Abstract
We tested the hypothesis that high fat (HF) feeding results in endothelial dysfunction in resistance arteries of epididymal white adipose tissue (eWAT) and is mediated by adipose tissue inflammation. When compared with normal chow (NC)-fed mice (n = 17), HF-fed male B6D2F1 mice were glucose intolerant and insulin resistant as assessed by glucose tolerance test (area under the curve; HF, 18,174 ± 1,889 vs. NC, 15,814 ± 666 mg·dl−1·min−1; P < 0.05) and the homeostatic model assessment (HF, 64.1 ± 4.3 vs. NC, 85.7 ± 6.4; P = 0.05). HF diet-induced metabolic dysfunction was concomitant with a proinflammatory eWAT phenotype characterized by greater macrophage infiltration (HF, 3.9 ± 0.8 vs. NC, 0.8 ± 0.4%; P = 0.01) and TNF-α (HF, 22.6 ± 4.3 vs. NC, 11.4 ± 2.5 pg/dl; P < 0.05) and was associated with resistance artery dysfunction, evidenced by impaired endothelium-dependent dilation (EDD) (maximal dilation; HF, 49.2 ± 10.7 vs. NC, 92.4 ± 1.4%; P < 0.01). Inhibition of nitric oxide (NO) synthase by Nω-nitro-l-arginine methyl ester (l-NAME) reduced dilation in NC (28.9 ± 6.3%; P < 0.01)- and tended to reduce dilation in HF (29.8 ± 9.9%; P = 0.07)-fed mice, eliminating the differences in eWAT artery EDD between NC- and HF-fed mice, indicative of reduced NO bioavailability in eWAT resistance arteries after HF feeding. In vitro treatment of excised eWAT arteries with recombinant TNF-α (rTNF) impaired EDD (P < 0.01) in NC (59.7 ± 10.9%)- but not HF (59.0 ± 9.3%)-fed mice. l-NAME reduced EDD in rTNF-treated arteries from both NC (21.9 ± 6.4%)- and HF (29.1 ± 9.2%)-fed mice (both P < 0.01). In vitro treatment of arteries with a neutralizing antibody against TNF-α (abTNF) improved EDD in HF (88.2 ± 4.6%; P = 0.05)-fed mice but was without effect on maximal dilation in NC (89.0 ± 5.1%)-fed mice. l-NAME reduced EDD in abTNF-treated arteries from both NC (25.4 ± 7.5%)- and HF (27.1 ± 16.8%)-fed mice (both P < 0.01). These results demonstrate that inflammation in the visceral adipose tissue resulting from diet-induced obesity impairs endothelial function and NO bioavailability in the associated resistance arteries. This dysfunction may have important implications for adipose tissue blood flow and appropriate tissue function.
Keywords: visceral adipose tissue, endothelium, tumor necrosis factor, high-fat feeding, obesity
as of 2008, an estimated 1.5 billion adults worldwide are overweight. This is more than double in the incidence of overweight since 1980. Of these individuals, over 500 million are clinically obese. Where being overweight/obesity was once a problem only of developed Western countries, a striking 65% of the world's population now live in countries where the consequences of being overweight/obesity are more deadly than the consequences of underfeeding (27). The consequences of being overweight/obesity include insulin resistance, type 2 diabetes, and cardiovascular disease, all of which have been associated with dysfunction in the adipose tissue (3).
Tissue-specific blood flow is an important determinant of metabolism and lipid handling in the adipose tissue (35, 36) with epinephrine-induced increases in tissue blood flow associated with increases in fatty acid release from the adipose (36). A similar dependence on tissue blood flow can be observed in skeletal muscle where the vasodilatory effects of insulin increase nutritive blood flow (7). The direct effects of insulin on muscle glucose uptake are significantly augmented by its effects to increase limb blood flow (2), as shown by euglycemic-hyperinsulinemic clamp studies in the absence and presence of nitric oxide (NO) synthase inhibition. Although impaired adipose tissue blood flow is associated with obesity (5, 18, 37, 38), currently, the mechanism for this impairment in blood flow is unclear. A reduced vasodilator capacity of the adipose tissue resistance vasculature may be a cause. Previous studies have demonstrated adipose tissue blood flow to be modulated by both local and systemic factors such as NO bioavailability, free fatty acids, and circulating catecholamines (1, 13). Blood flow may also be impacted by inflammation in the adipose tissue microenvironment. These factors may control blood flow in the adipose tissue by modulating vasodilation in the resistance arteries. Thus, because visceral, in contrast with subcutaneous, adiposity is associated with elevated disease risk (3, 16, 28), a direct examination of the effects of high-fat (HF) feeding on the function of the resistance vasculature of the visceral adipose is of critical importance.
