Abstract
During recovery from social stress in a visible burrow system (VBS), during which a dominance hierarchy is formed among the males, rats display hyperphagia and gain weight preferentially as visceral adipose tissue. By proportionally increasing visceral adiposity, social stress may contribute to the establishment of metabolic disorder. Amylin was administered to rats fed ad libitum during recovery from VBS stress in an attempt to prevent hyperphagia and the resultant gain in body weight and fat mass. Amylin treatment reduced food intake, weight gain, and accumulation of fat mass in male burrow rats, but not in male controls that spent time housed with a single female rather than in the VBS. Amylin did not alter neuropeptide Y (NPY), agouti-related peptide (AgRP), or proopiomelanocortin (POMC) mRNA expression in the arcuate nucleus of the hypothalamus as measured at the end of the recovery period, nor did it affect plasma corticosterone or leptin. Amylin exerted most of its effect on food intake during the first few days of recovery, possibly through antagonism of NPY and/or increasing leptin sensitivity. The potential for chronic social stress to contribute to metabolic disorder is diminished by amylin treatment, though the neuroendocrine mechanisms behind this effect remain elusive.
Keywords: metabolic disorder, energy expenditure, neuropeptide Y, leptin, visible burrow system
amylin is a peptide hormone cosecreted with insulin from pancreatic β-cells that acts as both a satiation and adiposity signal. Administration of amylin reduces food intake and decreases weight gain and fat mass, with relative preservation of lean mass (19, 22, 32). Conversely, recovery from social stress can cause precisely the opposite effects on food intake, body weight, and adiposity (10, 35). We use the visible burrow system (VBS) as a model of psychosocial stress. The VBS consists of a set of chambers and tubes that socially house 4 male and 2 female Long-Evans rats for 2 wk. In this situation, the male rats form a dominance hierarchy as they compete for access to females (5). When housed in the VBS, subordinate (SUB) rats lose a significant amount of weight, primarily from adipose mass, but also from lean mass. After removal from the VBS, rats are singly housed and allowed to recover for a few weeks, during which time SUB rats display hyperphagia and significant weight increase (36). SUB rats exposed to two cycles of VBS and recovery have greater amounts of adipose tissue than dominant (DOM) and control (CON) rats. In addition, a greater proportion of this fat is located in the visceral compartment, a pattern of deposition that has been associated with greater risk of metabolic dysfunction (4, 36).
The behavioral, metabolic, and endocrine responses to stress can contribute to the development of obesity and obesity-related diseases (7). Mediating factors in these responses include several peripheral and central “homeostatic” peptides, which are involved in affective and behavioral processes, as well as control of appetite and metabolic regulation. Some neuropeptides, such as orexigenic neuropeptide Y (NPY) and agouti-related peptide (AgRP), and the anorexigenic proopiomelanocortin (POMC) have well documented roles in both feeding and stress and are influenced by peripherally generated peptides like leptin and amylin (21, 33). The primary means by which amylin reduces weight gain is through inhibition of food intake (32), specifically through a reduction in meal size combined with a shortening of meal duration at higher doses (19, 22, 31). The hyperphagia of SUB rats during recovery is characterized by the ingestion of larger meals and longer meal duration with no increase in meal frequency (23). The driving force behind this hyperphagia may be related to the elevated NPY mRNA in the arcuate nucleus of the hypothalamus (ARC) and reduced plasma leptin seen in burrow rats at the beginning of recovery (23, 36). Additionally, amylin blocks NPY-induced hyperphagia (25).
The purpose of this study was to determine whether chronic peripheral perfusion of amylin during recovery from VBS could prevent or lessen the disproportionate increase in adipose mass observed in SUB rats during recovery. We hypothesized that amylin would successfully ameliorate the hyperphagia, body weight, and fat gain seen during this time period. We proposed that such and effect might occur via disruption of central orexigenic signaling, specifically NPY signaling in the ARC, since NPY gene expression is altered by both chronic social stress and amylin treatment. Furthermore, any effect on hypothalamic gene expression could be a downstream result of an effect on peripheral signals of stress or adiposity, such as corticosterone or leptin, respectively.
MATERIALS AND METHODS
Animals and housing.
All procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 1996). Long-Evans rats, ∼90 days of age, were obtained from Harlan (Indianapolis, IN). Under all housing conditions, rats were maintained under a 12:12-h light-dark cycle with ad libitum access to standard low-fat chow (Teklad Sterilizable Mouse/Rat Diet 7012; Harlan Teklad, Madison, WI) and water. Rats were individually housed upon arrival in conventional shoebox cages (18 × 24.5 × 18 cm) for 4 wk and then assigned to a visible burrow system (VBS) colony or a control pair (5). VBS colonies consisted of four weight-matched males and two females. The structure of the VBS has been previously described (35). Briefly, the VBS is constructed of black Plexiglas and consists of a large high-walled open-field chamber, and a series of clear tubes connecting the open field to two smaller chambers. The open field is lit by a 15-W bulb on a 12:12-h light-dark cycle, while the tubes and smaller chambers are kept dark to simulate an underground burrow system. Food and water are provided ad libitum in the open field and in both of the smaller chambers. Infrared cameras are suspended above each of the burrows to record behavior during the dark cycle. Control (CON) males were weight-matched to their respective VBS colony and housed with a single female in a shoebox cage for the duration of the VBS run. After 2 wk of housing in either the VBS or control pairs, males were removed, assessed for body composition, assigned to amylin or control treatment, and returned to their individual cages for a 2-wk recovery with ad libitum access to food and water. Rats were not fasted at any time during this study. After the 2-wk recovery, rats were removed from their home cages and immediately euthanized. Twenty colonies were involved in this study, with each colony yielding one DOM, three SUB, and two CON.
