BAL protein after sham compared with vehicle-treated groups (48.7 ± 1.7 vs. 21.1 ± 2.4†, P = 6.005 × 10−5). n ≥ 5/group, *P < 0.05 for IRI vs. sham, †P < 0.05 for vehicle vs. Bay-11.
To correlate the apoptotic phenotype with lung functional injury, we measured BAL protein in vehicle-treated and Bay-11-treated mice 24 h after ischemic AKI. There was no difference between Bay-11-treated and vehicle-treated mice after IRI (75.8 ± 6.6 vs. 53.1 ± 8.4, P = 0.09), again correlating the apoptotic phenotype to lung microvascular barrier dysfunction after ischemic AKI. Similar to vehicle-treated mice (48.7 ± 1.7 vs. 75.8 ± 6.6, P = 0.01), Bay-11-treated mice demonstrated an increase in BAL protein after IRI compared with sham (21.1 ± 2.4 vs. 53.1 ± 8.4, P = 0.006). However, Bay-11-treated animals also had decreased BAL protein after sham laparotomy compared with vehicle-treated groups (48.7 ± 1.7 vs. 21.1 ± 2.4, P = 6 × 10−5).
DISCUSSION
Despite advancements in renal replacement therapy, AKI remains a significant predictor of mortality. AKI alters the host innate and adaptive immune response, and experimental data have identified both soluble and cellular mediators of organ crosstalk activated by the postischemic kidney (16, 21, 33). Despite a clear clinical correlation between AKI and ALI, little is known about the pathophysiology of kidney-lung crosstalk after AKI. We have previously identified caspase-dependent pulmonary endothelial cell apoptosis resulting in microvascular barrier dysfunction after 24 h of ischemic AKI (3, 8, 9). Our laboratory is presently characterizing the pulmonary endothelial cell-specific phenotypical, functional, and transcriptional response to ischemic AKI both in vivo and in vitro in an attempt to understand how this cell-specific response can influence lung dysfunction after kidney IRI.
In this present study, we have taken a mechanistic approach to determining the predominant pathway to pulmonary apoptosis activated after ischemic AKI. In a murine model of kidney IRI-induced lung dysfunction, we have identified that 1) ischemic AKI induces circulatory TNF-α activation and pulmonary TNFR1 expression, 2) pulmonary apoptosis after ischemic AKI occurs in a TNFR1-dependent manner, 3) TNFR1 signaling disruption diminishes the onset of pulmonary apoptosis and microvascular barrier dysfunction, 4) TNFR1 proapoptotic signaling induces NF-κB (Complex I) activation, and 5) direct NF-κB inhibition augments caspase-3 activation, the proapoptotic phenotype, and lung microvascular injury.
The potential mediators implicated in this distant organ response remain unknown but may include the downregulation of pulmonary epithelial Na-K-ATPase and aquaporin 5 (25), the activation of proinflammatory cytokines such as IL-6 (14), and macrophage trafficking and activation that may confer proinflammatory pulmonary damage after ischemic AKI (19). Prior research identified TNF-α, a key cytokine that regulates numerous biological pathways including inflammation, proliferation, differentiation and cell death, in models of both local kidney damage and distant organ dysfunction after IRI (2, 13, 24, 27). Increased serum TNF-α after ischemic AKI has previously been reported by other investigators (13), and we have demonstrated the early activation of serum TNF-α with concurrent early and sustained activation of the lung TNFR1 receptor. This implicates the release of TNF-α from the postischemic kidney as a soluble mediator of organ crosstalk.
The role of local pulmonary TNF-α production after ischemic AKI remains unknown. We have previously developed techniques to isolate lung microvascular endothelial cells in vivo, and RT-PCR arrays identified an increase in TNF-α production after kidney IRI compared with sham (3). However, in this study, we found no increase in TNF-α of whole lung homogenate by ELISA or RT-PCR (data not included). Infiltrating lymphocytes could be another potential source of local TNF-α production in the lungs, and our laboratory has previously investigated the role of T cells as potential mediators of distant organ pulmonary injury after ischemic AKI (3, 15). These preliminary studies have identified trafficking of activated CD3+ T cells into the lungs after ischemic AKI, with a predominant CD8+ T cell population (22). Although these activated T cell populations were necessary for kidney IRI-induced lung apoptosis, we did not find increased expression of T cell TNF receptors or TNF-α, suggesting that T cell-mediated lung apoptosis may occur independently from local T cell TNF-α production after ischemic AKI (22).