Chronic low-grade inflammation associated with diet-induced obesity contributes to increased cardiovascular and metabolic disease risk and is characterized by increased circulating and adipose tissue cytokines and adhesion molecules as well as increased infiltration of macrophages in the adipose tissue (3, 24). Chief among the cytokines is TNF-α. When compared with subcutaneous adipose tissue, visceral adipose tissue of obese patients demonstrates a proinflammatory adipose tissue phenotype characterized by elevated TNF-α gene expression and macrophage infiltration concomitant with endothelial dysfunction in excised resistance arteries (12). Although a direct effect of this proinflammatory cytokine on vasodilator capacity of the adipose tissue resistance arteries is not known, TNF-α impairs endothelium-dependent dilation (EDD) and NO bioavailability in the coronary circulation of obese and type 2 diabetic rats (14, 31). Taken together, these studies provide evidence in support of our hypothesis that diet-induced obesity-mediated increases in TNF-α in the adipose tissue artery microenvironment contribute to impaired endothelial function in adipose tissue resistance arteries. Here, we demonstrate that TNF-α impairs EDD and NO bioavailability in resistance arteries excised from the epididymal white adipose tissue (eWAT), a visceral adipose depot of lean mice, but this in vitro effect of TNF-α is absent in the arteries isolated from the inflamed adipose tissue of glucose-intolerant insulin-resistant HF-fed mice.
METHODS
Ethical approval.
All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85-23, revised 1996) and were approved by the University of Utah and Veteran's Affairs Medical Center-Salt Lake City (VAMC-SLC) Animal Care and Use Committees.
Animals.
Male B6D2F1 mice were obtained from Charles River. All mice were housed in an animal care facility at the VAMC-SLC on a 12-h:12-h light:dark cycle. Young (5.7 ± 0.3 mo, n = 17) mice were fed normal rodent chow (NC, 8640 Harlan Teklad 22/5 Standard Rodent Chow; protein, 29%; carbohydrate, 55%; fat, 16% by kcal) or a HF (Harlan Teklad custom diet TD.96132, adjusted fat diet; protein: 18.7%; carbohydrate, 40.7%; fat, 40.7% by kcal) (24) diet ad libitum and housed in standard mouse cages. Before tissue harvest, mice were euthanized via exsanguinations by cardiac puncture while under isoflurane anesthesia (9, 10, 21).
Metabolic testing.
Glucose tolerance was assessed by an intraperitoneal glucose tolerance test (GTT) as described previously (24). Briefly, mice were fasted 2 h in the morning before baseline blood glucose was measured using a Precision Xceed Pro Glucose Analyzer in whole blood collected via a tail nick (∼5 μl). Glucose (2,000 mg/kg) was administered by intraperitoneal injection, and blood glucose was monitored at 15, 30, 45, 60, and 90 min after the injection. An additional 30 μl of blood was collected from the tail at times 0, 15, 60, and 90 of the GTT, plasma collected, and insulin determined by ELISA (Alpco No. 80-INSMSU-E01; Salem, NH). Insulin sensitivity and β-cell function were estimated from fasted blood glucose and plasma insulin using the Homestatic Model Assessment (HOMA) (39). Although the HOMA parameters can be approximated using simple linear equations yielding estimates of insulin resistance (IR), insulin sensitivity (S) and β-cell function (B) are as follows: 1) HOMA-IR = fasting insulin (μIU/ml) × fasting glucose (mmol/ml)/22.5; 2) HOMA-S = 100/HOMA-IR; 3) HOMA-B = 20 × fasting insulin (μIU/ml)/fasting glucose (mmol/ml) − 3.5.
An updated, more appropriate nonlinear model (HOMA2) was described by Levy et al. (25), and a computer-based calculator program was made available online (http://www.dtu.ox.ac.uk/homacalculator/index.php). This calculator was used to determine HOMA-S and HOMA-B from the same fasted blood sample collected.
Adipose tissue macrophage infiltration.
A portion of the epididymal adipose tissue was formalin fixed and paraffin embedded, and 7-μm sections were mounted on glass microscope slides. Immunohistochemistry was performed using primary antibodies against macrophages (F4/80: 1:1,000, ab6640; Abcam). Positive staining was visualized with 3,3′-diaminobenzidine (K4011; Dako), and sections were counterstained with Mayer's hematoxylin (S3309; Dako) to identify nuclei. Using ImageJ 1.42, a technician blinded to group identity outlined the adventitia with a pen tablet (Bamboo Fun; Wacom) and counted the total number of nuclei and the number of nuclei with positive staining for macrophages (23). Infiltration was expressed as the percentage of total cells (nuclei). For each animal, three serial sections were analyzed and the results were averaged.
