Abstract
We had previously proposed the presence of permanent stimulatory influences in the tissue microenvironment surrounding the aged mesenteric lymphatic vessels (MLV), which influence aged lymphatic function. In this study, we performed immunohistochemical labeling of proteins known to be present in mast cells (mast cell tryptase, c-kit, prostaglandin D2 synthase, histidine decarboxylase, histamine, transmembrane protein 16A, and TNF-α) with double verification of mast cells in the same segment of rat mesentery containing MLV by labeling with Alexa Fluor 488-conjugated avidin followed by toluidine blue staining. Additionally, we evaluated the aging-associated changes in the number of mast cells located by MLV and in their functional status by inducing mast cell activation by various activators (substance P; anti-rat DNP Immunoglobulin E; peptidoglycan from Staphyloccus aureus and compound 48/80) in the presence of ruthenium red followed by subsequent staining by toluidine blue. We found that there was a 27% aging-associated increase in the total number of mast cells, with an ∼400% increase in the number of activated mast cells in aged mesenteric tissue in resting conditions with diminished ability of mast cells to be newly activated in the presence of inflammatory or chemical stimuli. We conclude that higher degree of preactivation of mast cells in aged mesenteric tissue is important for development of aging-associated impairment of function of mesenteric lymphatic vessels. The limited number of intact aged mast cells located close to the mesenteric lymphatic compartments to react to the presence of acute stimuli may be considered contributory to the aging-associated deteriorations in immune response.
Keywords: mesenteric lymphatic vessels, mast cells, aging, compound 48/80, immunoglobulin E, substance P, peptidoglycan
the mesenteric lymphatic network is functionally the busiest part of the whole lymphatic system; it provides route for up to 90% of the daily formed lymph in whole body (4) through maintaining an effective intestinal water/lipid absorption. The mesenteric lymphatic compartments constantly operate with potential to be exposed to the pathogens consumed with food, and thus are continuously involved in the immune defense of the body at the interface between its inner spaces and biologically aggressive environment of the intestinal lumen. Each of the above-mentioned functions is affected by aging; however, the lymphatic-related component of the aging-associated dysfunctions is in large degree not investigated. In the gastrointestinal tract, the potential aging-associated alterations of active mesenteric lymph pumping, as well as the corresponding changes in mesenteric lymph flow, are basically unknown, and therefore they are largely ignored in clinical practice.
Recently, we performed series of experiments to evaluate the aging-associated alterations of the contractility of isolated rat mesenteric lymphatic vessels (MLV) (52) as well as rat MLV situated in situ (1). These data demonstrated a severe weakening of the lymphatic pump in aged MLV including diminished lymphatic contraction amplitude and, to greater degree, diminished contraction frequency. As a result of such aging-associated alterations in lymphatic inotropy and chronotropy, we observed depleted lymphatic pump flow in both isolated (52) and in situ preparations (1). As a consequence of the moderate aging-associated weakening of the active mesenteric lymphatic pump, the maximal systolic lymph flow velocity was significantly lower in aged MLV (1). At the same time diastolic lymph flow velocity was significantly higher in aged vessels correlative to observed enlargement of the aged MLV compared with adult vessels (1). All of these findings taken into consideration together may indicate lowered ability of the aged MLV to adapt their contractility to the situations with increased volumetric loads, which may occur during development of acute or chronic inflammation and local edema in the gut. At the same time, careful comparative evaluation of the parameters of the lymphatic contractility in aged MLV revealed important differences between isolated and in situ preparations. The nonspecific nitric oxide (NO) synthases blockade was able to improve, to some degree, contractility and pumping of isolated aged rat MLV (52), whereas in situ the same lymphatic vessels were able to contract (at least for the duration of experiment) with the same contraction frequency as their adult counterparts (1). Subsequently, due to elimination of the NO in aged MLV in situ, their pumping went all the way up to similar levels in adult vessels. We linked these profound differences in parameters of lymphatic contractility and pumping in aged MLV in isolated preparations versus in situ to the presence of permanent stimulatory influences of some cellular elements in the tissue microenvironment surrounding the aged MLV. Such influences in aged MLV counterbalance to some degree inhibitory effects of disturbed NO production in aged lymphatic vessels (1, 21). As a result of our recent studies (1), we focused our current research efforts on understanding the nature of the aging-associated alterations of lymphatic functions in MLV through investigations of the mechanisms of potential interaction of the aged contractile lymphatic vessels and aged tissues surrounding them.
One of the first candidates for us to investigate was mast cells situated in close proximity to MLV with their potential involvement into “lymphatic vessel-tissue” interactions in aged animals described above. Mast cells are long-lived tissue resident cells, which are strategically located at sites where host tissue comes in contact with external allergens and microbes such as upper dermis, respiratory tract, and bowel mucosa (3, 14, 43). Previous mast cell studies were limited to the context of allergy and asthma, but over the past decades mast cells have been extensively researched and have been found to play an important role in modulating innate immunity and in shaping the host adaptive immune response. Mast cells can produce, store, and release upon activation numerous inflammatory and vasoactive mediators that continuously modulate the immune system and promote inflammation (2, 28, 41, 42). Close proximity of mast cells to blood vessels is important in hypersensitivity and inflammatory reactions where release of histamine from activated mast cells increases vascular permeability, causing increased efflux of fluid and immune cells from the circulation to the interstitial tissue (13, 43, 44).
Although there has been extensive research on mast cells and the blood vasculature, few studies have investigated the modulatory role of mast cells on collecting lymphatic vessels. It has been known that mast cells are often situated in higher number in close proximity to the lymphatic vessel wall (35, 55, 72). In the paravascular zones of the mesentery, mast cells are particularly associated with lymphatic vessels, rather than with blood vessels (75, 76). Although mast cells produce and secrete various potent vasoactive mediators mentioned above, it was proposed by several investigators that at least some of them may influence lymphatic contractile/pumping function. In particular it has been shown that histamine is a potent dose-dependent modulator of lymphatic contractility (16–18, 33, 49, 54–57, 59, 70, 71). Other mast cell-derived mediators like heparin (40, 55), serotonin (15, 33, 54, 70), leukotrienes B4 and C4 (17, 33), and thromboxane A2 (59, 65) are also shown to have physiological effects on lymphatic contractility. However, until now, in only one study (59) the activation and degranulation of mast cells has been directly linked to subsequent changes in lymphatic contractility mediated by histamine. Although it assumes that mast cell activation influences lymph contractility and flow, and should subsequently modulate immune reactions in the mesentery, lipid absorption, and interstitial fluid dynamics, there are no currently available data that directly establish links between all of these processes. Moreover, the functional status of mast cells located in close proximity to aged lymphatic vessels has never been evaluated in comparison with that in the adult body, and therefore the aging-associated alterations in the regulatory interactions between lymphatic vessels and mast cells located nearby them remain undiscovered.
The objectives of our current study were to identify and reconfirm the presence of the mast cells located by rat mesenteric collecting lymphatic vessels and to evaluate the potential aging-associated changes in their number and functional status, which may be responsible for observed aging-associated alterations of lymphatic pumping in situ (1).
MATERIALS AND METHODS
Animals and surgery.
For the current studies, we used Fischer-344 male rats, a commonly used rat strain in aging-related research (51, 69) (animals obtained from aged rat colony maintained by National Institutes of Health National Institute on Aging). The animals ranged in age groups from adulthood to aged (9- and 24 mo old). All animal procedures for the current studies were reviewed and approved by our Institutional Animal Care and Use Committee.