Food intake, body weight, and body composition.
Rats were weighed every other day during the VBS and daily during recovery. Total daily food intake was measured by weighing the food hoppers once a day during recovery. Whole body composition was measured by placing each rat into a Plexiglas tube, which was then inserted into an EchoMRI (Echo Medical Systems, Houston, TX) whole body composition analyzer system. This analysis provides estimates of fat mass, lean mass, and water content. Whole body composition was assessed at four time points: prior to entering the VBS, at the end of the VBS, and at 1 and 2 wk of recovery.
Determining social status.
The hierarchical status of rats housed in the VBS was determined by previously described criteria, including observation of agonistic interactions between males, body weight, coat condition, and wounding patterns (5). Dominant (DOM) rats will either maintain their weight or have a minimal loss (3–5%). They engage in more offensive behaviors, and the few wounds that they receive are on their head (consistent with aggressive behavior). SUB rats will lose an average of 5–15% of their body weight and engage in more defensive behaviors, including avoidance, fleeing, and assuming supine, submissive postures. SUB receive more wounds than DOM, generally located on their back and flanks, consistent with avoidance and flight behaviors. Control males (CON) were housed with a single female in conventional caging. Wounds were counted by visual inspection every other day during weighing. CON rats were handled and placed individually in their home cages during the wound count.
Osmotic pump implantation.
After 2 wk of VBS housing, pumps were implanted subcutaneously in male DOM, SUB, and CON rats while under brief isoflurane anesthesia. Osmotic pumps (Alzet, Palo Alto, CA) delivered 0.9% saline (blank) or amylin (infused at a rate of 100 μg·kg−1·day−1; Amylin Pharmaceuticals, San Diego, CA) during the 2-wk recovery from the VBS. Animals were matched by body weight and composition and divided evenly into blank and amylin groups.
Stress test and hormone measurements.
A restraint-stress test was performed on day 7 of recovery. Rats were restrained in clear, ventilated, Plexiglas tubes for 1 h. Tubes were 17 cm long with an inner diameter of 7 cm. Blood was collected at three time points: immediately upon restraint, after 1 h of restraint, and 1 h after release from restraint. Approximately 50 μl of blood was collected from the tip of the tail into tubes containing 4 μl of 100 mM EDTA. Samples were placed on ice after collection and centrifuged, and plasma was removed and stored at −20°C until assayed. Plasma corticosterone (CORT) was measured using a radioimmunoassay kit (CORT DA; MP Biomedicals, Solon, OH). Plasma leptin levels were determined from trunk blood collected on the final day of recovery using a rat leptin RIA kit (Millipore, Billerica, MA).
In situ hybridization.
All males were euthanized after 14 days of recovery from the VBS. Brains were immediately removed, flash frozen, and stored at −20°C. Brains were sectioned coronally at 14 μm with a Leica 3050 S cryostat, thaw-mounted onto Fisherbrand Superfrost Plus slides (Fisher, Hampton, NJ), and stored at −20°C. Sections were fixed in a 4% formaldehyde PBS solution for 10 min, then rinsed in PBS, acetylated in 0.1 M triethanolamine with 0.25% acetic anhydride for 10 min, rinsed in 2× SSC, dehydrated through an ethanol series, and delipidated with chloroform. All pretreatment solutions were prepared with diethyl pyrocarbonate-treated water.
Antisense 35S-labeled probes for NPY (courtesy of James Herman, University of Cincinnati), POMC, and AgRP (both POMC and AgRP courtesy of Streamson Chua, Albert Einstein College of Medicine) were used. Probes were generated by in vitro transcription from cDNA templates using appropriate polymerases obtained from Ambion (Austin, TX). The reaction was digested with DNase, and the probe was precipitated with ammonium acetate and ethanol. The final concentration of the probe in the hybridization mix was 106 counts per minute (cpm)/50 μl based on incorporated cpm, as determined by trichloroacetic acid precipitation. The remainder of the hybridization mix consisted of 10% dextran sulfate; 50% deionized formamide; 335 mM NaCl; 20 mM Tris-HCl, pH 7.4; 5 mM EDTA; 1× Denhardt's solution; 0.1 mg/ml tRNA; 20 mM DTT; 30 mM fish ssDNA. After pipetting of 50 μl of hybridization mix onto each slide, slides were coverslipped and incubated at 55°C for 14 h in hybridization chambers containing blotting paper soaked in 50% formamide.
