Abstract
The effects of estradiol on neuropeptide Y (NPY) neurotransmission in skeletal muscle resistance vessels have not been described. The purpose of this study was to determine the effects of long-term estradiol supplementation on NPY overflow, degradation, and vasoconstriction in gastrocnemius first-order arterioles of adult female rats. Female rats (4 mo; n = 34) were ovariectomized (OVX) with a subset (n = 17) receiving an estradiol pellet (OVE; 17β-estradiol, 4 μg/day). After conclusion of the treatment phase (8 wk), arterioles were excised, placed in a physiological saline solution (PSS) bath, and cannulated with micropipettes connected to albumin reservoirs. NPY-mediated vasoconstriction via a Y1-agonist [Leu31Pro34]NPY decreased vessel diameter 44.54 ± 3.95% compared with baseline; however, there were no group differences in EC50 (OVE: −8.75 ± 0.18; OVX: −8.63 ± 0.10 log M [Leu31Pro34]NPY) or slope (OVE: −1.11 ± 0.25; OVX: −1.65 ± 0.34% baseline/log M [Leu31Pro34]NPY). NPY did not potentiate norepinephrine-mediated vasoconstriction. NPY overflow experienced a slight increase following field stimulation and significantly increased (P < 0.05) over control conditions in the presence of a DPPIV inhibitor (diprotin A). Estradiol status did not affect DPPIV activity. These data suggest that NPY can induce a moderate decrease in vessel diameter in skeletal muscle first-order arterioles, and DPPIV is active in mitigating NPY overflow in young adult female rats. Long-term estradiol supplementation did not influence NPY vasoconstriction, overflow, or its enzymatic breakdown in skeletal muscle first-order arterioles.
Keywords: neuropeptide Y, dipeptidyl peptidase IV, arteriole, estrogen, estradiol, steroid, sex, sympathetic, supplementation
neuropeptide Y (NPY) is a multifunctional polypeptide that exists in the central and peripheral nervous systems (26, 45). In the skeletal muscle vasculature, NPY has the capacity to stimulate vasoconstriction through its postjunctional Y1-receptor, modulate the release of sympathetic neurotransmitters via its prejunctional Y2 receptor (48), and influence the magnitude of response to other sympathetic neurotransmitters (11, 41). The efficacy of NPY as a vasoconstrictor may depend on several factors such as vessel type (33, 47), vascular system (13, 33), and sex (15–17) with the latter inclusive of the cumulative effects of sex steroids.
Seminal work by Jackson and colleagues (16, 17) determined that female rats differed from male rats in some measures of NPY neurotransmission including NPY receptor actions, total NPY, and NPY degradation in whole skeletal muscle vascular beds. In female rats, ovariectomy resulted in an increase in NPY-mediated vasoconstriction compared with ovary-intact rats (15). Estradiol supplementation in the ovariectomized model reversed the increase in NPY-mediated vasoconstriction. However, Y1-receptor protein expression decreased with estradiol in white muscle only, whereas Y1-receptor protein in red muscle failed to change with estradiol treatment (15). Estradiol modulates some aspects of NPY neurotransmission in whole muscle vascular beds, but an estradiol effect may be predicated on the tissue or blood vessel type under study.
There is a paucity of literature examining the actions of NPY in sympathetic neurotransmission of small caliber resistance vessels in skeletal muscle. This level of the vasculature is of great significance as it is at this level that the majority of regulatory control occurs with respect to systemic blood pressure (40). The effects of estradiol on NPY neurotransmission in resistance vessels have not been described. It is unclear if estradiol possesses a modulatory function on NPY neurotransmission at specific levels of the skeletal muscle resistance vasculature.
The purpose of this study was to examine the effects of estradiol on NPY overflow, postjunctional (Y1) receptor activity, and enzymatic activity of a protease [dipeptidyl peptidase IV (DPPIV)] that degrades NPY in red gastrocnemius first-order arterioles. Estradiol is a prolipolytic steroid that enhances fat metabolism in skeletal muscle (44). Therefore, we hypothesized that arterioles from oxidative muscle [red gastrocnemius (6)] would be more likely to exhibit potential vascular change as a result of estradiol supplementation. We hypothesized that long-term estradiol supplementation would attenuate NPY overflow and Y1-receptor actions, the primary receptor responsible for NPY-mediated vasoconstriction and potentiation of adrenergic vasoconstriction (48). In addition to influences on NPY overflow and Y1-receptor actions, we hypothesized that estradiol would promote NPY metabolism through greater DPPIV activity. The sum of these effects would provide evidence of a direct role for estradiol in mitigating NPY neurotransmission in resistance vessels.
METHODS
Animals.
Six-month-old (197 ± 3 days) female Fischer-344 rats were used in this study. Rats (n = 34) were ovariectomized (OVX) at ∼4 mo of age (141 ± 3 days) with a subset (OVE) receiving a 17β-estradiol pellet (60-day release, 4 μg/day; Innovative Research of America, Sarasota, FL) immediately after ovariectomy. 17β-Estradiol pellets were inserted subcutaneously at the dorsoscapular region just behind the left ear above the shoulder using a 10-gauge trochar. Animal behavior, food and water intake, appearance, and surgical incisions were monitored for 10 days postoperatively. Animals were housed for 8 wk following the procedure to allow for vascular adaptations associated with long-term estradiol treatment to occur (21). Rats were housed at the university's animal care facility under static environmental conditions (22°C; 12:12 h light/dark cycle). Water and rat chow were provided ad libitum. Animal care and experimental protocols were approved by the University of Arkansas Institutional Animal Care and Use Committee.
Body weight (12, 37), uterine weight (21, 35), and bone morphology (4, 36, 43, 46) are responsive to estradiol and can be used as measures to ascertain estradiol status. Body weight was recorded before the euthanizing procedure. Rats were euthanized with an overdose of pentobarbital (40 mg/kg ip) followed by pneumothorax with the depth of anesthesia determined through flexor withdrawal reflex in response to a foot pinch. The uterus was removed and surrounding connective tissue trimmed away to assess uterine weight.
Evaluation of bone microarchitectural properties via bone histomorphometry.