The rats were anesthetized with a solution containing a combination of fentanyl/droperidol (0.3 ml/kg im) and diazepam (2.5 mg/kg im). The animal was then positioned on its back, and the ventral chest wall was opened by lateral incision. Immediately after animals ceased breathing and were subsequently euthanized, the sternum and approximately half of the ribs were excised and the inferior vena cava was cut to drain blood. A midline abdominal incision was made, and the whole gut was removed by cutting at the root of the mesentery after clamping it to avoid excess bleeding.
The average body weight of the animals used in this study was 400–420 g. It should be noted that for the Fischer-344 National Institutes of Health (NIH) National Institute on Aging rat strain, the majority of the weight gain occurs before 7 mo of age (69). Therefore, both 9- and 24-mo age groups are within ranges of weight slightly above 400 g and are not significantly different.
Immunohistochemical labeling and toluidine blue staining of mast cells by mesenteric lymphatic vessels.
To identify and reconfirm the presence of the mast cells located by rat mesenteric collecting vessels, we performed immunohistochemical labeling of the segments of rat mesentery containing collecting lymphatic vessels for common protein targets shown to be present in mast cells. We labeled specimens for mast cell tryptase, c-kit (CD 117), prostaglandin D2 synthase, histidine decarboxylase, histamine, transmembrane protein 16 A (TMEM 16A), and TNF-α. To verify that immunohistochemically labeled cells were mast cells, we at the same time labeled specimens with Alexa Fluor 488-conjugated avidin. Avidin has a high isoelectric point, which makes it to bind preferentially to the intensely negative charged granules containing heparin sulphate inside the mast cells (5, 9, 68). For additional confirmation that immunohistochemically labeled cells were mast cells, we finally stained all mesenteric segments with acidic solution of toluidine blue (pH −2.2). Toluidine blue is commonly used for labeling of mast cells. Toluidine blue is one of the most common stains for staining acid mucopolysaccharides and glycoaminoglycans components of mast cells granules. Toluidine blue stains mast cells red-purple (metachromatic staining) and the background blue (orthochromatic staining) (23, 36, 62).
The exteriorized gut with mesentery was rinsed three times in PBS (Catalog No. 6505; EMD Chemicals, Gibbstown, NJ) and pinned down in a sylgard-coated 10-mm Petri dish. The whole exteriorized mesentery was fixed by 4% paraformaldehyde at room temperature for 2 h, and then it was washed three times for 30 min each wash with 0.3% Triton X-100 (Catalog No. T8787; Sigma Aldrich, St. Louis, MO) in PBS. Segments of mesentery excluding the gut (with approximate size 3 × 3 cm) containing MLV were cut and put in a 24 well plate and blocked with 5% donkey serum (Catalog No. GTX27475; Genete, Irvine, CA) in 0.1% Triton X in PBS for 1 h at room temperature. Primary antibody incubation with the following antibodies was performed overnight at 4°C in 0.5% donkey serum in 0.1% Triton X-100 in PBS: rabbit polyclonal anti-rat mast cell tryptase (Catalog No. sc-32889, dilution 1:100; Santa Cruz Biotechnologies, Santa Cruz, CA), rabbit polyclonal anti-rat c-kit (Catalog No. sc 5535, dilution 1:50; Santa Cruz Biotechnologies), rabbit polyclonal anti-rat prostaglandin D2 synthase (Catalog No. sc-30067, dilution 1:100; Santa Cruz Biotechnologies, Santa Cruz, CA), rabbit polyclonal anti-rat histidine decarboxylase (Catalog No. sc-68940, dilution 1:100; Santa Cruz Biotechnologies, Santa Cruz, CA), rabbit polyclonal anti-rat histamine (Catalog No. ab43870, dilution 1:100; Abcam, Cambridge, MA), rabbit polyclonal anti-rat TMEM 16A (Catalog No. ab53212, dilution 1:50; Abcam, Cambridge, MA), and rabbit polyclonal anti-rat TNF-α (Catalog No. ab66579, dilution 1:50; Abcam, Cambridge, MA). On the next day segments were washed three times with 0.1% Triton X-PBS for 20 min for each wash and incubated with donkey anti-rabbit Alexa Fluor 647-conjugated secondary antibodies (Catalog No. A31573; Life Technologies, Grand Island, NY) and with Alexa Fluor 488-conjugated avidin at the same time (Catalog No. A21370, dilution 1:200; Life Technologies) in 0.1% Triton X-100 in PBS for 1 h at room temperature. We used ChromPure rabbit IgG (Catalog No. 011-000-003; Jackson ImmunoResearch Laboratories, West Grove, PA) as a primary antibody control at the highest dose as that for primary protein targets and Alexa Fluor 647 donkey anti-rabbit IgG (Catalog No. A31573, dilution 1:200; Life Technologies) as a secondary antibody control, respectively. After final washing three times for 20 min each with 0.1% Triton X-PBS, the segments of mesentery were mounted on glass slides and imaged using an Olympus DP72 fluorescent camera and Olympus CKX41 fluorescent microscope equipped with filters suitable for Alexa Fluor 488 and Alexa Fluor 647 imaging. The same parameters of image capturing were used for all segments treated with the same primary antibody and for corresponding controls.
Previously imaged slides were then stained with an acidic solution of toluidine blue (Catalog No. 198161, pH 2.2–2.3; Sigma Aldrich) for 5 min, washed with distilled water for 10 min, air dried, and imaged using an Olympus CKX41 fluorescent microscope under its bright field mode at the exact same position it was imaged before, to confirm that fluorescent staining of mast cells coincided with violet/purple staining of mast cell granules labeled by toluidine blue.
For each selected primary antibody, therefore, we performed sets of images with mast cells labeled by antibody, avidin, and toluidine blue. For each of such sets we used mesenteric specimen from at least three animals of each selected age; from one animal we were able to perform labeling for at least three selected antibody. For presentation we selected the most representative images of mast cells located in close proximity to rat MLV with vessels located as much as possible outside of the mesenteric adipose tissue.
To verify that mast cells are not present in walls of isolated MLV we performed similar immunohistochemical labeling of MLV segments carefully dissected out of surrounding tissues as described above for labeling of whole mesenteric tissue segments. These experiments demonstrated absence of mast cells in walls of MLV segments isolated by the same procedures as done for studies described in Ref. 52; images of mast cells-negative isolated MLV segments are not presented in results. All mast cells shown on representative images of MLV on Figs. 1 and 2 are located outside lymphatic vessels, and their overlap with vessel wall and/or vessel lumen exists on figures solely as a result of the two-dimensional nature of images.