The next day, coverslips were removed, and slides were washed in 2× SSC, and then incubated for 30 min in 37°C RNase A (100 μg/ml). Slides were then rinsed in room temperature 0.2× SSC, incubated in 65°C 0.2 × SSC for 1 h, and finally dehydrated through an ethanol series and dried before placing on film.
Image analysis.
Hybridized slides were exposed on Kodak BioMAX MR film for 4 days for NPY and POMC, and 6 days for AgRP. The resulting film images of brain sections were illuminated on a light box and captured by a digital video camera. Semiquantitative analyses of autoradiograph images were performed using Scion Image (Scion, Frederick, MD) software. The arcuate and dorsomedial nuclei of the hypothalamus were identified from the captured autoradiograph images of the tissue using the Paxinos and Watson rat brain atlas (30). Measurements from both nuclei were taken from ∼3 mm caudal to bregma. The signal from each section was corrected by subtracting the background tissue area from the same section to give the corrected gray level. The corrected gray level was then multiplied by the area of the signal to give the integrated gray level. For the sake of presentation, results were then converted to percentage of the average of CON blank.
Statistical analysis.
Data are presented as means ± SE. Body weight and body composition changes during the VBS were analyzed with a one-way ANOVA. Food intake and body weight during recovery were analyzed using a three-way repeated-measures ANOVA with least significant difference (LSD) post hoc comparisons. Factorial ANOVAs were used to assess body composition, plasma hormone levels, and mRNA expression after recovery with LSD post hoc comparisons. P < 0.05 was considered significant. Statistics were carried out using Statistica 6 (StatSoft, Tulsa, OK). Graphs were generated using Prism 5 (GraphPad Software, San Diego, CA).
RESULTS
Body weight.
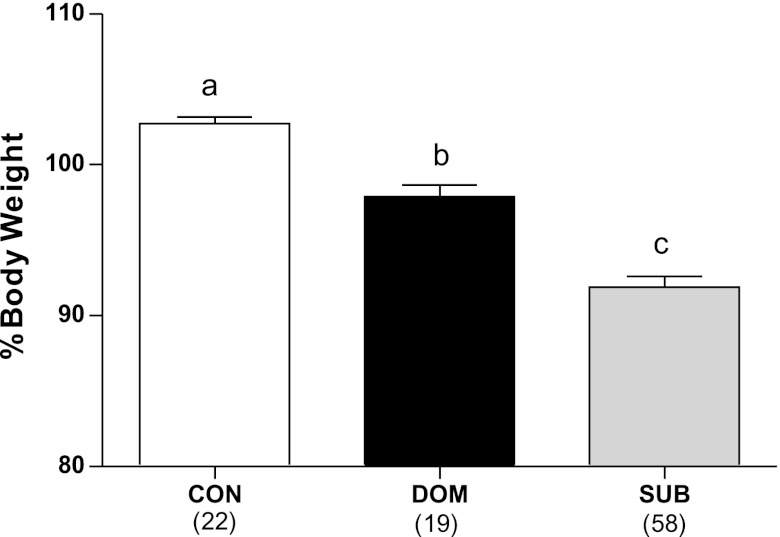
During the VBS, body weight was calculated from basal levels (recorded the day before rats were placed in the VBS). There was a main effect of status on weight (F2,87 = 71.05, P < 0.001), with SUB having lost a higher proportion of their original weight than DOM, which, in turn, lost more than CON (Fig. 1).
Fig. 1.
Burrow rats had lost a significant percent of their original body weight after 2 wk in the visible burrow system (VBS) compared with controls. DOM, dominant; SUB, subordinate; CON, control. a,b,cDifferent letters indicate significant differences between the groups (P < 0.05). Numbers in parentheses represent group size.
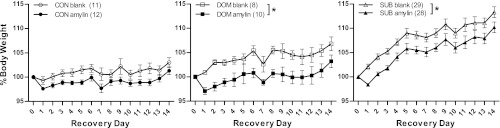
During recovery, the body weight was calculated as the percentage of weight on the last day of the burrow to account for the implantation of amylin at the beginning of recovery. A three-way repeated-measures ANOVA revealed that both status (F2,91 = 43.1, P < 0.001; CON: 102 ± 0.9, DOM: 105 ± 1.1, SUB: 112 ± 0.6) and treatment (F1,91 = 7.29, P < 0.01) had an effect on body weight during recovery. Amylin significantly reduced overall weight gain during recovery in DOM and SUB, but not in CON (Fig. 2).
Fig. 2.
Amylin suppressed weight gain in DOM and SUB, but not CON over the course of the 2-wk recovery from VBS. There were no differences on individual days. *P < 0.005 amylin vs. blank within status group over entire 2-wk recovery. Numbers in parentheses represent group size.
Body composition.