Right femurs from OVX (n = 5) and OVE (n = 5) rats were dissected,fixed in 10% formalin for 3 days, dehydrated, and embedded in methylmethacrylate at low temperature. The distal femoral metaphysis was sectioned frontally with a microtome (Leica RM2255, Leica Microsystems, Bannockburn), and histological slides were created. Two 9-μm-thick histological sections stained with Goldner's trichrome were used for measurement of bone microarchitectural properties [i.e., bone volume to total volume ratio (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular number (Tb.N /mm2), and trabecular separation (Tb.Sp, μm)] using the OsteoMeasure bone histomorphometry analysis system (OsteoMetrics, Decatur, GA).
Vessel preparation.
Red gastrocnemius first-order arterioles were removed and placed in a cold (4°C) Krebs-Ringer physiological saline solution (in mmol/l: 119 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, 5.5 glucose, and 2 glycerol). Arterioles designated for DPPIV activity analysis were homogenized in 200 μl of warm (37°C) Krebs-Ringer physiological saline solution, centrifuged for 3 min (2,000 g), and the supernatant removed. All samples were flash frozen and stored at −80°C.
NPY sampling and analysis.
Arterioles for NPY analysis were transferred to a vessel chamber (Living Systems, Burlington, VT) with the ends secured to micropipettes using 11–0 ophthalmic suture. The vessel bath contained a Krebs-Ringer physiological saline solution (37°C, pH 7.4, bubbled with 5% CO2-30% O2), and the vessel was perfused with Krebs-Ringer physiological saline solution containing 1% albumin (37°C, pH 7.4) (34). The vessel chamber was transferred to the stage of an inverted microscope (Olympus CKX41, Melville, NY). A sampling port was placed within the vessel chamber juxtaposed to the suspended vessel. The micropipettes were connected to independent reservoir systems. Initial luminal pressure was set at 60 cmH2O for 30 min. Luminal pressure was then increased to 90 cmH2O, which is a pressure associated with normal in vivo conditions (49). The bath solution was replaced at 15-min intervals during equilibration. The arterioles were considered viable if they were able to constrict by 10% in response to phenylephrine (10 μmol/l) and to dilate by 20% to acetylcholine (1 μmol/l) (39).
Field stimulation was delivered via two parallel platinum electrodes placed on either side of the vessel. The electrical current was supplied using a DS3 isolated constant current stimulator (Digitimer, Letchworth Garden City, UK) interfaced with a Powerlab 16/30 with Chart software (version 5.2; ADI Instruments, Colorado Springs, CO). The exocytosis of large dense-cored vesicles that store NPY occurs at higher neural frequencies (7, 20, 24–25); therefore, 60 Hz, 32 mA, and 200 impulses were selected to elicit NPY release (8). Bath samples (200 μl) were taken before field stimulation (baseline), immediately following the cessation of field stimulation (0 s), and 30 s postfield stimulation. For experiments assessing the effects of DPPIV activity on NPY overflow, a DPPIV inhibitor, diprotin A (4 μmol/l; Sigma-Aldrich, St. Louis, MO), was added to the vessel bath and allowed to incubate for 20 min. Field stimulation and sampling time points were performed as previously stated. Samples were flash frozen and stored at −80°C. A peptide enzyme immunoassay (S-1145; Bachem, King of Prussia, PA) was used to determine NPY overflow as previously described (8). The assay had a minimum detectable concentration of 0.04–0.06 ng/ml.
DPPIV activity analysis.
DPPIV samples were brought to room temperature along with assay components. Incubation buffer (50 mmol/l Tris·HCl, pH 8.3), substrate solution (20 mmol/l glycyl-l-proline-4-methoxy-2-naphthylamide), and DPPIV sample were added to the sample wells of a black 96-well microplate, while standard solution (50 mmol/l 4-methoxy-2-naphthylamine), “stopping” solution (100 mmol/l citrate, pH 4.0), substrate solution, and incubation buffer were added to the standard wells. The microplate incubated on a heating block (37°C) for 30 min, and the reaction was terminated with stopping solution. Fluorescence was excited at 360 and measured at 440 nm on a FLX800 fluorometer (Biotek Instruments, Winooski, VT). The minimum detection limit for enzyme activity of this assay is 5 μmol·l−1·min−1 (38).
Vasomotor response.
Cumulative concentration response curves were performed to elucidate arteriole response to an NPY analogue. Vessel (luminal) diameter was measured through the use of video calipers (307A Horizontal Video Calipers, Colorado Video, Boulder, CO). NPY-mediated vasoconstriction was assessed using the postjunctional Y1-receptor agonist [Leu31Pro34]NPY (1 pmol/l-10 μmol/l; Bachem). This Y1-receptor agonist was equipotent to NPY in stimulating Y1-receptor-mediated actions such as vasoconstriction (19) or potentiation of adrenergic vasoconstriction (50). An α-adrenoceptor agonist norepinephrine (NE, 100 fmol/l-100 μmol/l; Sigma-Aldrich) was used to establish a baseline EC50 and slope to examine the efficacy of [Leu31Pro34]NPY to potentiate adrenergic vasoconstriction. Y1-receptor-mediated potentiation of adrenergic vasoconstriction was determined by adding a single concentration of [LeuPro34]NPY (98 nmol/l) 5 min before performing the second adrenergic cumulative concentration response curve. This concentration of Y1-receptor agonist was within a range of concentrations capable of eliciting potentiation of NE-mediated vasoconstriction (13, 28). The vessel bath was rinsed five times following each cumulative concentration response curve. Data were presented as a percentage of the maximum vasoconstriction achieved with KCl (80 mmol/l) and NE (10 μmol/l). The concentration response curves were fit using GraphPad Prism 5 for windows (San Diego, CA) using the equation below.
where Y is the percentage of maximum vasoconstriction, X is the drug concentration, “minimum” and “maximum” represent the respective range of percentage of maximum vasoconstriction, EC50 is the drug concentration that elicits 50% of maximum vasoconstriction, and the Hill slope represents the steepness of the curve.
Statistical analysis.
Data were expressed as means ± SE. NPY overflow mean data were derived using difference scores from baseline (Δ) NPY levels (expressed in ng/ml). NPY overflow data during control and DPPIV inhibition conditions were analyzed using a repeated measures one-way analysis of variance (SAS 9.1.3, Cary, NC). Differences in animal descriptive characteristics, bone microarchitectural properties, DPPIV activity, and vessel protein were determined using independent samples t-tests. One-way multivariate analyses of variance were performed (EC50 and slope) for the respective cumulative concentration response curves to detect differences in vasomotor response. The criteria for determining statistical significance (α = 0.05) was the same for all comparisons.