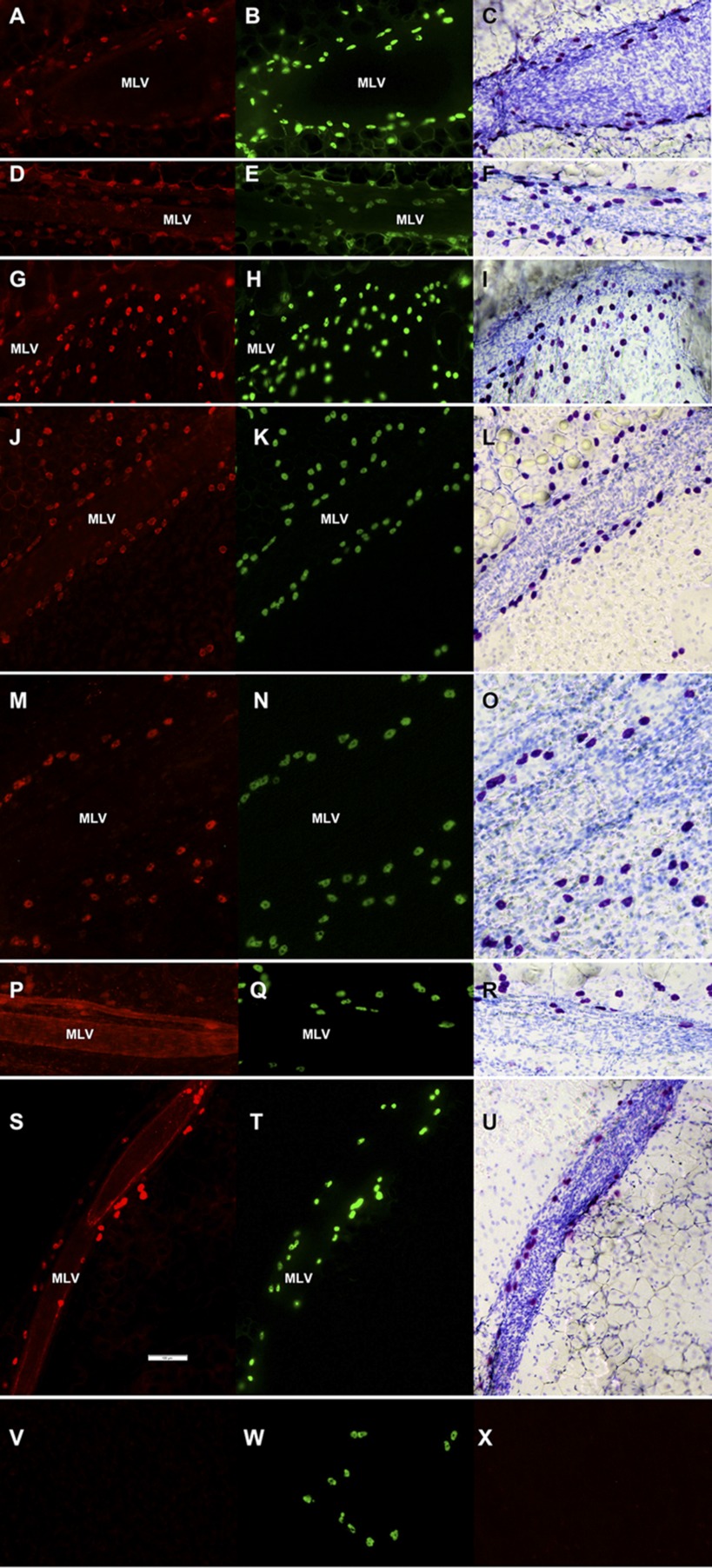
Fig. 1.
Immunohistochemical labeling (representative images of segments of rat mesentery in close proximity to mesenteric lymphatic vessels) of protein targets shown to be present in mast cells (A, D, G, J, M, P, S) together with the labeling of the same segment by Alexa Fluor 488-conjugated avidin (B, E, H, K, N, Q, T) followed by staining of the same segment with toluidine blue (C, F, I, L, O, R, U). Specimens are labeled for mast cell tryptase (A), c-kit (CD 117) (D), prostaglandin D2 synthase (G), histidine decarboxylase (J), histamine (M), transmembrane protein 16 A (P), and TNF-α (S). V, primary antibodies control. W, same segment labeled by Alexa Fluor 488-conjugated avidin to confirm presence of mast cells. X, secondary antibodies control. Scale bar on S corresponds to 100 μm and applies to all images. G, H, and I: images from 24-mo-old rat; the rest are taken from 9-mo-old animals. Mast cells were always found to lie on or outside the lymphatic wall. MLV, mesenteric lymphatic vessel.
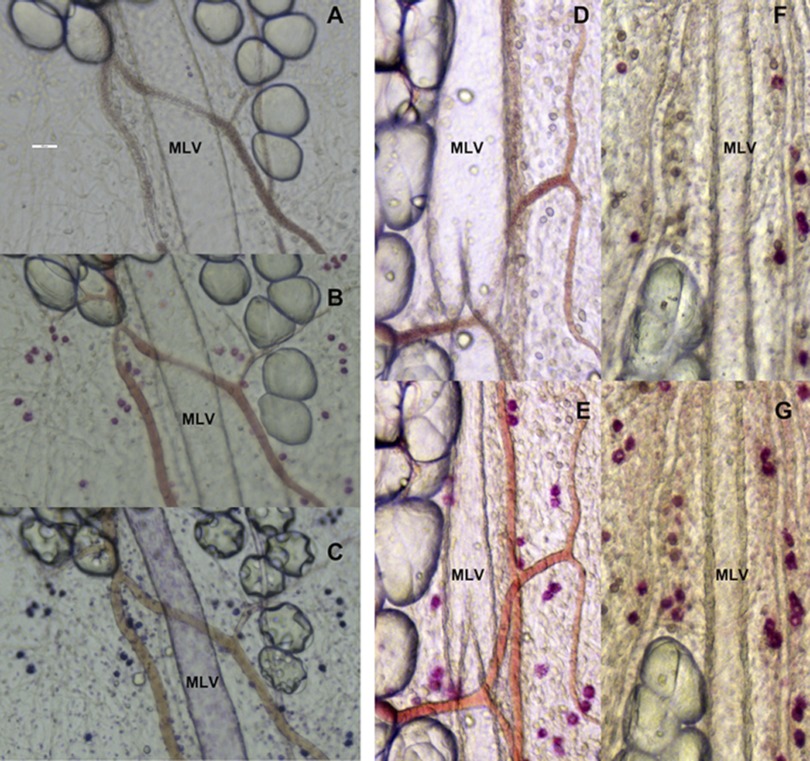
Fig. 2.
Representative images of the activation of mast cells located in close proximity to rat mesenteric lymphatic vessels in adult and aged segments of mesentery. A: segment (9 mo old) of mesentery before activation. B: same segment with mast cells activated by compound 48/80 stained with the ruthenium red. C: same segment stained with toluidine blue. D: segment (9 mo old) of mesentery before activation. E: same segment activated by substance P in 10−5 M. F: segment (24 mo old) of mesentery before activation. G: same segment activated by substance P in 10−5 M. Scale bar on A corresponds to 100 μm and applies to all images.
Mast cell activation experiments with use of ruthenium red staining of live isolated mesenteric tissue segments.
To evaluate the potential aging-associated changes in the number of mast cells located by MLV and in their functional status, we performed various studies of mast cell activation using ruthenium red as the commonly accepted marker of degranulated mast cells. Ruthenium red is a cationic dye that is able to enter activated cells only and thus has been used in the past to selectively stain for activated mast cells and is quantitative to degree of activation (37–39, 63, 67). In our experiments we mimicked acute inflammatory conditions by adding substance P, an inflammatory neuropeptide; PGN, the infective component of gram-positive bacteria Staphylococcus aureus; and anti-rat DNP IgE followed by DNP-HSA as an allergic stimulus to compare potential aging-associated differences in mast cell activation in response to acute inflammation. Substance P, peptidoglycan, and IgE are associated with inflammatory, infective, and allergic reactions and are also known to activate mast cells (6, 10, 11, 31, 32, 34, 47, 50, 58, 74). Compound 48/80, a chemical activator of mast cells (30, 64, 73), was used as a positive control, and physiological salt solution (PSS) was used as a negative control (sham) for these studies. Subsequent toluidine blue staining served for double verification of mast cells as well as for total mast cells count in each mesenteric segment.