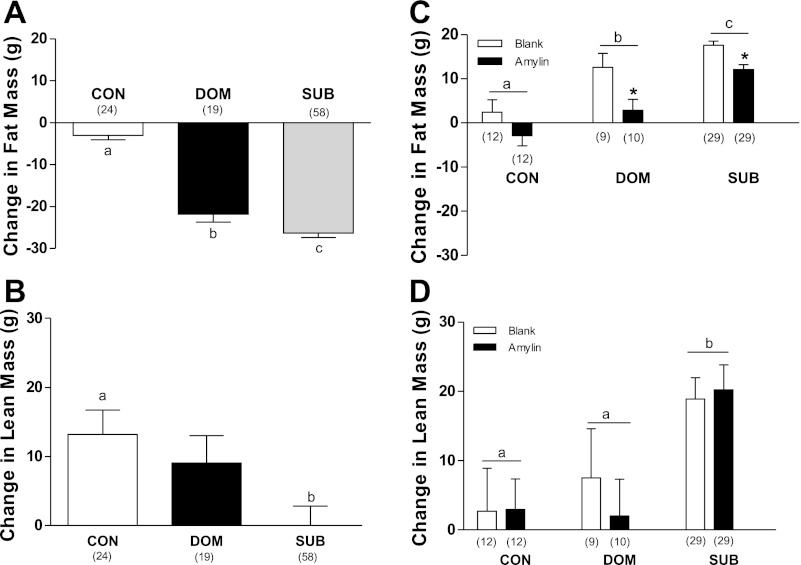
Body composition was determined as the difference in grams between the start and end of the burrow (burrow change), and as difference in grams between the end of the burrow and the end of recovery (recovery change). There was a main effect of status on changes in fat mass (F2,98 = 91.44, P < 0.001) and lean mass (F2,98 = 4.41, P = 0.015) composition during the burrow. While changes in fat composition differed significantly between all three groups (P < 0.05) (Fig. 3A), changes in lean only differed significantly between CON and SUB (P < 0.05) (Fig. 3B).
Fig. 3.
The VBS had a significant effect on fat mass (A) and lean mass (B). During recovery, both status and treatment affected changes in fat mass (C), while only status significantly affected lean mass (D). a,b,cDifferent letters indicate significant differences between status groups (P < 0.05). *P < 0.05 between treatments within status group. Numbers in parentheses represent group size.
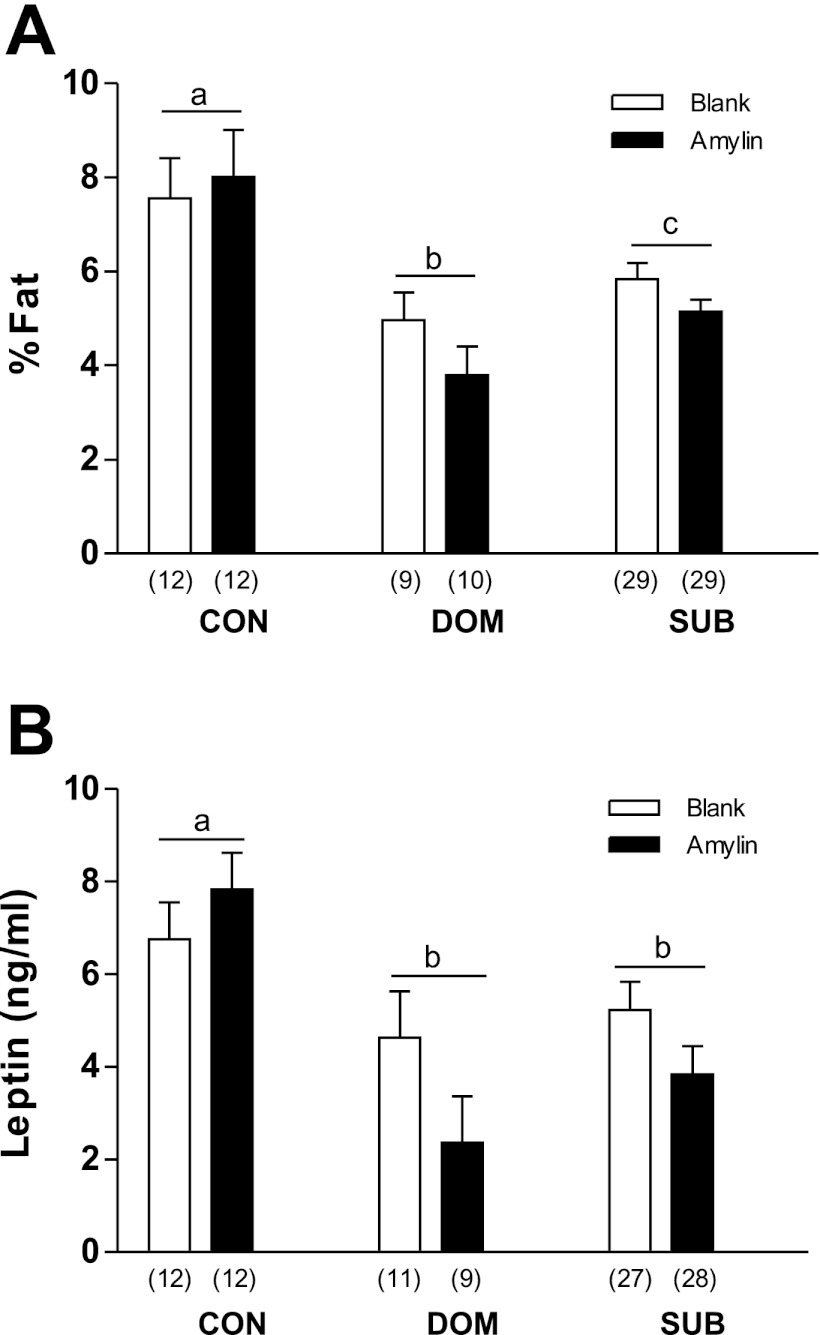
Changes in fat mass during recovery reflected main effects of both treatment (F1,95 = 20.54, P < 0.001) and status (F2,95 = 43.07, P < 0.001) (Fig. 3C), with all three status groups differing significantly. Amylin suppressed fat mass gain in burrow rats, but not in CON. While lean mass was not affected by amylin, it was affected by status (F2,95 = 9.88, P < 0.001). SUB gained significantly more lean tissue than CON and DOM during the recovery period (Fig. 3D). At the end of recovery, the percentage of body weight as fat was significantly lower in DOM and SUB than CON (Fig. 4A), but it was not significantly different between treatments.