TNF-α signaling in many organ injury models can trigger both a regenerative and cell-death response, and these pleiotropic effects are attributed to its ability to activate both apoptotic and survival pathways through the TNFR1 receptor (7). TNFR1 is a prominent death receptor pathway activated after inflammation and apoptosis, and we have previously identified robust AKI-induced TNFR1 pathway activation by global gene expression profiling (9). The TNFR1 receptor is critical to pulmonary apoptosis after ischemic AKI, with significantly fewer TUNEL-positive cells at 24 h in TNFR1−/− mice compared with sham. We hypothesize that the increase in caspase-3-positive cells in TNFR1−/− mice results from TNFR1-independent activation of caspase-3, perhaps to serve in a nonapoptotic function such as cellular inflammation (20). Inhibition of TNF-α-TNFR1 binding with Etanercept produced an attenuated proapoptotic phenotype that was not as complete as in TNFR1−/− mice. This would imply either incomplete TNF-α-TNFR1 binding blockade by Etanercept or potential TNFR1 receptor activation independent of TNF-α; further studies are needed to clarify this discrepancy. Despite these confounding data, pretreatment with Etanercept attenuated lung microvascular barrier dysfunction measured by BAL protein. We have previously correlated pulmonary apoptosis with lung microvascular barrier injury (9); however, this study highlights specific molecular mechanisms and implicates the critical role of TNFR1-mediated lung apoptosis in mediating lung injury following ischemic AKI.
TNFR1 signaling includes activation of two pathways: Complex I (TNFR1, TRADD, RIP, TRAF2, and c-IAIP1) at the membrane, which results in NF-κB activation and prosurvival signals, and Complex II (FADD, procaspases 8 and 10) in the cytosol, which promotes the apoptotic cascade. TNFR1 activation initiates a complex signaling caspase between these two prosurvival and proapoptotic pathways, and the current paradigm suggests that Complex II (apoptotic) activation occurs depending on the balance between levels of NF-κB and C-FLIP isoform expression (28). Complex I activation produces TRAF1, TRAF2, and c-IAP1 and c-IAP2 induction, which suppress caspase-8 activation and thus apoptosis (32). In our present study, we have shown NF-κB activation independent of caspase-8 activation, yet this occurred concurrently with cellular apoptosis and activation of caspase-3, the executioner caspase. In both TNFR1−/− mice and in WT mice after treatment with Etanercept, ischemic AKI was associated with fewer TUNEL-positive cells and also decreased NF-κB activation. Furthermore, direct inhibition of NF-κB with Bay-11 resulted in an apoptotic phenotype with TUNEL-positive cells and activation of caspase-3. Therefore, the role of NF-κB activation in TNFR1-mediated lung apoptosis remains unknown, prompting the exploration of further downstream regulators of Complex 1/Complex 2 signaling after ischemic AKI. This proapoptotic phenotype was associated with lung microvascular barrier dysfunction, further implicating lung apoptosis as a critical mediator of lung injury after ischemic AKI.
Caspase-8 serves as an initiator caspase in the caspase cascade and, when activated, cleaves (and activates) caspase-3, the main effector caspase that stimulates the caspase cascade and subsequent apoptosis. However, in our model, we have demonstrated signs of apoptosis and caspase-3 activity in the absence of caspase-8 activation. Several plausible explanations for caspase-8-independent caspase-3 activation include 1) difficulty in detecting initiator caspases by immunoblotting (12), 2) concurrent nondeath receptor-dependent pathways to apoptosis (such as the mitochondrial pathway) occurring after IRI, which also employ caspase-3, and 3) additional newly identified mediators of caspase-8-independent caspase-3 activation after TNFR1 proapoptotic signaling (4). Alternatively, caspase enzymes also serve nonapoptotic functions, such as processing of cytokines after inflammation and the proliferation of T cells. Therefore, the inability of Etanercept to completely suppress caspase-3 activation despite decreased phenotypical evidence of apoptosis may be due to nonapoptotic functions of caspase-3 (17).