RESULTS
Animal characteristics.
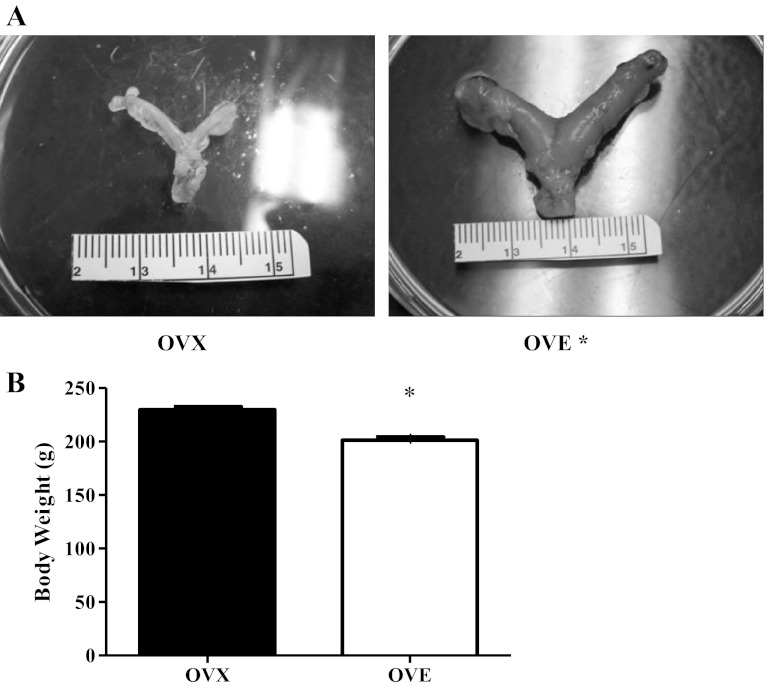
OVE (n = 17) rats had substantially larger uteri (Fig. 1A; OVE: 933 ± 17 mg, OVX: 185 ± 5 mg, P < 0.05) and lower body weights at the conclusion of the 8-wk period (Fig. 1B; OVE: 201 ± 3 g, OVX: 230 ± 3 g, P < 0.05) compared with OVX (n = 17). Estradiol supplementation significantly enhanced bone volume-to-total volume ratio in the OVE group versus OVX group by augmenting (P < 0.05) trabecular thickness and number (Table 1). Furthermore, there was a tendency (P = 0.07) for augmented trabecular separation in the OVE group versus OVX group. These data support a difference in estradiol status between OVE and OVX groups.
Fig. 1.
Animal characteristics: uterine weight (A) and body weight (B). Rats with estradiol supplementation (OVE: n = 17) had larger uteri and lower body weight compared with their ovariectomized (OVX: n = 17) counterparts. Bars indicate means ± SE. *Significant difference from OVX (P < 0.05).
Table 1.
Microarchitectural properties of the femoral distal metaphysis
| OVX | OVE | |
|---|---|---|
| BV/TV, % | 2.8 ± 0.8 | 12.4 ± 1.4* |
| Tb.Th, μm | 21.5 ± 2.4 | 29.3 ± 1.8* |
| Tb.N, mm | 1.2 ± 0.3 | 4.6 ± 0.7* |
| Tb.Sp, μm | 1314.0 ± 516.2 | 213.5 ± 37.8† |
Values represent means ± SE. OVX, ovariectomized; OVE, ovariectomized and receiving 17β-estradiol; BV/TV, bone volume-to-total volume ratio; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation.
P < 0.05 vs. OVX group,
P < 0.10 vs. OVX group.
NPY overflow and metabolism.
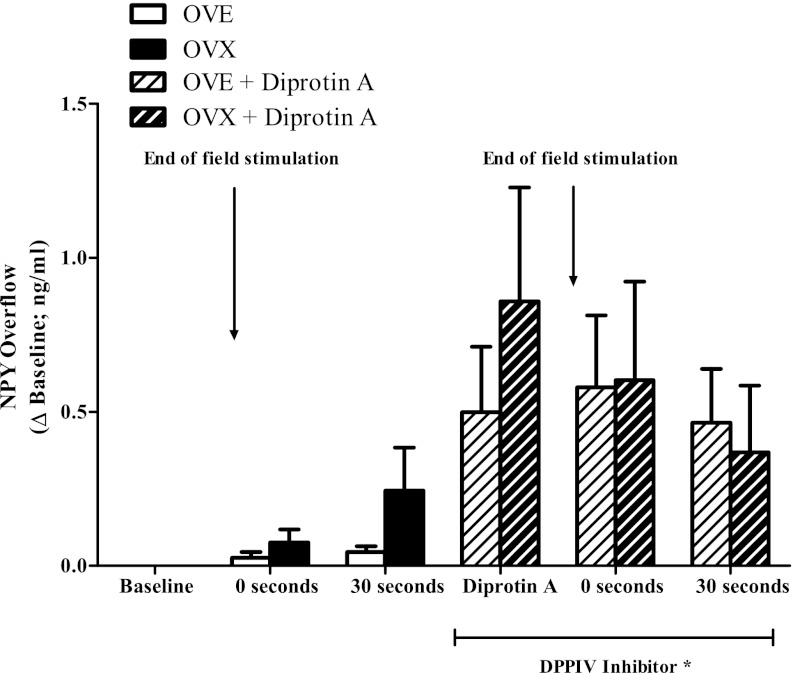
Absolute NPY overflow before field stimulation (control baseline) did not differ between groups (OVX: 2.86 ± 0.54 ng/ml; OVE: 2.12 ± 0.47 ng/ml). NPY overflow increased over and above baseline values following field stimulation in OVX and OVE rats (Fig. 2). NPY overflow exhibited a gradual increase with the greatest concentration detected at 30 s postfield stimulation (OVX: 0.24 ± 0.14 ng/ml; OVE: 0.04 ± 0.02 ng/ml).
Fig. 2.
Neuropeptide Y (NPY) overflow in gastrocnemius first-order arterioles of OVX (n = 9) and OVE (n = 11) rats. NPY overflow was greater with DPPIV inhibition (diprotin A) compared with control condition regardless of estradiol status. Bars indicate means ± SE. *Significant difference from control conditions (baseline, 0 s, and 30 s; P < 0.05).