Fig. 4.
At the end of recovery, % body fat (A) and plasma leptin concentrations (B) were lower in burrow rats. a,b,cDifferent letters indicate significant differences between status groups (P < 0.05). Numbers in parentheses represent group size.
Food intake.
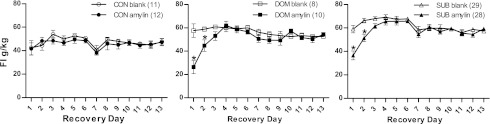
To account for the difference in body weight among the groups, food intake was calculated as grams of food consumed per kilogram body weight. During recovery, chronic peripheral infusion of amylin produced a significant treatment × status × time interaction (F12,492 = 4.01, P < 0.001). DOM and SUB displayed hyperphagia relative to CON, which was blocked by amylin for the first 2 days of recovery. Also, amylin significantly reduced food intake on recovery days 1 and 2 in the DOM and SUB groups, but not in CON (Fig. 5).
Fig. 5.
Amylin significantly reduced food intake in burrow rats during the first 2 days of recovery. FI, food intake. *P < 0.05 between blank and amylin on that day within status group. Numbers in parentheses represent group size.
Circulating hormones.
Plasma leptin (ng/ml) was measured at the end of recovery. While leptin levels were not influenced by amylin treatment, there was a main effect of status (F2,51 = 4.18, P = 0.021) with DOM and SUB having a significantly lower leptin concentration than CON, consistent with the lower percent of body weight as fat in DOM and SUB (Fig. 4B).
Corticosterone (CORT, ng/ml) was measured on day 7 of recovery during a restraint stress test. As expected, restraint stress caused a significant increase in CORT levels, which decreased to baseline levels after an hour of recovery (F2, 110 = 39.59), and there were no effects of status or treatment on CORT levels (Fig. 6).
Fig. 6.
Plasma CORT levels were elevated after 1 h of restraint. 0 = before restraint; 60 = after 1 h of restraint; 120 = 1 h after removal from restraint. *P < 0.05 different from other time points. Numbers in parentheses represent group size.
mRNA expression in the arcuate nucleus of the hypothalamus.
Amylin had no overall effect on expression of NPY, AgRP, or POMC (reported as percent of CON blank average). Status did not have an effect on NPY mRNA expression; however, status did significantly influence expression of AgRP (F2,31 = 3.43, P = 0.045) and POMC (F2,37 = 5.57, P = 0.008). Fig. 7 shows that AgRP expression is less in DOM than in SUB and that POMC expression is less in DOM than SUB and CON.
Fig. 7.
After 2 wk of recovery from VBS, POMC, and AgRP mRNA expression in the ARC was reduced in DOM rats . a,bDifferent letters indicate significant differences between status groups (P < 0.05). Numbers in parentheses represent group size.
DISCUSSION
The main interest in amylin lies with its ability to suppress weight gain, with an emphasis on inhibiting fat accumulation, while sparing lean tissue, an effect achieved in large part by a marked reduction in food intake (2, 20, 32). Considering the hyperphagia displayed by burrow rats during recovery, one might presume that amylin would have less impact on these hyperphagic rats than on control rats. However, weight and fat gain and food intake were reduced only in the burrow rats. These counterintuitive results may stem from interplay between the effects of amylin and the residual effects of the social stress experienced by the burrow rats. Furthermore, the data suggest that such an interaction may be strongest early on in recovery. Possible central and peripheral mechanisms through which amylin exerts more influence over burrow than control rats are discussed below.