The exteriorized gut with mesentery was rinsed in warm standard PSS of (in mM) 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.2 NaH2PO4, 5.0 dextrose, 2.0 sodium pyruvate, 0.02 EDTA, and 3.0 MOPS with pH adjusted to 7.36 at 37°C, and at least five segments of mesentery (without gut) from each animal (both 9- and 24 mo old) were cut and fixed into specially designed tissue chambers. These custom designed chambers were developed to be used to treat equal-size segments of mesentery in fixed position over time and to perform imaging of tissue structures after several subsequent treatments.
Ruthenium red (0.00125%) in PSS (Catalog No. R2751; Sigma Aldrich) was added to tissue chambers containing segments of mesentery, which were initially incubated with it for 30 min at 37°C, then washed with warm PSS three times for 5 min each wash and imaged using an Olympus CKX41 fluorescent microscope under its bright field mode. These images represent “before-treatment” conditions.
The following biological activators diluted in warm PSS were added to three separate tissue chambers containing mesenteric tissue from the same rat and incubated at 37°C: substance P (Catalog No. s6883; 10−5 M; Sigma Aldrich,) for 1 h; anti-rat DNP-IgE (Catalog No. 04-8888, dilution 1:200; Life Technologies) for 1 h followed by DNP-HSA (2,4-dinitrophenyl conjugated to human serum albumin; Catalog No. D-5059-10, 5 μg/ml; Biosearch Technologies, Novato, CA) for 1 h; and peptidoglycan from Staphylococcus aureus (Catalog No. 77140, 100 μg/ml; Sigma Aldrich) for 1 h. In a separate tissue chamber, Compound 48/80 (Catalog No. C2313, 10 μg/ml; Sigma Aldrich) diluted in PSS was added and incubated at 37°C for 1 h, which served as positive control, while in another chamber plain PSS was added and incubated at 37°C for 2 h as the sham/negative control. All doses and durations of treatments by mast cell activators were considered as mentioned in previous literature cited in the previous paragraph. Because total treatment with anti-rat DNP IgE followed by DNP-HSA took 2 h, we performed our sham control experiments for 2 h. After treatment, chambers containing live mesentery were washed with warm PSS three times for 5 min each and 0.00125% ruthenium red in PSS was added to all chambers. Tissue segments were incubated in ruthenium red for 30 min at 37°C, then washed with warm PSS three times for 5 min each wash and imaged using an Olympus CKX41 fluorescent microscope under its bright field mode at the same exact same position as before treatment. These images represent “after-treatment” conditions.
During activation experiments with live tissue segments, we used experimental conditions similar to that which we demonstrated as the ability of isolated mesenteric lymphatic vessels to maintain normal function up to 1 wk (20) and to be preserved during 2-day transportation (19). In the current study we used much shorter periods to keep the mesenteric tissue samples alive in before- as well as after-treatment conditions. Tissue segments were alive as evidenced by our numerous observations of contracting MLV inside mesenteric segments during the experiments as well as by the fact that the mast cells, known to be extremely sensitive to tissue damage/inflammation, did not activate/degranulate in sham-treated tissue samples as a result of experimental conditions.
The mesenteric tissue segments were then fixed with 4% paraformaldehyde, stained with acidic solution of toluidine blue for 5 min as described above, and washed with distilled water for 10 min. The sections were again imaged using an Olympus CKX41 fluorescent microscope under its bright field mode at the same exact same position as during ruthenium red staining procedures.
Data analysis and statistics for mast cell activation experiments.
Captured sets of images (ruthenium red staining before activation, ruthenium red staining after activation, and toluidine blue staining) were analyzed using a Windows-operating computer. Any pink or red stained cells were considered as activated. Such activated cells were manually counted for the same specimen for untreated as well as for treated conditions. We counted all mast cells visible in the field of view near MLVs and in adjacent areas of mesentery. Multiple images from each used mesenteric segment representing all quadrants of the segment of tissue were included in analysis. The total number of mast cells in any particular tissue segment was counted using final violet/purple staining of mast cells by acidic solution of toluidine blue. Ratio of activated cells in treated and untreated conditions to total number of cells was calculated and expressed in percentages.
The degree of mast cell activation was assessed by measuring pixel intensity of ruthenium red staining by NIH ImageJ software. For this type of analysis, the colored red, green, blue images of the same specimens used for cell count were converted to grayscale 8-bit images and phase inverted, then mean pixel intensity of mast cells was measured for the same specimen in untreated and treated conditions (67).
Fraction of cells activated by treatment (FCAT) was calculated by the following formula: (number of activated mast cells in treated conditions) − (number of activated mast cells in untreated conditions)/total number of mast cells and expressed in percentage.
Statistical differences were determined by two-way ANOVA, regression analysis, and paired Student's t-test (JMP software version 9.0.0. for Windows) as appropriate and considered significant at P ≤ 0.05. At least one but not more than three segments of mesentery were used for any single type of treatment in each animal; therefore, n in results for each type of treatment and each animal age represents the number of mesenteric segments used. A minimum of four animals was used for each given type of treatment for each animal age.
RESULTS
Immunohistochemical labeling and toluidine blue staining of mast cells by mesenteric lymphatic vessels.
In this study, we performed, for the first time, immunohistochemical labeling of protein targets shown to be present in mast cells with double verification of their presence in the same segment of rat mesentery containing MLV by labeling it with Alexa Fluor 488-conjugated avidin followed by staining of the same segment with toluidine blue. After initial optimizations of the experimental protocols we performed successful immunohistochemical labeling of specimens from at least three rats of each age for each protein mentioned above. Representative images are provided in Fig. 1. We found that mast cells are located in close proximity to rat MLV in segments of mesentery obtained from both age groups; however, it was impossible to detect any potential measureable differences between specimens from adult and aged rats using the immunohistochemical labeling technique.
Mast cell activation experiments with the use of ruthenium red staining of live isolated mesenteric tissue segments.
In this study for the first time we evaluated the aging-associated changes in the number of mast cells located by MLV and in their functional status by using an experimental approach of mast cell activation induced by various activators and staining by ruthenium red, a commonly used dye to stain degranulated mast cells, followed by staining of the same segment with toluidine blue. Figure 2 illustrates this experimental approach and provides the representative images of the observed differences between 9- and 24-mo-old mesenteric segments. Figure 2, A–C, demonstrates subsequent series of representative images of one segment of 9-mo-old mesenteric before and after activation by compound 48/80 followed by toluidine blue staining. Figure 2, D–G, demonstrates pairs of representative images of the same segments of rat mesentery obtained from 9- (D and E) and 24-mo-old (F and G) rats before and after their activation by substance P in 10−5 M. Major difference between 9- and 24-mo segments relays to the number of activated mast cells before experimental stimulation, which is significantly greater in 24-mo-old animals (compare Fig. 2, D and F).
Using toluidine blue staining, we were able to calculate the total number of mast cells in all mesenteric segments used in experiments with mast cells activation. We found significant 27% increase in the number of mast cells located by MLV in 24-mo-old segments versus 9-mo-old segments: 56 ± 3 and 44 ± 2 cells/segment correspondingly with n = 84 for 24-mo-old specimens and n = 73 for 9-mo-old specimens.