The enzymatic breakdown of NPY can impact the physiological response as only the full-length peptide stimulates vasoconstriction. The DPPIV inhibitor diprotin A was added to the vessel bath to determine the effects of DPPIV activity on NPY bioavailability according to estradiol status. Absolute NPY overflow with DPPIV inhibition (DPPIV baseline) before field stimulation was similar across groups (OVX: 3.44 ± 0.56 ng/ml; OVE: 2.56 ± 0.66 ng/ml). DPPIV inhibition resulted in an increase in NPY overflow at 0 s (OVX: 0.60 ± 0.29 ng/ml; OVE: 0.58 ± 0.23 ng/ml) and 30 s (OVX: 0.37 ± 0.20 ng/ml; OVE: 0.46 ± 0.18 ng/ml) following field stimulation in both groups (Fig. 2: P < 0.05).
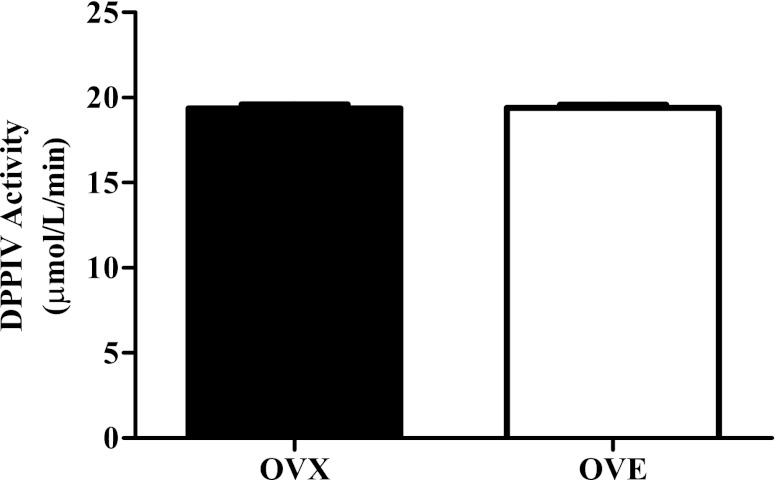
Whole vessel homogenates were analyzed to directly assess DPPIV activity (Fig. 3). DPPIV activity did not differ between OVX (19.37 ± 0.21 μmol·l−1·min−1, n = 13) and OVE rats (19.39 ± 0.18 μmol·l−1·min−1, n = 15). Total vessel protein content was quantified to rule out the possibility of differences in enzyme activity with respect to vascular smooth muscle. Arteriole protein content did not differ between OVX (39.72 ± 0.45 μg/ml) and OVE (39.37 ± 0.40 μg/ml) groups. DPPIV activity was similar between groups, and its proteolytic actions played an integral role in modulating the bioavailability of NPY regardless of estradiol status in this young-adult cohort.
Fig. 3.
DPPIV activity in gastrocnemius first-order arterioles of OVX (n = 13) and OVE (n = 15) rats. Estradiol supplementation did not influence DPPIV activity. Bars indicate means ± SE.
NPY vasomotor response.
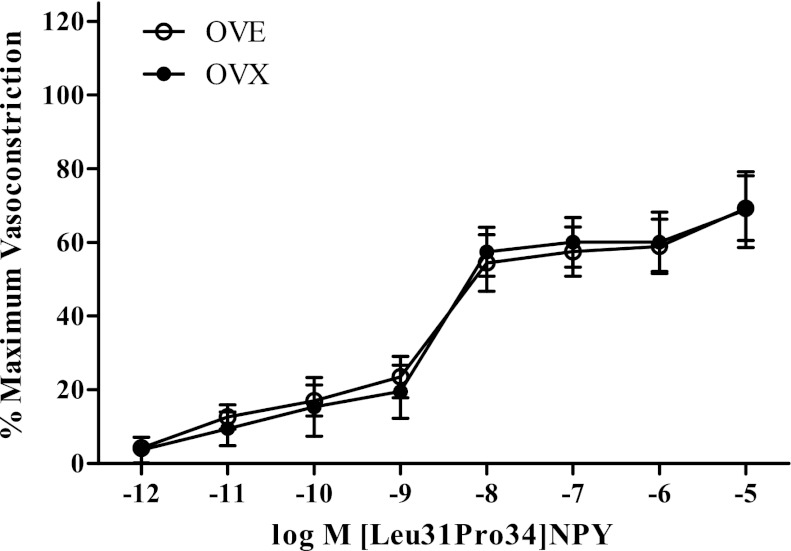
Diameter measurements of red gastrocnemius first-order arterioles at resting and maximal vasoconstriction conditions did not differ between OVX (282.43 ± 12.28 μm and 110.08 ± 6.89 μm, respectively) and OVE (293.56 ± 8.61 μm and 96.15 ± 4.48 μm, respectively) rats. The cumulative concentration response curves were performed to determine the effects of long-term estradiol supplementation on Y1-receptor activity in skeletal muscle arterioles. The Y1-receptor agonist [Leu31Pro34]NPY elicited a decrease in vessel diameter that was 69% of maximum vasoconstriction (Fig. 4) at the highest concentration tested. Estradiol status did not affect the maximum amount of Y1-mediated vasoconstriction with OVE (69 ± 9% of maximum vasoconstriction; n = 9) producing similar magnitudes of vasoconstriction to that observed in OVX (69 ± 10% of maximum vasoconstriction; n = 7). The sensitivity of Y1-receptor actions did not differ between OVE (EC50: −8.75 ± 0.18 log M [Leu31Pro34]NPY; Hill slope: −1.11 ± 0.25% maximum vasoconstriction (%max VC)/log M [Leu31Pro34]>NPY) and OVX rats (EC50: −8.63 ± 0.10 log M [Leu31Pro34]NPY; Hill slope: −1.65 ± 0.34% max VC/log M [Leu31Pro34]NPY).
Fig. 4.
Cumulative concentration response curve for Y1-receptor agonist. [Leu31Pro34]NPY-mediated vasoconstriction was similar in OVX (n = 7) and OVE (n = 9) rats. Bars indicate means ± SE.