The VBS influences several factors that may lead to the hyperphagia seen during recovery in burrow rats. Burrow rats have elevated circulating corticosterone (CORT) levels on the fourth day of being in the burrow, and CORT levels remain elevated at least until the last day of the burrow (1, 5, 6, 12, 23). The present data indicate that baseline CORT has returned to normal by the 7th day of recovery. Corticosterone stimulates food intake and increases expression of the orexigenic neuropeptides NPY and AgRP in the ARC (34, 39). In turn, NPY exerts positive feedback on the hypothalamic-pituitary-adrenal (HPA) axis, increasing plasma ACTH and CORT (15). Thus, chronically elevated CORT levels may create an orexigenic drive through upregulation of NPY and AgRP in socially stressed rats. The loss of fat mass seen in both DOM and SUB indicates a negative energy balance, which may also create an orexigenic drive through similar mechanisms (29). Indeed, singly housed males weight-matched to SUB VBS rats also show hyperphagia when given ad libitum access to standard chow (36).
In opposition to the potentially orexigenic effects of VBS stress, burrow rats also show an increase in expression of CRF in the paraventricular nucleus of the hypothalamus and oval nucleus of the bed nucleus of the stria terminalis (1, 6). Intracerebroventricular administration of CRF reduces food intake (28) and blocks NPY-induced hyperphagia (13). The net effect of stress on the burrow rats is an inhibition of food intake with a likely increase in energy expenditure during the burrow (16, 23, 24). Subsequently, body weight and fat mass are reduced during VBS; with SUB rats affected more than DOM, and with SUB even losing lean mass compared with CON. Immediately upon removal from the burrow, SUB and DOM have increased expression of NPY mRNA in the ARC (23), and decreased plasma leptin (36). It is at this point that burrow rats evince a strong orexigenic drive (23, 26, 36), which can be transiently blocked by amylin.
Amylin has an effect, direct or indirect, on neuronal activity and feeding-related peptide expression in the ARC (8, 32, 38). While amylin is known to affect feeding-related peptides in several other hypothalamic areas, previous findings of the effect of VBS on NPY in the ARC led us to focus on this area. Also, preliminary data from another study in our laboratory show a trend for increased AgRP and decreased POMC expression in the ARC of SUB rats immediately at the end of the VBS (unpublished data). Similar to the effects of CRF (13), intracerebroventricular administration of amylin counteracts NPY-induced feeding (25). While NPY mRNA expression in the ARC is increased on the last day of the burrow, 1 wk later, expression in burrow rats is back to CON levels despite continued hyperphagia (23). The continued hyperphagia may be attributable to AgRP since elevated AgRP mRNA expression persists after NPY expression has returned to baseline in rats refed after fasting (29). Amylin had no effect on NPY mRNA expression at the end of recovery, although the coincidence of amylin's transient inhibition of food intake and heightened NPY expression during early recovery warrants further investigation. As of yet, there are no data suggesting interaction between amylin and AgRP. However, we did find that expression of AgRP and POMC mRNA was lower in DOM rats at the end of recovery. Measurements from earlier time points in the VBS/recovery cycle will be necessary for a proper interpretation of these results. Previously, Roth et al. (32) reported elevated levels of both POMC and NPY in the ARC of diet-induced obese amylin-treated rats. The discordance with the present study may be due to differences in dose (100 μg·kg−1·day−1 in the present study instead of 300 μg·kg−1·day−1), use of diet-induced obese vs. VBS rats, overall magnitude of weight loss, and/or treatment regimen (2 wk in the present study vs. 24 days). We used a lower dose of amylin in the present study because of concerns that a higher dose would cause SUB rats to continue losing weight during recovery, which might result in debilitating and possibly fatal weight loss. This lower dose may also partially explain the absence of feeding inhibition in CON rats.
Recovery from VBS and amylin treatment modifies total food intake by way of their opposing effects on satiety. The hyperphagia of SUB rats during recovery is characterized by the ingestion of larger meals and longer meal duration, with no increase in daily meal frequency (23), an effect that may be mediated, at least in part, by NPY, since NPY administration increases food intake by increasing meal size (17). SUB meal patterns also display a shift in circadian rhythm by consuming fewer meals in the dark phase than DOM and CON (23). Amylin treatment, on the other hand, decreases food intake primarily through reductions in meal size, along with a shortening of meal duration at higher doses (19, 22, 31). Amylin also produces a more pronounced reduction in food intake during the light phase (2), and this may further normalize the meal patterns of SUB rats during recovery. A limited set of preliminary data collected from a subset of animals in this study suggests that on the second day of recovery amylin-treated VBS rats ate much smaller meals (∼0.5 g per meal) compared with untreated rats (1.5–2 g per meal).
While satiety signals may ultimately converge in the hypothalamus, many of these signals originate in the periphery. Leptin, a hormone secreted from adipocytes, decreases meal size without any effect on meal frequency (9). The reduction in body weight and fat mass in burrow rats coincides with a reduction in plasma leptin at the end of the VBS (36) that persists through 2 wk of recovery. Although this decrease in plasma leptin is unaltered by amylin administration, it is possible that amylin administration increases sensitivity to leptin by up-regulating leptin receptor expression in the hypothalamus (21). Indeed, there is an additive effect of amylin and leptin on body weight and food intake, suggesting increased leptin sensitivity in the ARC (37). Leptin signaling can also be mediated by the HPA axis (11, 18, 39), suggesting a mechanism by which social stress may enhance leptin and/or amylin sensitivity in VBS rats.