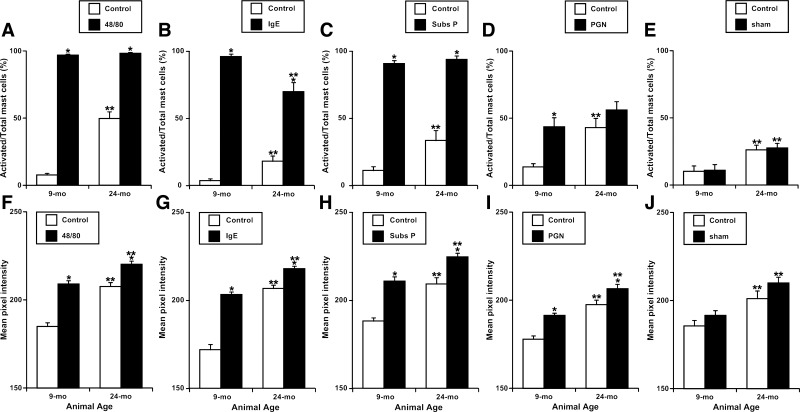
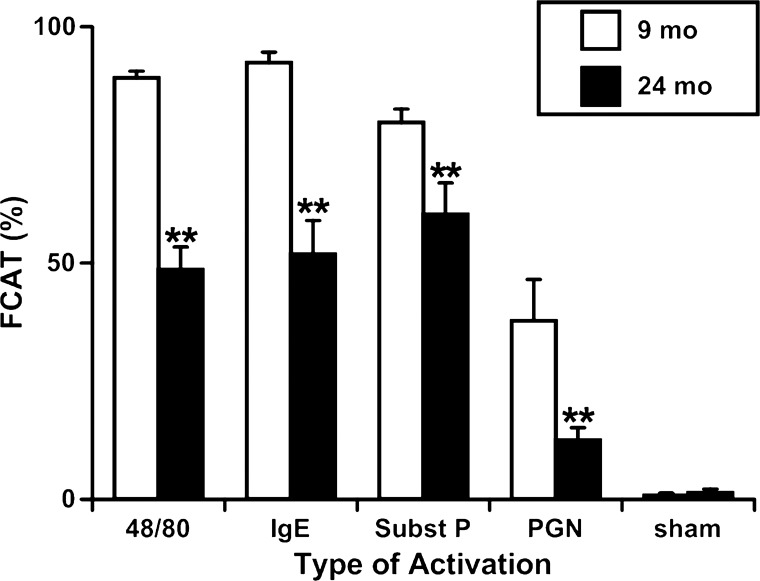
We determined that in all mesenteric segments in control conditions (before treatment) the number of activated mast cells in 24-mo-old segments was in average 403% higher than in 9-mo-old segments before treatment. The numbers of mast cells activated in 24-mo-old segments in untreated conditions for each type of treatment were statistically nonsignificant between the different types of treatment-namely, compound 48/80, substance P, PGN, IgE, and sham-treated samples. For activation by compound 48/80, the ratio of activated cells to total cells in 9-mo specimens was increased 1,258% after activation compared with control (before activation) conditions, whereas in the 24-mo-old specimens we observed only 198% increase. For Immunoglobulin E, substance P, and peptidoglycan these numbers were 2,667/386%, 818/280%, and 318/130%, correspondingly, whereas in sham control specimens routine incubation of the sample in PSS without adding mast cells activators did not induce any changes (Fig. 3, A–E). To better illustrate the observed phenomena we calculated parameter called as fraction of cells activated after treatment. We found that activation by compound 48/80 was able to activate in 9-mo mesenteric segments 89.2 ± 1.4% of available intact mast cells, whereas in 24-mo segments only 48.6 ± 4.8%. For Immunoglobulin E, substance P, and peptidoglycan these numbers were 92.4 ± 2.2/51.9 ± 7.1%, 79.7 ± 2.8/60.3 ± 6.6%, and 37.6 ± 8.8/12.5 ± 2.6%, correspondingly, whereas in sham control specimens routine incubation of the sample without adding mast cells activators did not induce any significant changes (Fig. 4).
Fig. 3.
A-J: Quantitative analysis of mast cells activation induced by various activators in adult (9 mo) and aged (24 mo) segments of rat mesentery. 48/80, compound 48/80; subs P, substance P. Values are means ± SE. *Significant differences (P < 0.05) before and after treatment with same age group; **significant differences between 9- and 24-mo age groups.
Fig. 4.
Aging-associated alterations in the fraction of mast cells activated by various treatments (FCAT) in adult (9-mo-old) and aged (24-mo-old) segments of rat mesentery. Values are means ± SE. **Significant differences (P < 0.05) between 9- and 24-mo-old age groups. Subst P, substance P.
Trends similar to that described above were observed with analysis of the mean pixel intensities of mast cells in conditions before and after treatment of 9- and 24-mo mesenteric segments by various activators. Mean pixel intensity of stained cells gives a measure of the degree of activation of mast cells as a result of treatment. Because increased number of mesenteric mast cells was found to be activated in basal conditions in 24-mo-old segments, the mean pixel intensity of mast cells before treatment is significantly raised compared with that of 9-mo-old segments. After treatment with mast cell activators, the mean pixel intensity of mast cells increased in both age groups, but the change in intensity from untreated to treated conditions is much less in 24-mo-old segments as compared with 9-mo-old segments. Hence, although the mean pixel intensity after treatment may be more in 24-mo-old rats (this is because there were already more activated cells in basal conditions), the change in mean intensity as a result of treatment is significantly less in old rats. Table 1 presents the raw data of analyses of functional status of rat mesenteric mast cells before and after treatment by various substances. Additionally, numbers of used segments/animals are provided in the Table 1. All illustrative calculations of the percentage of change of cell numbers and mean pixel intensity between adult and aged mesenteric segments described in the text section above are based on the data presented in this table.
Table 1.
Evaluation of the functional status of rat mesenteric mast cells located in close proximity to mesenteric lymphatic vessels before and after treatment by various substances
| Age/Parameter/Treatment | Compound 48/80 | IgE | Substance P | Peptidoglycan | Sham |
|---|---|---|---|---|---|
| Ratio activated/total mast cells (%) | |||||
| Before treatment, 9 mo | 7.7 ± 1.2 | 3.6 ± 1.4 | 11.1 ± 3.0 | 13.7 ± 2.4 | 10.2 ± 4.0 |
| After treatment, 9 mo | 96.9 ± 0.7 | 96.0 ± 1.9 | 90.8 ± 2.2 | 43.6 ± 6.7 | 11.0 ± 4.2 |
| Before treatment, 24 mo | 49.7 ± 5.0 | 18.1 ± 3.9 | 33.4 ± 7.5 | 43.0 ± 6.8 | 26.2 ± 3.4 |
| After treatment, 24 mo | 98.3 ± 0.6 | 69.9 ± 6.8 | 93.8 ± 2.7 | 56.1 ± 6.1 | 27.8 ± 3.4 |
| Mean pixel intensity of mast cells | |||||
| Before treatment, 9 mo | 184.9 ± 2.1 | 171.9 ± 3.0 | 188.2 ± 1.9 | 177.9 ± 1.8 | 185.6 ± 3.1 |
| After treatment, 9 mo | 209.0 ± 1.8 | 203.4 ± 1.4 | 210.8 ± 2.6 | 191.4 ± 2.5 | 191.6 ± 2.7 |
| Before treatment, 24 mo | 207.6 ± 2.2 | 206.7 ± 2.0 | 209.3 ± 3.6 | 197.4 ± 2.5 | 201.1 ± 4.3 |
| After treatment, 24 mo | 220.2 ± 1.8 | 217.9 ± 1.5 | 224.6 ± 2.2 | 206.5 ± 2.5 | 210.0 ± 3.2 |
| Number of segments/animals used | |||||
| 9 mo | 27/9 | 12/4 | 12/4 | 12/4 | 10/4 |
| 24 mo | 29/10 | 14/5 | 12/4 | 20/6 | 9/4 |
Values are means ± SE.