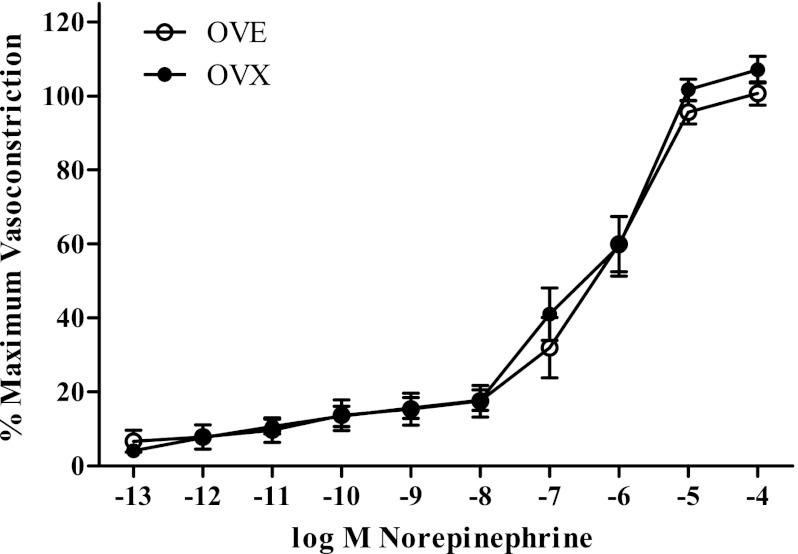
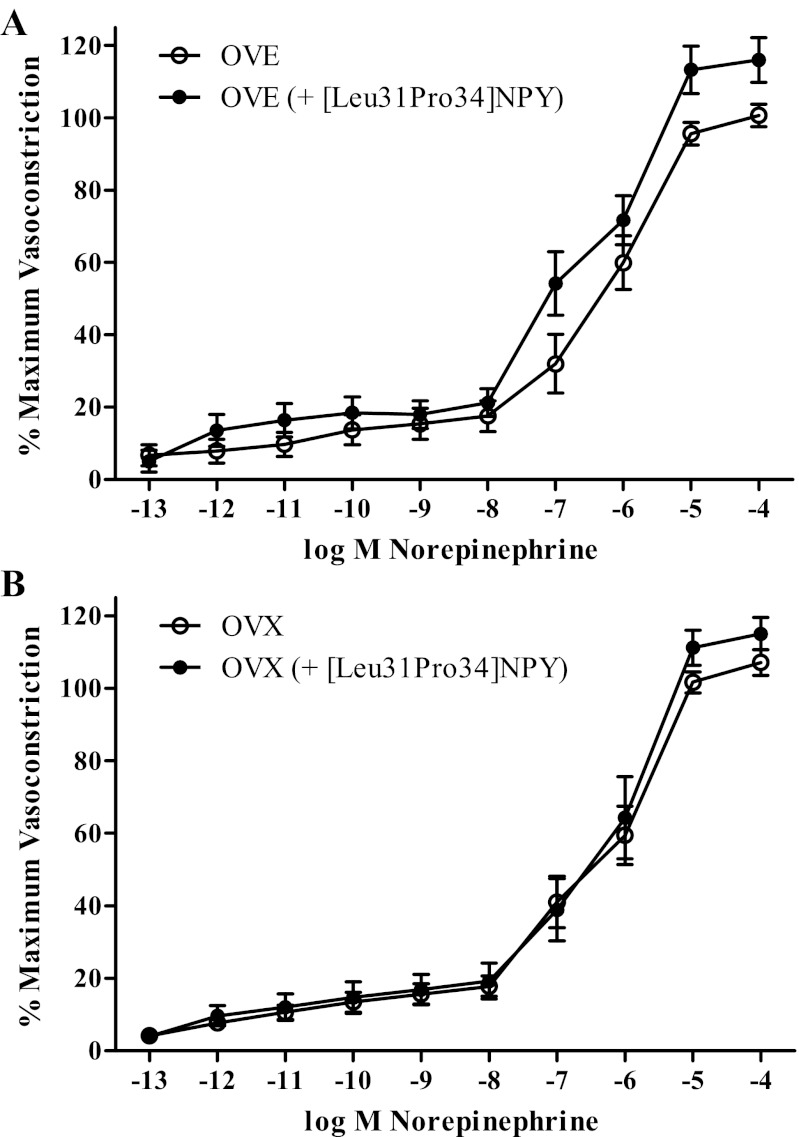
A final set of cumulative concentration response curves was performed to assess the effects of estradiol supplementation on Y1-receptor-mediated potentiation of adrenergic vasoconstriction. OVE (n = 12; EC50: −6.31 ± 0.19 log M NE; slope: −1.19 ± 0.21% max VC/log M NE) did not differ from OVX (n = 13; EC50: −6.16 ± 0.19 log M NE; slope: −1.28 ± 0.28% max VC/log M NE) in NE-stimulated vasoconstriction (Fig. 5). Interestingly, neither OVE (n = 11; EC50: −6.40 ± 0.14 log M NE; slope: −0.78 ± 0.14% maximum vasoconstriction/log M NE) nor OVX (n = 10; EC50: −6.18 ± 0.20 log M NE; slope: −1.34 ± 0.34% maximum vasoconstriction/log M NE) exhibited NPY potentiation via [Leu31Pro34]NPY of NE-stimulated vasoconstriction (Fig. 6).
Fig. 5.
Cumulative concentration response curve for α-adrenergic receptor agonist. Norepinephrine-mediated vasoconstriction did not differ between OVX (n = 13) and OVE (n = 12) rats. Bars indicate means ± SE.
Fig. 6.
Cumulative concentration response curves for α-adrenergic vasoconstriction in the presence of a Y1-receptor agonist in OVE (A: n = 11) and OVX rats (B: n = 10). [Leu31Pro34]NPY failed to potentiate norepinephrine-mediated vasoconstriction in OVX and OVE rats. Bars indicate means ± SE.
DISCUSSION
The purpose of this study was to examine the effects of estradiol on NPY overflow, metabolism, and Y1-mediated vasoconstriction in red gastrocnemius first-order arterioles. We found that NPY overflow in ovariectomized rats was detectable and was strongly inhibited by dipeptidyl peptidase IV activity. However, estradiol replacement did not alter NPY overflow or inhibition by dipeptidyl peptidase IV. Y1-receptor-mediated vasoconstriction occurred in first-order arterioles of ovariectomized rats but was not influenced by estradiol replacement. Although estradiol did not affect these measures of NPY neurotransmission, estradiol supplementation in this study significantly influenced variables that are known to be responsive to estradiol such as body weight (12, 37), uterine weight (21, 35), and bone morphology (4, 36, 43, 46). The estradiol dosage was similar to dosages used in other studies that detected estradiol effects on vascular physiology (15, 42). To our knowledge this is the first study to directly evaluate the role of estradiol in mediating NPY overflow, metabolism and Y1-mediated vasoconstriction. We found that estradiol replacement in ovariectomized rats does not affect any of these variables in the red gastrocnemius first-order arteriole.