In addition to suppressing food intake, amylin prevents reductions in energy expenditure that would be expected in rats with reduced weight, such as the burrow rats in this study (14, 22, 27, 32). Administration of amylin to anesthetized rats increases V̇o2, heart rate, and body temperature, and these effects are blocked by pretreatment with a β-adrenergic receptor antagonist (27). Because of the loss of adipose during VBS housing, SUB and DOM have a greater proportion of lean, metabolically active, tissue than CON males, which may, in turn, render them more sensitive to the effects of amylin on energy expenditure.
Perspectives and Significance
Amylin attenuates the hyperphagia, weight gain, and fat gain in rats recovering from social stress. Although the mechanisms through which amylin is able to achieve these effects preferentially in burrow rats remain elusive, the transient suppression of food intake at the beginning of recovery provides some clues as to what those mechanisms may be. Additionally, this transient effect may provide clues to the mechanism through which hyperphagia is sustained throughout recovery. One might speculate that during the burrow, despite the negative energy balance of the rats, stress is inhibiting any potential orexigenic drive. Negative energy balance and heightened CORT could drive up expression of NPY and AgRP during the VBS, but the orexigenic effects of these peptides are blocked by a stress-induced increase in CRF. Once the rat is removed from the stressful environment, inhibition is lifted, and the rat engages in hyperphagia. This could be attributed to a reduction in CRF release, allowing increased NPY and AgRP to drive hyperphagia. Amylin appears to function by transiently suppressing this effect during the first days of recovery, perhaps by inhibiting NPY. The subsequent decrease in NPY after 1 wk of recovery suggests that the orexigenic drive has switched to another mechanism, perhaps one that can resist the effects of amylin. Validation of such proposed mechanisms will require experiments beyond the scope of this study, which was intended to test the hypothesis that amylin will prevent weight gain and hyperphagia in recovering VBS rats. Although we now have confirmation of the effect of amylin in recovering rats, the results from the end-point of our study point to the necessity of earlier measurements to confirm the suspected mechanisms discussed here. Thus, the effects of amylin during recovery would be clarified by future studies measuring the effects of VBS early on in recovery, specifically focusing on NPY, AgRP, POMC, and leptin receptor expression during the peak of amylin's effect on food intake. Further experiments would also benefit from the addition of weight-matched controls to distinguish the effects of weight loss from the effects of social stress on hyperphagia, feeding-related peptides, and interactions with amylin.
GRANTS
This study was supported by National Institutes of Health Grant R01 DK-066596-06.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S., K.A.S., S.J.M., E.G.K., and R.R.S. performed experiments; M.S., K.A.S., and S.J.M. analyzed data; M.S., K.A.S., S.J.M., and R.R.S. interpreted results of experiments; M.S. prepared figures; M.S. drafted manuscript; M.S., K.A.S., S.J.M., E.G.K., and R.R.S. edited and revised manuscript; M.S., K.A.S., S.J.M., E.G.K., and R.R.S. approved final version of manuscript; K.A.S., S.J.M., E.G.K., and R.R.S. conception and design of research.
ACKNOWLEDGMENTS
The authors would like to thank Jonathan Roth of Amylin Pharmaceuticals for supplying the amylin and for his comments on the manuscript.
Present address of E. Krause: College of Pharmacy, University of Florida, Gainesville, FL 32610.
Present address of S. Melhorn: Harborview Medical Center, University of Washington, Seattle, WA 98104.