DISCUSSION
Dysfunctional lymphatic transport can result in a wide range of disturbances including edema, altered lymphocyte circulation, depressed immune function, and impaired lipid metabolism. We recently demonstrated that aging is accompanied by a significant decrease in lymphatic pumping of various lymphatic vessels especially in experimental conditions, which correspond to situations with their higher volumetric loads in situ (1, 21, 52). We believe that such aging-induced changes in function of the lymphatic vessels may be a contributory cause to several aging-associated cardiovascular, metabolic, and immune derangements. Our recent in situ observations (1) performed in aged rats suggested the existence of altered functional behavior/status of some cellular elements in the mesenteric bed located in close proximity to mesenteric lymphatic vessels. Such aging-associated changes in tissue microenvironment influence lymphatic pumping function and hence play a significant contributory role to various inflammatory and immune disorders of the elderly. Of the cellular elements present in mesenteric tissue, mast cells are unique immune cells that serve as the first line of defense against many pathogens and allergens and are the depot of various vasoactive and inflammatory mediators that is known to selectively influence lymphatic vessel contractile function (discussed in the introduction). Therefore, in this study we targeted our research efforts to these cells as potentially influenced by aging and therefore acting on adjacent aged mesenteric lymphatic vessels.
In this study we for the first time systematically identified mast cells in close proximity to mesenteric lymphatic vessels by using whole mount immunohistochemical labeling and subsequent staining by metachromatic dyes. Mast cells were seen to line MLV or lie close to them. These cells stained positive with the following proteins shown to be present in mast cells: mast cell tryptase, c-kit (CD 117), prostaglandin D2 synthase, histidine decarboxylase, histamine, TMEM 16A, and TNF-α. We double-verified the presence of mast cells in the same segment of rat mesentery containing MLV by labeling it with Alexa Fluor 488-conjugated avidin followed by staining of the same segment with toluidine blue. Presence of mast cells near MLVs makes them capable of modulating lymphatic pump function through release of their various mediators, which are known to influence lymphatic smooth muscle activity.
Aging is considered to be a chronic inflammatory process with a shift toward a proinflammatory cytokine profile in tissues that may account for numerous deleterious vascular changes associated with aging (7, 8, 12). The increased state of preactivation as well as increased number of mast cells in aged mesentery, which we for the first time confirmed in this study, indicate existence of chronic inflammatory environment in mesentery since activated mast cells would have released their preformed inflammatory mediators like histamine, proteases, and cytokines like TNF-α. Such an aging-associated chronic inflammatory environment, sign of which is an increased state of activation of aged mast cells in close proximity to aged MLV, may be one of the important causes of alterations in lymphatic pump function in aged animals. Previously we demonstrated that application of NG-nitro-l-arginine methyl ester in situ caused an abrupt and significant increase in contraction frequency of aged MLVs but not in isolated vessels (1). This proves that in the immediate vicinity of aged MLV, there are some counterbalancing mediators that have effects opposite to the lymphatic inhibitory effects of NO in aged rats. Such counterbalancing effects do not exist in isolated MLV preparations (52) because carefully dissected lymphatic vessels do not have mast cells in their walls and surrounding tissues are eliminated during vessel isolation procedure. The presence of increased numbers of activated mast cells by aged MLVs indirectly confirms the existence of the increased local concentrations of histamine and other mediators, which are known to influence lymphatic contractility and in particular are able to induce positive chronotropic effect on lymphatic vessels (discussed in introduction). These mediators have potential to further increase contraction frequency of aged MLV in time when the inhibitory effect of NO is removed by NG-nitro-l-arginine methyl ester administration (1). In light of our present findings-there is an average ∼400% increase in the number of activated mast cells in aged mesentery in addition to a 27% increase in their density near aged lymphatic vessels-we may consider the cumulative action of mast cells mediators chronically released from activated mast cells in resting conditions as an important mechanism of aged-induced shifts in lymphatic contractile function. Further detailed investigations are necessary to determine how different mast cell-derived mediators and by which mechanisms influence function of the aged lymphatic vessels.
Although there has been a great deal of work done on inflammation in vascular aging, there is lack of sufficient research that may apply to the aging of the lymphatic system. Despite a chronic inflammatory state, aging has been thought to involve immunosenescence with deficient innate as well as adaptive immune response (24, 25, 53, 66). Mast cells are considered to be the first line of defense against allergens and pathogens and thus are important initiators of innate immune response (14, 42, 43, 45, 46). Various inflammatory and chemotactic molecules produced and released from mast cells in response to foreign allergens or pathogens help recruitment of other antigen presenting cells at the site of inflammation and participate in shaping the host adaptive response (13, 29, 60, 61). Historically studies on mast cells in aging have been limited to dermal and lung mast cells in context of studies on allergy and asthma (26, 27, 48). In this study we have for the first time analyzed the aging-associated changes in mast cell number and their behavior in rat mesentery near lymphatic vessels. In this study we also have compared the responses of mesenteric mast cells with different acute stimuli in adult and aged animals. We used known mast cell activators: substance P, an inflammatory neurotransmitter; anti-rat DNP IgE, an allergic mediator; PGN from Staphyloccus aureus, a classical toll-like receptors 2 ligand; and compound 48/80, standard chemical activator to stimulate mast cells ex vivo in live mesenteric tissue containing MLV. Unlike in vitro experiments where the total number of cells is arbitrary, our current ex vivo preparation helped us to evaluate the number of preactivated cells as well as the number of intact cells available in the particular tissue bed to be newly activated by acute inflammatory stimuli. We found that despite the fact that 24-mo-old rats had ∼27% increase in total mast cell number in the mesenteric bed, the number of intact cells available to react to the presence of acute stimuli is significantly decreased in aged rats. We predict that the presence of extensive degranulation of mast cells in basal conditions in 24-mo-old rats promotes a chronic inflammatory environment, but the reduced availability of intact mast cells to react to acute noxious stimuli is one of the major contributory reasons of delayed immune response to acute inflammation in the elderly. We discovered that a smaller fraction of previously nonactivated mast cells were activated as a result of either biological or chemical stimulation in 24-mo-old rats as compared with 9-mo-old animals (FCAT on Fig. 4). However, in case of IgE stimulation, the diminished ability of aged mast cells to be activated (lower number of activated mast cells after treatment) also has its own impact on age-associated alterations in the functional status of mast cells and in their acute response to acute stimulation. The mean pixel intensity as a result of treatment (change of intensity after treatment from before treatment conditions) is always significantly less in 24-mo-old rats compared with 9-mo-old rats. The presence of extensive degranulation in basal conditions in aged mesentery increased the mean pixel intensity of mesenteric mast cells after treatment in 24-mo-old rats as compared with 9-mo-old rats, but the increase in mean pixel intensity as a result of activation due to treatment was always significantly more in young rats as compared with old rats. Taken together our findings indicate that in aging, mesenteric mast cells have reduced ability to be activated by acute inflammatory stimuli. Although deficient immune cell function has been reported in aging (24, 25, 53, 66), there is no detailed information on the aging-associated alterations of mast cells and how they affect the function of mesenteric lymphatic vessels. The limited number of aged mast cells located in the mesentery to react to the presence of acute stimuli may be considered contributory to the aging-associated deteriorations in immune response. Further investigations will be able to answer the important questions on the mechanisms of such effects discovered in this study.