NPY overflow.
Our lab previously detected low levels (<0.05 ng/ml) of NPY overflow following field stimulation in adult, ovary-intact rat skeletal muscle arterioles (8). While characteristics related to NPY overflow have received little attention, there was evidence to suggest that estradiol depletion led to an increase in NPY content of skeletal muscle tissue (15). The present results indicate that long-term estradiol supplementation does not influence NPY overflow from red skeletal muscle arterioles of ovariectomized rats. Indeed, NPY overflow patterns in ovariectomized (OVX and OVE) rats were similar to those previously observed in ovary-intact rats (8). Furthermore, these results are congruent with norepinephrine release data in rat tail artery, which was independent of sex steroid influences (9).
Estradiol affects the expression of many vascular proteins (30); therefore, it was plausible that estradiol was an underlying mechanism behind the sex differences observed in some measures of NPY neurotransmission (16, 17). The contrary results of the current study versus those using whole muscle homogenate may be attributed to several factors. First, whole muscle homogenates do not discriminate between vessel type (arteries, veins, capillaries, venules); thus it is difficult to pin down the source behind the differences in NPY content. Whole muscle homogenate will also include blood elements that contain NPY originating from nonneural sources (platelets, adrenal gland). One or more of these sources may be sensitive to estradiol supplementation, which could affect the expression of NPY and subsequent detection. The present study possesses experimental control for extraneous sources of NPY such as those related to blood elements. Therefore, the NPY concentration recorded is indicative of the NPY overflow characteristic of an isolated red skeletal muscle first-order arteriole, which appears to be independent of estradiol supplementation.
DPPIV activity.
Our data indicate that low levels of NPY detection in young adult female rats occur, in part, as a product of high protease activity under normal physiological conditions. Once DPPIV activity is marginalized, NPY concentration rises significantly over baseline levels in both OVX and OVE rats. This effect was irrespective of estradiol status; and it was consistent with previous observations in ovary-intact female rats, which exhibited a similar increase in NPY overflow with DPPIV inhibition (8).
The actions of DPPIV can significantly impact the response initiated by NPY. In female rat tail artery, peptidase inhibition in combination with exogenous NPY increased adrenergic vasoconstriction following transmural nerve stimulation, whereas the same condition had no effect on adrenergic vasoconstriction in ovariectomized rats (10). Similarly, peptidase inhibition resulted in decreased blood flow in hind leg conduit arteries of female rats (17). The present findings fail to support a link between long-term estradiol supplementation and NPY metabolism in red skeletal muscle first-order arterioles. While no effect was observed with estradiol supplementation, the results do reveal some interesting developments in our concept of NPY in the vasculature.
The physiological significance of an augmented role for DPPIV in the female rat resistance vasculature is that female rats would have less Y1-receptor actions, and therefore, less NPY-mediated vasoconstriction. Systemic blood pressure is maintained, in part, through the actions of arterioles, which control blood flow into the capillary beds (40). While it is difficult to determine the role of DPPIV activity in influencing blood flow through the resistance vasculature, the present results do suggest a significant role for DPPIV in modulating the amount of bioavailable NPY.
The stark similarities in DPPIV activity through direct (enzymatic assay) and indirect (NPY assay following DPPIV inhibition) measurements provide evidence to support the idea that estradiol supplementation does not affect DPPIV mechanisms in first-order arterioles. Prior study of whole skeletal muscle failed to detect a difference in DPPIV activity with 2 wk of estradiol supplementation (15). A unique addition of the present study was to extend DPPIV analysis directly to isolated first-order arterioles. Although DPPIV is active in modulating NPY in red skeletal muscle first-order arterioles of young adult female rats, it is independent of influences related to long-term estradiol supplementation.
NPY vasoconstriction.
The efficacy of NPY as a vasoconstrictor depends on the level (artery, arteriole) and location (mesentery, brain, skeletal muscle) of the vasculature under study. Very little is known about the behavior of NPY in the skeletal muscle arterioles of female rats. A prior study (18) of male skeletal muscle arterioles revealed an inverse relationship between the magnitude of vasoconstriction (vessel diameter changes) and vessel size (first-, second-, third-order arterioles). In the current study, the amount of vasoconstriction observed in female rat skeletal muscle first-order arterioles was similar to previous measurements of the same vessel type observed in males (18). The cornerstone of the present study was the long-term effect of estradiol on NPY neurotransmission. The available studies on sex differences (16–17) in NPY neurotransmission implicate sex hormone status as a possible candidate for the underlying difference between male and female rats. The present data fail to support a link with respect to estradiol supplementation and Y1-receptor activity as there were no differences between OVX and OVE groups.
The absence of an estradiol effect on sympathetic neurotransmission is not unprecedented with similar null results having been observed in adrenergic vasoconstriction (3, 22, 29, 42). Estradiol supplementation over a wide range of treatment durations (∼1–60 days) did not alter the adrenergic postjunctional response in female rats (3, 29, 42). The effects of estradiol supplementation on NPY-mediated vasoconstriction are less clear and have received little attention. In white vastus muscle, estradiol depletion increased Y1-receptor protein expression and actions (15). However, estradiol depletion failed to change Y1-receptor protein in red vastus muscle. It is possible that Y1 mechanisms are dependent on the type of tissue (red or white muscle) or blood vessel under study. Our results are consistent with other in vitro studies that failed to detect a difference in sympathetic (adrenergic) vasoconstriction following estradiol supplementation (3, 29, 42). Y1-receptor activation can induce a moderate amount of vasoconstriction in isolated first-order arterioles of young adult female rats, and long-term estradiol supplementation does not impact Y1-receptor sensitivity in these vessels.
NPY potentiation of adrenergic vasoconstriction.
In many vascular beds, NPY fails to cause direct vasoconstriction but retains influence on vessel diameter through indirect effects, most notably through potentiation of NE-induced vasoconstriction at low concentrations (1–100 nmol/l) (1, 2, 13, 47). In the present study, the Y1-receptor agonist did not potentiate NE-mediated vasoconstriction in OVX and OVE groups. The concentration used (98 nmol/l) was within the previously mentioned range of concentrations where this Y1-receptor agonist potentiates NE-mediated vasoconstriction (13, 28).