REFERENCES
- 1. Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwan BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci 17: 4895– 4903, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnelo U, Permert J, Adrian TE, Larsson J, Westermark P, Reidelberger RD. Chronic infusion of IAPP causes anorexia in rats. Am J Physiol Regul Integr Comp Physiol 271: R1654– R1659, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, Hucking K, Ader M. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity 14: 16S– 19S, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen BS, Sakai RR. Visible Burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology 20: 117– 134, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav 89: 301– 310, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab 21: 159– 165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davidowa H, Ziska T, Plagemann A. Arcuate neurons of overweight rats differ in their responses to amylin from controls. Neuroreport 15: 2801– 2805, 2004 [PubMed] [Google Scholar]
- 9. Flynn MC, Scott TR, Pritchard TC, Plata-Salaman CR. Mode of action of OB protein (leptin) on feeding. Am J Physiol Regul Integr Comp Physiol 275: R174– R179, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 290: R1284– R1293, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Gardner JD, Rothwell NJ, Luheshi GN. Leptin affects food intake via CRF-receptor-mediated pathways. Nat Neurosci 1: 103, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwan BS, Haider SG, Markham CM, Blanchard RJ, Blanchard DC, Sakai RR. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod 67: 1750– 1755, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Heinrichs SC, Cole BJ, Merlo Pich E, Menzaghi F, Koob GF, Hauger RL. Endogenous corticotropin-releasing factor modulates feeding induced by neuropeptide Y or a tail-pinch stressor. Peptides 13: 879–884. [DOI] [PubMed] [Google Scholar]
- 14. Isaksson B, Wang F, Permert J, Olsson M, Fruin B, Herrington MK, Enochsson L, Erlanson-Albertsson C, Arnelo U. Chronically administered islet amyloid polypeptide in rats serves as an adiposity inhibitor and regulates energy homeostasis. Pancreatology 5: 29– 36, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Kakui N, Kitamura K. Direct evidence that stimulation of Neuropeptide Y Y5 receptor activates hypothalmo-pituitary-adrenal axis in conscious rats via both corticotropin-releasing factor and arginine vasopressin-dependent pathway. Endocrinology 148: 2854– 2862, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Kramer M, Hiemke C, Fuchs E. Chronic psychosocial stress and antidepressant treatment in tree shrews: time-dependent behavioral and endocrine effects. Neurosci Biobehav Rev 23: 937– 947, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Leibowitz SF, Alaxander JT. Analysis of neuropeptide Y-induced feeding: dissociation of Y1 and Y2 receptor effects on natural meal patterns. Peptides 12: 1251– 1260, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Lu X, Kim C, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA 103: 1593– 1598, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E. Amylin decreases meal size in rats. Physiol Behav 50: 1197– 1202, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract lesioned rats. Int J Obes Relat Metab Disord 20: 1005– 1011, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Lutz TA. The role of amylin in energy homeostasis. Am J Physiol Regul Integr Comp Physiol 298: R1475– R1248, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Mack C, Wilson J, Athanacio J, Reynolds J, Laugero K, Guss S, Vu C, Roth J, Parkes D. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Am J Physiol Regul Integr Comp Physiol 293: R1855– R1863, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. Am J Physiol Regul Integr Comp Physiol 299: R813– R822, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moles A, Bartolomucci A, Garbugino L, Conti R, Caprioli A, Coccurello R, Rizzi R, Ciani B, D'Amato FR. Psychosocial stress affects energy balance in mice: modulation by social status. Psychoneuroendocrinology 31: 623– 633, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Morris MJ, Nguyen T. Does neuropeptide Y contribute to the anorectic action of amylin? Peptides 22: 541– 546, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Nguyen MM, Tamashiro KL, Melhorn SJ, Ma LY, Gardner SR, Sakai RR. Androgenic influences on behavior, body weight, and body composition in a model of chronic social stress. Endocrinology 148: 6145– 6156, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Osaka T, Tsukamoto A, Koyama Y, Inoue S. Central and peripheral administration of amylin induces energy expenditure in anesthetized rats. Peptides 29: 1028– 1035, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharm Res 43: 425– 473, 1991 [PubMed] [Google Scholar]
- 29. Palou M, Sanchez J, Rodriguez AM, Priego T, Pico C, Palou A. Induction of NPY/AgRP orexigenic peptide expression in rat hypothalamus is an early event in fasting: relationship with circulating leptin, insulin and glucose. Cell Physiol Biochem 23: 115– 124, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Reidelberger RD, Kelsey L, Heimann D. Effects of amylin-related peptides on food intake, meal patterns, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 282: R1395– R1404, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Roth JD, Huges H, Kendall E, Baron AD, Anderson CM. Antiobesity effects of beta-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology 147: 5855– 5864, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Schwartz MW, Woods SC, Porte D, Jr, Seely RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661– 671, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Shimizu H, Arima H, Watanabe M, Goto M, Banno R, Sato I, Ozaki N, Nagasaki H, Oiso Y. Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via adenosine monophosphateactivated protein kinase signaling in the arcuate nucleus of rats. Endocrinology 149: 4544– 4553, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, YunMa L, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav 80: 683– 693, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Tamashiro KL, Nguyen MM, Ostrander MM, Gardener SR, Ma LY, Woods SC, Sakai RR. Social stress and recovery: implications for body weight and body composition. Am J Physiol Regul Integr Comp Physiol 293: R1864– R1874, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Turek VF, Trevaskis JL, Levin BE, Dunn-Maynell AA, Irani B, Gu G, Wittmer C, Griffin PS, Vu C, Parkes DG, Roth JD. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology 151: 143– 152, 2010 [DOI] [PubMed] [Google Scholar]
- 38. van Rossum D, Menard DP, Fournier A, St-Pierre S, Quirion R. Autoradiographic distribution and receptor binding profile of [125I]Bolton Hunter-rat amylin binding sites in the rat brain. J Pharmacol Exp Ther 270: 779– 787, 1994 [PubMed] [Google Scholar]
- 39. Zakrzewska KE, Cusin I, Stricker-Krongrad A, Boss O, Ricquier D, Jeanrenaud B, Rohner-Jeanrenaud F. Induction of obesity and hyperleptinemia by central glucocorticoid infusion in the rat. Diabetes 48: 365– 370, 1999 [DOI] [PubMed] [Google Scholar]