The present study is limited without cell-specific assays to directly link apoptosis and lung injury with a certain cell type. However, we have previously identified lung microvascular endothelial cells undergoing apoptosis in vivo following ischemic AKI and hypothesize that these microvascular endothelial cells, with their critical role in maintaining the lung semipermeable barrier, are specific targets of lung apoptosis and injury after kidney IRI. In an effort to advance our understanding of the pathophysiology of indirect lung injury after ischemic AKI, our laboratory has recently focused on identifying lung microvascular endothelial cell-specific changes in ischemic AKI utilizing parallel in vivo and in vitro methods. Ischemic AKI incites specific transcriptional and phenotypic alterations in lung microvascular endothelial cells, including activation of proinflammatory and proapoptotic genes, along with cytoskeletal rearrangement and apoptosis in vitro (3). RT-PCR arrays performed on isolated lung microvascular endothelial cells in vivo at 24 h of kidney IRI identified activation of genes related to the TNF superfamily, apoptosis, and TNF-α, which follows the same pattern as our data performed on whole lung tissue (3). Future endeavors of our laboratory remain focused on understanding the lung endothelial cell-specific response to ischemic AKI.
In summary, our research has identified a critical role for the TNFR1 receptor in pulmonary apoptosis after ischemic AKI. Kidney-lung crosstalk after kidney IRI represents a complex biological process activated by several key soluble and cellular mediators. Future endeavors to characterize the intricate balance between programmed cell death and cell survival in organ crosstalk may improve outcomes in this formidable clinical challenge.
GRANTS
This work was supported by grants from the NIH/NHLBI K08HL089181 and the American Vascular Association/American College of Surgeons Lifeline Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.E.W., R.J.S., and H.T.H. conception and design of research; L.E.W., R.J.S., and Y.C. performed experiments; L.E.W., R.J.S., Y.C., and H.T.H. analyzed data; L.E.W., R.J.S., and H.T.H. interpreted results of experiments; L.E.W. and Y.C. prepared figures; L.E.W. and R.J.S. drafted manuscript; L.E.W., F.A.M., and H.T.H. edited and revised manuscript; L.E.W., Y.C., F.A.M., and H.T.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Stephen Jones for expert assistance with the statistical analyses.
REFERENCES
- 1. Cataisson C, Pearson AJ, Torgerson S, Nedospasov SA, Yuspa SH. Protein kinase C alpha-mediated chemotaxis of neutrophils requires NF-kappa B activity but is independent of TNF alpha signaling in mouse skin in vivo. J Immunol 174: 1686–1692, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Donnahoo KK, Meng X, Ayala A, Cain MP, Harken AH, Meldrum DR. Early kidney TNF-α expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol Regul Integr Comp Physiol 277: R922–R929, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Feltes CM, Hassoun HT, Lie ML, Cheadle C, Rabb H. Pulmonary endothelial cell activation during experimental acute kidney injury. Shock 36: 70–76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geering B, Gurzeler U, Federzoni E, Kaufmann T, Simon HU. A novel TNFR1-triggered apoptosis pathway mediated by class IA PI3Ks in neutrophils. Blood 117: 5953–5962, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Golks A, Brenner D, Krammer PH, Lavrik IN. The c-FLIP-NH2 terminus (p22-FLIP) induces NF-kappaB activation. J Exp Med 203: 1295–1305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grounds MD, Davies M, Torrisi J, Shavlakadze T, White J, Hodgetts S. Silencing TNFalpha activity by using Remicade or Enbrel blocks inflammation in whole muscle grafts: an in vivo bioassay to assess the efficacy of anti-cytokine drugs in mice. Cell Tissue Res 320: 509–515, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Han D, Ybanez MD, Ahmadi S, Yeh K, Kaplowitz N. Redox regulation of tumor necrosis factor signaling. Antioxid Redox Signal 11: 2245–2263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hassoun HT, Grigoryev DN, Lie ML, Liu M, Cheadle C, Tuder RM, Rabb H. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol 293: F30–F40, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Hassoun HT, Lie ML, Grigoryev DN, Liu M, Tuder RM, Rabb H. Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am J Physiol Renal Physiol 297: F125–F137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hodgetts S, Radley H, Davies M, Grounds MD. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul Disord 16: 591–602, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med 36: S146–S151, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Kaufmann SH, Lee SH, Meng XW, Loegering DA, Kottke TJ, Henzing AJ, Ruchaud S, Samejima K, Earnshaw WC. Apoptosis-associated caspase activation assays. Methods 44: 262–272, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 14: 1549–1558, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int 74: 901–909, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Ko GJ, Jang HR, Huang Y, Womer KL, Liu M, Higbee E, Xiao Z, Yagita H, Racusen L, Hamad AR, Rabb H. Blocking Fas ligand on leukocytes attenuates kidney ischemia-reperfusion injury. J Am Soc Nephrol 22: 732–742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ko GJ, Rabb H, Hassoun HT. Kidney-lung crosstalk in the critically ill patient. Blood Purif 28: 75–83, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kohler C, Orrenius S, Zhivotovsky B. Evaluation of caspase activity in apoptotic cells. J Immunol Methods 265: 97–110, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Koyner JL, Murray PT. Mechanical ventilation and the kidney. Blood Purif 29: 52–68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int 55: 2362–2367, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ 14: 44–55, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Li X, Hassoun HT, Santora R, Rabb H. Organ crosstalk: the role of the kidney. Curr Opin Crit Care 15: 481–487, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Lie ML, Santora RJ, Rabb H, Hassoun HT. Distant organ t cell trafficking and activation during ischemic acute kidney injury (AKI). J Surg Res 158: 291, 2010 [Google Scholar]
- 23. Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol 13: 1350–1357, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Meldrum KK, Meldrum DR, Meng X, Ao L, Harken AH. TNF-α-dependent bilateral renal injury is induced by unilateral renal ischemia-reperfusion. Am J Physiol Heart Circ Physiol 282: H540–H546, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int 63: 600–606, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Ricci Z, Ronco C. Pulmonary/renal interaction. Curr Opin Crit Care 16: 13–18, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Serteser M, Koken T, Kahraman A, Yilmaz K, Akbulut G, Dilek ON. Changes in hepatic TNF-alpha levels, antioxidant status, and oxidation products after renal ischemia/reperfusion injury in mice. J Surg Res 107: 234–240, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Sheikh MS, Huang Y. Death receptor activation complexes: it takes two to activate TNF receptor 1. Cell Cycle 2: 550–552, 2003 [PubMed] [Google Scholar]
- 29. Sio SW, Ang SF, Lu J, Moochhala S, Bhatia M. Substance P upregulates cyclooxygenase-2 and prostaglandin E metabolite by activating ERK1/2 and NF-kappaB in a mouse model of burn-induced remote acute lung injury. J Immunol 185: 6265–6276, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Walters DM, Wills-Karp M, Mitzner W. Assessment of cellular profile and lung function with repeated bronchoalveolar lavage in individual mice. Physiol Genomics 2: 29–36, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281: 1680–1683, 1998 [DOI] [PubMed] [Google Scholar]
- 33. White LE, Chaudhary R, Moore LJ, Moore FA, Hassoun HT. Surgical sepsis and organ crosstalk: the role of the kidney. J Surg Res 167: 306–315, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564–2575, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ, Haitsma JJ, van der Poll T, Juffermans NP, Zweers MM, Schultz MJ. Recombinant human soluble tumor necrosis factor-alpha receptor fusion protein partly attenuates ventilator-induced lung injury. Shock 31: 262–266, 2009 [DOI] [PubMed] [Google Scholar]