While the potentiation of adrenergic vasoconstriction is an oft-described product of NPY in the vasculature (23), the lack of potentiation in vessels that exhibit vasoconstriction to NPY has been observed before (5, 14, 27). Moreover, this lack of a contributory effect of NPY on adrenergic vasoconstriction in these studies was noted in multiple animal models across diverse vascular beds. In agreement with these findings, the present results revealed a potent vasoconstrictor effect for NPY with no discernible influence on adrenergic vasoconstriction in isolated red skeletal muscle first-order arterioles of female rats.
Limitations.
The enzymatic action of DPPIV results in a C-truncated fragment of NPY. It is unclear as to the binding capacity of the assay and the truncated product of DPPIV activity (NPY3–36); however, we interpreted the increase in detectable NPY as an increase in the bioavailability of the full-length peptide based on the known actions of DPPIV with respect to NPY metabolism. Some measures of NPY neurotransmission in skeletal muscle may depend on the type of muscle (red or white) under study (15). The present study was concerned with a resistance vessel that supplies primarily oxidative muscle (6). It is unknown if NPY neurotransmission of white gastrocnemius first-order arterioles is responsive to estradiol supplementation.
Conclusions.
In young adult female rats, long-term estradiol supplementation did not influence NPY overflow, breakdown, or Y1-receptor-mediated vasoconstriction in isolated skeletal muscle first-order arterioles. NPY overflow was detected following field stimulation and increased with DPPIV inhibition regardless of estradiol status. The putative postjunctional receptor for NPY, Y1, induced a moderate vasoconstrictive response in young adult female rat resistance vessels.
Perspectives and Significance
The observation that estradiol promotes vasorelaxation through modulation of endothelial nitric oxide synthase activity (31) underscores the potential for sex steroids to influence physiological processes beyond those associated with reproduction. Today, there is a level of expedition to develop our understanding on the vascular effects of sex steroids, specifically estradiol and progesterone, as subjects such as hormone replacement therapy become more common and polarizing in an aging female population. Cardiovascular risk increases with age for both males and females, and increased sympathetic nerve activity in the vasculature is a contributor to age-related changes in blood pressure (32). While limited evidence suggests that sex steroids such as estradiol may attenuate various measures of NPY in females (15), the collective data inclusive of data from the present manuscript suggest that the influence of estradiol is variable and possibly dependent on multiple factors (e.g., tissue type, vessel type). Future research will require both functional and molecular components to identify the vascular regions responsive to sex steroids and the associated signaling mechanisms involved.
GRANTS
This project was supported by the National Institute on Aging Grant 5R03AG-033245 and the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlements Proceeds Act of 2000.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.W.E., A.J.S., R.P., and H.A.K. conception and design of research; K.W.E., A.J.S., E.S., T.B., R.P., and H.A.K. performed experiments; K.W.E., A.J.S., E.S., T.B., R.P., and H.A.K. analyzed data; K.W.E., E.S., T.B., R.P., and H.A.K. interpreted results of experiments; K.W.E., T.B., and R.P. prepared figures; K.W.E. drafted manuscript; K.W.E., A.J.S., E.S., T.B., R.P., and H.A.K. edited and revised manuscript; K.W.E., A.J.S., E.S., T.B., R.P., and H.A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Ryan Bailey for technical assistance during the project.
REFERENCES
- 1.Abel PW, Han C. Effects of neuropeptide Y on contraction, relaxation, and membrane potential of rabbit cerebral arteries. J Cardiovasc Pharmacol 13: 52–63, 1989 [PubMed] [Google Scholar]
- 2.Andriantsitohaina R, Stoclet JC. Enhancement by neuropeptide Y (NPY) of the dihydropyridine-sensitive component of the response to alpha 1-adrenoceptor stimulation in rat isolated mesenteric arterioles. Br J Pharmacol 99: 389–395, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandin L, Bergstrom G, Manhem K, Gustafsson H. Oestrogen modulates vascular adrenergic reactivity of the spontaneously hypertensive rat. J Hypertens 21: 1695–1702, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Cai DJ, Zhao Y, Glasier J, Cullen D, Barnes S, Turner CH, Wastney M, Weaver CM. Comparative effect of soy protein, soy isoflavones, and 17beta-estradiol on bone metabolism in adult ovariectomized rats. J Bone Miner Res 20: 828–839, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Clarke J, Benjamin N, Larkin S, Webb D, Maseri A, Davies G. Interaction of neuropeptide Y and the sympathetic nervous system in vascular control in man. Circulation 83: 774–777, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Donoso MV, Brown N, Carrasco C, Cortes V, Fournier A, Huidobro-Toro JP. Stimulation of the sympathetic perimesenteric arterial nerves releases neuropeptide Y potentiating the vasomotor activity of noradrenaline: involvement of neuropeptide Y-Y1 receptors. J Neurochem 69: 1048–1059, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Evanson KW, Stone AJ, Hammond AL, Kluess HA. Neuropeptide Y overflow and metabolism in skeletal muscle arterioles. J Physiol 589: 3309–3318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Villalon AL, Buchholz JN, Duckles SP, Krause DN. Noradrenaline content and release in male and female rat tail arteries. J Cardiovasc Pharmacol 29: 93–96, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Glenn TC, Krause DN, Duckles SP. Vascular responses to neuropeptide Y are greater in female than male rats. Naunyn Schmiedebergs Arch Pharmacol 355: 111–118, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Gustafsson H, Nilsson H. Endothelium-independent potentiation by neuropeptide Y of vasoconstrictor responses in isolated arteries from rat and rabbit. Acta Physiol Scand 138: 503–507, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Haim S, Shakhar G, Rossene E, Taylor AN, Ben-Eliyahu S. Serum levels of sex hormones and corticosterone throughout 4- and 5-day estrous cycles in Fischer 344 rats and their simulation in ovariectomized females. J Endocrinol Invest 26: 1013–1022, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Han S, Yang CL, Chen X, Naes L, Cox BF, Westfall T. Direct evidence for the role of neuropeptide Y in sympathetic nerve stimulation-induced vasoconstriction. Am J Physiol Heart Circ Physiol 274: H290–H294, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Hanko JH, Tornebrandt K, Hardebo JE, Kahrstrom J, Nobin A, Owman C. Neuropeptide Y induces and modulates