In conclusion, in this study, we performed, for the first time, immunohistochemical labeling of protein targets shown to be present in mast cells with double verification of their presence in the same segment of rat mesentery containing MLV by labeling of it with Alexa Fluor 488-conjugated avidin followed by staining of the same segment with toluidine blue. Specimens were successfully labeled for mast cell tryptase, c-kit (CD 117), prostaglandin D2 synthase, histidine decarboxylase, histamine, TMEM 16A, and TNF-α. Additionally, for the first time we evaluated the aging-associated changes in the number of mast cells located in close proximity to MLV and in their functional status by using an experimental approach of mast cell activation in live mesenteric tissue with ruthenium red staining followed by subsequent staining with toluidine blue. We found the aging-associated 27% increase in the total number of mast cells, with ∼400% increase in the number of activated mast cells in aged mesenteric tissue in resting conditions. During the stimulation of mast cells ex vivo in live mesenteric tissue containing MLV by substance P, an inflammatory neurotransmitter; anti-rat DNP IgE, an allergic mediator; PGN from Staphyloccus aureus, a toll-like receptor 2 ligand; and compound 48/80, a standard chemical mast cell activator, we found diminished ability of aged mast cells to react to the presence of acute inflammatory or chemical stimuli; the fraction of cells activated by treatment was greatly diminished in aged specimens after activation of any type. We conclude that higher degree of resting activation of mast cells in aged mesenteric tissue is important for development of aging-associated impairment of function of mesenteric lymphatic vessels reported by us earlier. The limited number of intact mast cells located close to the mesenteric lymphatic compartments to react to the presence of active stimuli may be considered contributory to the aging-associated deteriorations in immune response.
GRANTS
This work was supported in part by NIH Grants NIH RO1-AG-030578 and HL-094269 and by Texas A&M Health Science Center College of Medicine and Department of Systems Biology and Translational Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.C. and A.A.G. conception and design of research; V.C. and A.A.G. performed experiments; V.C. and A.A.G. analyzed data; V.C. and A.A.G. interpreted results of experiments; V.C. and A.A.G. prepared figures; V.C. and A.A.G. drafted manuscript; V.C. and A.A.G. edited and revised manuscript; V.C. and A.A.G. approved final version of manuscript.
REFERENCES
- 1.Akl TJ, Nagai T, Cote GL, Gashev AA. Mesenteric lymph flow in adult and aged rats. Am Physiol Heart Circ Physiol 301: H1828–H1840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin K. The role of mast cells in allergic inflammation. Respir Med 106: 9–14, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Beil WJ, Schulz M, Wefelmeyer U. Mast cell granule composition and tissue location—a close correlation. Histol Histopathol 15: 937–946, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Benoit JN, Zawieja DC. Gastrointestinal lymphatics. In: Physiology of the gastrointestinal tract, edited by Johnson L. New York: Raven Press, 1994, p. 1669–1692 [Google Scholar]
- 5.Bergstresser PR, Tigelaar RE, Tharp MD. Conjugated avidin identifies cutaneous rodent and human mast cells. J Invest Dermatol 83: 214–218, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Bohn A, Konig W. Monoclonal murine anti-DNP IgE: in vitro histamine release of rat mast cells in the presence of reaginic rat and mouse sera. Agents Actions 16: 485–490, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am 23: 15–39, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol 8: 131–136, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Bussolati G, Gugliotta P. Nonspecific staining of mast cells by avidin-biotin-peroxidase complexes (ABC). J Histochem Cytochem 31: 1419–1421, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Coleman JW, Huang Q, Stanworth DR. The mast cell response to substance P: effects of neuraminidase, limulin, and some novel synthetic peptide antagonists. Peptides 7: 171–175, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Columbo M, Horowitz EM, Kagey-Sobotka A, Lichtenstein LM. Substance P activates the release of histamine from human skin mast cells through a pertussis toxin-sensitive and protein kinase C-dependent mechanism. Clin Immunol Immunopath 81: 68–73, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB 17: 1183–1185, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Dawicki W, Jawdat DW, Xu N, Marshall JS. Mast cells, histamine, and IL-6 regulate the selective influx of dendritic cell subsets into an inflamed lymph node. J Immunol 184: 2116–2123, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol 19: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Dobbins DE. Receptor mechanisms of serotonin-induced prenodal lymphatic constriction in the canine forelimb. Am J Physiol Heart Circ Physiol 274: H650–H654, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Dobbins DE, Buehn MJ, Dabney JM. Constriction of perfused lymphatics by acetylcholine, bradykinin and histamine. Microcirc Endothelium Lymphatics 6: 409–425, 1990 [PubMed] [Google Scholar]
- 17.Ferguson MK, Shahinian HK, Michelassi F. Lymphatic smooth muscle responses to leukotrienes, histamine and platelet activating factor. J Surg Res 44: 172–177, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Fox JLR, von der Weid PY. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol 136: 1210–1218, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gashev AA, Davis MJ. Long-distance transportation of live isolated lymphatic vessels. Lymphat Res Biol 8: 189–192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gashev AA, Davis MJ, Gasheva OY, Nepiushchikh ZV, Wang W, Dougherty P, Kelly KA, Cai S, von der Weid PY, Muthuchamy M, Meininger CJ, Zawieja DC. Methods for lymphatic vessel culture and gene transfection. Microcirculation 16: 615–628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasheva OY, Knippa K, Nepiushchikh ZV, Muthuchamy M, Gashev AA. Age-related alterations of active pumping mechanisms in rat thoracic duct. Microcirculation 14: 827–839, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Ghannadan M, Baghestanian M, Wimazal F, Eisenmenger M, Latal D, Kargul G, Walchshofer S, Sillaber C, Lechner K, Valent P. Phenotypic characterization of human skin mast cells by combined staining with toluidine blue and CD antibodies. J Invest Dermatol 111: 689–695, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol 17: 457–462, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol 43: 718–728, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunin AG, Kornilova NK, Vasilieva OV, Petrov VV. Age-related changes in proliferation, the numbers of mast cells, eosinophils, and cd45-positive cells in human dermis. J Gerontol A Biol Sci Med Sci 66: 385–392, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Hart PH, Grimbaldeston MA, Hosszu EK, Swift GJ, Noonan FP, Finlay-Jones JJ. Age-related changes in dermal mast cell prevalence in BALB/c mice: functional importance and correlation with dermal mast cell expression of Kit. Immunology 98: 352–356, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvima IT, Nilsson G. Mast cells as regulators of skin inflammation and immunity. Acta Derm Venereol 91: 644–650, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Heib V, Becker M, Warger T, Rechtsteiner G, Tertilt C, Klein M, Bopp T, Taube C, Schild H, Schmitt E, Stassen M. Mast cells are crucial for early inflammation, migration of Langerhans cells, and CTL responses following topical application of TLR7 ligand in mice. Blood 110: 946–953, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Horsfield GI. The effect of compound 48/80 on the rat mast cell. J Pathol 90: 599–605, 1965 [DOI] [PubMed] [Google Scholar]