vasoconstriction in intracranial and peripheral vessels of animals and man. J Auton Pharmacol 6: 117–124, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Jackson DN, Ellis CG, Shoemaker JK. Estrogen modulates the contribution of neuropeptide Y to baseline hindlimb blood flow control in female Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 298: R1351–R1357, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Jackson DN, Milne KJ, Noble EG, Shoemaker JK. Gender-modulated endogenous baseline neuropeptide Y Y1-receptor activation in the hindlimb of Sprague-Dawley rats. J Physiol 562: 285–294, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson DN, Milne KJ, Noble EG, Shoemaker JK. Neuropeptide Y bioavailability is suppressed in the hindlimb of female Sprague-Dawley rats. J Physiol 568: 573–581, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshua IG. Neuropeptide Y-induced constriction in small resistance vessels of skeletal muscle. Peptides 12: 37–41, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Duran WR, Kobayashi I, Daniels AJ, Duran WN. Microcirculatory dynamics of neuropeptide Y. Microvasc Res 48: 124–134, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Lacroix JS. Adrenergic and non-adrenergic mechanisms in sympathetic vascular control of the nasal mucosa. Acta Physiol Scand Suppl 581: 1–63, 1989 [PubMed] [Google Scholar]
- 21.LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, Muller-Delp JM. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 297: R1713–R1723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luksha L, Poston L, Gustafsson JA, Aghajanova L, Kublickiene K. Gender-specific alteration of adrenergic responses in small femoral arteries from estrogen receptor-beta knockout mice. Hypertension 46: 1163–1168, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Lundberg JM, Franco-Cereceda A, Lacroix JS, Pernow J. Neuropeptide Y and sympathetic neurotransmission. Ann NY Acad Sci 611: 166–174, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Lundberg JM, Pernow J, Franco-Cereceda A, Rudehill A. Effects of antihypertensive drugs on sympathetic vascular control in relation to neuropeptide Y. J Cardiovasc Pharmacol 10, Suppl 12: S51–S68, 1987 [PubMed] [Google Scholar]
- 25.Lundberg JM, Rudehill A, Sollevi A, Theodorsson-Norheim E, Hamberger B. Frequency- and reserpine-dependent chemical coding of sympathetic transmission: differential release of noradrenaline and neuropeptide Y from pig spleen. Neurosci Lett 63: 96–100, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Lundberg JM, Terenius L, Hokfelt T, Goldstein M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett 42: 167–172, 1983 [DOI] [PubMed] [Google Scholar]
- 27.Lundberg JM, Terenius L, Hokfelt T, Martling CR, Tatemoto K, Mutt V, Polak J, Bloom S, Goldstein M. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand 116: 477–480, 1982 [DOI] [PubMed] [Google Scholar]
- 28.McAuley MA, Westfall TC. Possible location and function of neuropeptide Y receptor subtypes in the rat mesenteric arterial bed. J Pharmacol Exp Ther 261: 863–868, 1992 [PubMed] [Google Scholar]
- 29.Mehrotra S, Gupta S, Villalon CM, Boomsma F, Saxena PR, MaassenVanDenbrink A. Rat carotid artery responses to alpha-adrenergic receptor agonists and 5-HT after ovariectomy and hormone replacement. Headache 47: 236–246, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev 60: 210–241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology 147: 5557–5563, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Pernow J, Ohlen A, Hokfelt T, Nilsson O, Lundberg JM. Neuropeptide Y: presence in perivascular noradrenergic neurons and vasoconstrictor effects on skeletal muscle blood vessels in experimental animals and man. Regul Pept 19: 313–324, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Pourageaud F, De Mey JG. Vasomotor responses in chronically hyperperfused and hypoperfused rat mesenteric arteries. Am J Physiol Heart Circ Physiol 274: H1301–H1307, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Rachon D, Seidlova-Wuttke D, Vortherms T, Wuttke W. Effects of dietary equol administration on ovariectomy induced bone loss in Sprague-Dawley rats. Maturitas 58: 308–315, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23: 279–302, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Roepke TA. Oestrogen modulates hypothalamic control of energy homeostasis through multiple mechanisms. J Neuroendocrinol 21: 141–150, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scharpe S, De Meester I, Vanhoof G, Hendriks D, van Sande M, Van Camp K, Yaron A. Assay of dipeptidyl peptidase IV in serum by fluorometry of 4-methoxy-2-naphthylamine. Clin Chem 34: 2299–2301, 1988 [PubMed] [Google Scholar]
- 39.Schneider F, Bucher B, Schott C, Andre A, Julou-Schaeffer G, Stoclet JC. Effect of bacterial lipopolysaccharide on function of rat small femoral arteries. Am J Physiol Heart Circ Physiol 266: H191–H198, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation 12: 33–45, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Small DL, Bolzon BJ, Cheung DW. Endothelium-independent potentiating effects of neuropeptide Y in the rat tail artery. Eur J Pharmacol 210: 131–136, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Stice JP, Eiserich JP, Knowlton AA. Role of aging versus the loss of estrogens in the reduction in vascular function in female rats. Endocrinology 150: 212–219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 328: 688–696, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Tarnopolsky MA. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med Sci Sports Exerc 40: 648–654, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y–a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296: 659–660, 1982 [DOI] [PubMed] [Google Scholar]
- 46.Tivesten A, Moverare-Skrtic S, Chagin A, Venken K, Salmon P, Vanderschueren D, Savendahl L, Holmang A, Ohlsson C. Additive protective effects of estrogen and androgen treatment on trabecular bone in ovariectomized rats. J Bone Miner Res 19: 1833–1839, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Vu HQ, Budai D, Duckles SP. Neuropeptide Y preferentially potentiates responses to adrenergic nerve stimulation by increasing rate of contraction. J Pharmacol Exp Ther 251: 852–857, 1989 [PubMed] [Google Scholar]
- 48.Wahlestedt C, Grundemar L, Hakanson R, Heilig M, Shen GH, Zukowska-Grojec Z, Reis DJ. Neuropeptide Y receptor subtypes, Y1 and Y2. Ann NY Acad Sci 611: 7–26, 1990 [DOI] [PubMed] [Google Scholar]
- 49.Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol 463: 631–646, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia J, Neild TO, Kotecha N. Effects of neuropeptide Y and agonists selective for neuropeptide Y receptor sub-types on arterioles of the guinea-pig small intestine and the rat brain. Br J Pharmacol 107: 771–776, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]