- 31.Jawdat DM, Albert EJ, Rowden G, Haidl ID, Marshall JS. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J Immunol 173: 5275–5282, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Jawdat DM, Rowden G, Marshall JS. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J Immunol 177: 1755–1762, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Johnston MG, Kanalec A, Gordon JL. Effects of arachidonic acid and its cyclo-oxygenase and lipoxygenase products on lymphatic vessel contractility in vitro. Prostaglandins 25: 85–98, 1983 [DOI] [PubMed] [Google Scholar]
- 34.Kirshenbaum AS, Swindle E, Kulka M, Wu Y, Metcalfe DD. Effect of lipopolysaccharide (LPS) and peptidoglycan (PGN) on human mast cell numbers, cytokine production, and protease composition. BMC Immunol 9: 45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kluth W. [The presence of mast cells in the thoracic duct.] Zentralbl Allg Pathol 87: 139–141, 1951 [PubMed] [Google Scholar]
- 36.Kruger PG, Bloom GD. Structural features of histamine release in rat peritoneal mast cells. A study with toluidine blue. Int Arch Allergy Appl Immunol 46: 740–752, 1974 [DOI] [PubMed] [Google Scholar]
- 37.Kubes P. Ruthenium red as measure of mast cell activation. Methods Enzymol 301: 22–27, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Kubes P, Gaboury JP. Rapid mast cell activation causes leukocyte-dependent and -independent permeability alterations. Am J Physiol Heart Circ Physiol 271: H2438–H2446, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Lagunoff D. Vital staining of mast cells with ruthenium red. J Histochem Cytochem 20: 938–944, 1972 [DOI] [PubMed] [Google Scholar]
- 40.Lobov GI, Pan′kova MN. Heparin inhibits contraction of smooth muscle cells in lymphatic vessels. Bull Exp Biol Med 149: 4–6, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Malaviya R, Abraham SN. Mast cell modulation of immune responses to bacteria. Immunol Rev 179: 16–24, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Malaviya R, Georges A. Regulation of mast cell-mediated innate immunity during early response to bacterial infection. Clin Rev Allergy Immunol 22: 189–204, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol 4: 787–799, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol 114: 21–27, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Marshall JS, King CA, McCurdy JD. Mast cell cytokine and chemokine responses to bacterial and viral infection. Curr Pharm Des 9: 11–24, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev 173: 131–140, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Micklefield G, Konig W, Pfeiffer P, Bohn A. Interaction of monoclonal antibodies with the IgE-receptor on rat mast cells and rat basophilic leukemia (RBL) cells. Agents Actions 17: 418–426, 1986 [DOI] [PubMed] [Google Scholar]
- 48.Migally NB, Tucker A, Greenlees K, Wright M, Zambernard J. Density and ultrastructure of mast cells in lung vessels of aging rats exposed to and recovering from chronic hypoxia. Cell Tissue Res 232: 601–608, 1983 [DOI] [PubMed] [Google Scholar]
- 49.Mislin H. [The contractile properties of lymphatic vessels.] Angiologica 8: 207–211, 1971 [PubMed] [Google Scholar]
- 50.Mousli M, Bronner C, Bueb JL, Tschirhart E, Gies JP, Landry Y. Activation of rat peritoneal mast cells by substance P and mastoparan. J Pharmacol Exp Ther 250: 329–335, 1989 [PubMed] [Google Scholar]
- 51.Nadon NL. Maintaining aged rodents for biogerontology research. Lab Anim (NY) 33: 36–41, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation 18: 463–473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nomellini V, Gomez CR, Kovacs EJ. Aging and impairment of innate immunity. Contrib Microbiol 15: 188–205, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Ohhashi T, Kawai Y, Azuma T. The response of lymphatic smooth muscles to vasoactive substances. Pflügers Arch 375: 183–188, 1978 [DOI] [PubMed] [Google Scholar]
- 55.Orlov R, Lobov G. [Ionic mechanisms of the electrical activity of the smooth-muscle cells of the lymphatic vessels.] Fiziol Zh SSSR Im I M Sechenova 70: 712–721, 1984 [PubMed] [Google Scholar]
- 56.Pan′kova MN, Lobov GI, Chikhman VN, Solnyshkin SD. [Effects of histamine on contractile activity of lymphatic node capsules. The NO role.] Ross Fiziol Zh I M Sechenova 97: 633–640, 2011 [PubMed] [Google Scholar]
- 57.Petunov SG, Egorova AA, Orlov RS, Nikitina ER. Effect of histamine on spontaneous contractions of mesenteric lymphatic vessels and lymph nodes of white rats: endothelium-dependent responses. Dokl Biol Sci 432: 176–180, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Pietrzak A, Wierzbicki M, Wiktorska M, Brzezinska-Blaszczyk E. Surface TLR2 and TLR4 expression on mature rat mast cells can be affected by some bacterial components and proinflammatory cytokines. Mediators Inflamm 2011: 427473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plaku KJ, von der Weid PY. Mast cell degranulation alters lymphatic contractile activity through action of histamine. Microcirculation 13: 219–227, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Raible DG, Schulman ES, DiMuzio J, Cardillo R, Post TJ. Mast cell mediators prostaglandin-D2 and histamine activate human eosinophils. J Immunol 148: 3536–3542, 1992 [PubMed] [Google Scholar]
- 61.Ramos CD, Heluy-Neto NE, Ribeiro RA, Ferreira SH, Cunha FQ. Neutrophil migration induced by IL-8-activated mast cells is mediated by CINC-1. Cytokine 21: 214–223, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Schueller E, Peutsch M, Bohacek LG, Gupta RK. A simplified toluidine blue stain for mast cells. Can J Med Technol 29: 137–138, 1967 [PubMed] [Google Scholar]
- 63.Shepherd RK, Duling BR. Use of Ruthenium Red staining to detect mast cell degranulation in vivo. Microcirculation 2: 363–370, 1995 [DOI] [PubMed] [Google Scholar]
- 64.Singleton EM, Clark SL., Jr The response of mast cells to compound 48/80 studied with the electron microscope. Lab Invest 14: 1744–1763, 1965 [PubMed] [Google Scholar]
- 65.Sinzinger H, Kaliman J, Mannheimer E. Regulation of human lymph contractility by prostaglandins and thromboxane. Lymphology 17: 43–45, 1984 [PubMed] [Google Scholar]
- 66.Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity 24: 491–494, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Steiner DR, Gonzalez NC, Wood JG. Mast cells mediate the microvascular inflammatory response to systemic hypoxia. J Appl Physiol 94: 325–334, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Tharp MD, Seelig LL, Jr, Tigelaar RE, Bergstresser PR. Conjugated avidin binds to mast cell granules. J Histochem Cytochem 33: 27–32, 1985 [DOI] [PubMed] [Google Scholar]
- 69.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 54: B492–B501, 1999 [DOI] [PubMed] [Google Scholar]
- 70.Unthank JL, Hogan RD. The effect of vasoactive agents on the contractions of the initial lymphatics of the bat's wing. Blood Vessels 24: 31–44, 1987 [DOI] [PubMed] [Google Scholar]
- 71.Watanabe N, Kawai Y, Ohhashi T. Dual effects of histamine on spontaneous activity in isolated bovine mesenteric lymphatics. Microvasc Res 36: 239–249, 1988 [DOI] [PubMed] [Google Scholar]
- 72.Wilhelm DL, Yong LC, Watkins SG. The mast cell: distribution and maturation in the rat. Agents and Actions 8: 146–152, 1978 [DOI] [PubMed] [Google Scholar]
- 73.Wu J, Chase JD, Zhu Z, Holzapfel TP. Temperature rise in a tissue-mimicking material generated by unfocused and focused ultrasonic transducers. Ultrasound Med Biol 18: 495–512, 1992 [DOI] [PubMed] [Google Scholar]
- 74.Wu L, Feng BS, He SH, Zheng PY, Croitoru K, Yang PC. Bacterial peptidoglycan breaks down intestinal tolerance via mast cell activation: the role of TLR2 and NOD2. Immunol Cell Biol 85: 538–545, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Yong LC, Watkins S, Wilhelm DL. The mast cell: distribution and maturation in the peritoneal cavity of the adult rat. Pathology 7: 307–318, 1975 [DOI] [PubMed] [Google Scholar]
- 76.Yong LC, Watkins SG, Wilhelm DL. The mast cell: II. Distribution and maturaiton in the peritoneal cavity of the young rat. Pathology 9: 221–232, 1977 [DOI] [PubMed] [Google Scholar]