Plasma and adipose tissue TNF-α.
TNF-α concentrations were measured in plasma and adipose tissue lysates by ELISA (Quantikine Mouse TNF-α Immunoassay; R&D Systems, Minneapolis, MN) according to manufacturer instructions.
Vascular function.
To assess endothelial function, arteries were excised, cleared of surrounding adipocytes, and cannulated in the stage of a pressure myograph (DMT). Arteries were preconstricted with 2 μM phenylephrine, and EDD as well as the NO contribution to dilation was measured in response to the cumulative addition of Ach (1 × 10−9 to 1 × 10−4 mol/l) in the absence or presence of the NO synthase inhibitor Nω-nitro-l-arginine methyl ester (l-name) (0.1 mmol/l, 30 min), as described previously (10, 21). To determine the effect of TNF-α on vasodilation, a second arterial segment from the same eWAT was incubated intraluminally with recombinant TNF-α (rTNF; 1 ng/ml; R&D Systems) or a neutralizing antibody against mouse TNF-α (abTNF; 20 μg/ml; R&D Systems) for 1 h before performing the aforementioned ACh dose responses. Endothelium-independent dilation was assessed in response to sodium nitroprusside (SNP; 1 × 10−10 to 1 × 10−4 mol/l) (10, 21). Vessel diameters were measured by MyoView software (DMT). All dose response data are presented as percentages of possible dilation after preconstriction to phenylephrine. Arteries failing to achieve ≥20% preconstriction were excluded. Sensitivity was defined as the concentration of ACh or SNP that elicited 50% of the maximal response (IC50). Sensitivities (IC50s) were calculating using BioDataFit 1.02. A regression was used to fit a sigmoidal model to individual dose responses yielding a dose for the half maximal response in log M units.
Skeletal muscle lipid accumulation.
Quadriceps muscle was excised and weighed. Lipid was extracted from ∼100 mg of tissue as described previously (33). Triglyceride content was assessed using a commercially available assay kit (Cayman Chemicals) and expressed as a percentage of starting tissue mass.
Statistics.
To assess differences in glucose tolerance, plasma insulin during the GTT, and endothelium-dependent and -independent dilation, repeated-measures ANOVA tests were performed with least significant difference post hoc where appropriate. To determine differences in animal and artery characteristics, adipose tissue macrophage infiltration, TNF-α concentrations, and IC50s, ANOVAs with least significant difference post hoc tests were performed. Data are presented as means ± SE. Significance was set at P < 0.05.
RESULTS
At euthanasia, total body mass was greater in HF- compared with NC-fed mice (Table 1). Daily caloric intake in kilocalories per day did not differ between NC- and HF-fed mice (Table 1). HF was associated with a large increase in adiposity such that the mass of the eWAT was doubled after HF feeding and accounted for ∼4% of total body mass compared with only ∼2% in the NC-fed mice (Table 1). Soleus muscle mass was also greater in HF- compared with NC-fed mice (Table 1). No other differences in tissue masses were found after HF diet (gastrocnemius muscle, heart, liver; all P > 0.24 vs. NC). Triglyceride content of the quadriceps muscle was increased after HF feeding (Table 1).
Table 1.
Body and tissue masses, food intake, and skeletal muscle triglyceride content from normal chow and high fat-fed B6D2F1 mice
| Normal Chow | High-Fat Diet | |||
|---|---|---|---|---|
| Body mass, g | 34.9 ± 1.5 | 37.8 ± 0.9* | ||
| Food intake, kcal/day | 12.2 ± 0.4 | 11.8 ± 0.4 | ||
| Quadriceps muscle triglyceride content, mg TG/g muscle | 1.35 ± 0.07 | 1.66 ± 0.13* | ||
| Tissue | Weight, g | %Body mass | Weight, g | %Body mass |
| Epididymal adipose tissue | 0.89 ± 0.13 | 2.1 ± 0.3 | 1.56 ± 0.26* | 4.1 ± 0.6* |
| Gastrocnemius muscle | 0.19 ± 0.01 | 0.55 ± 0.02 | 0.20 ± 0.01 | 0.52 ± 0.02 |
| Soleus muscle | 0.012 ± 0.001 | 0.036 ± 0.004 | 0.015 ± 0.001* | 0.042 ± 0.003 |
| Heart | 0.20 ± 0.01 | 0.53 ± 0.02 | 0.19 ± 0.01 | 0.49 ± 0.03 |
| Liver | 1.78 ± 0.18 | 5.0 ± 0.5 | 1.91 ± 0.15 | 5.0 ± 0.3 |
Values are means ± SE. TG, triglyceride.
Significant difference between normal chow and high fat-fed mice, P < 0.05.
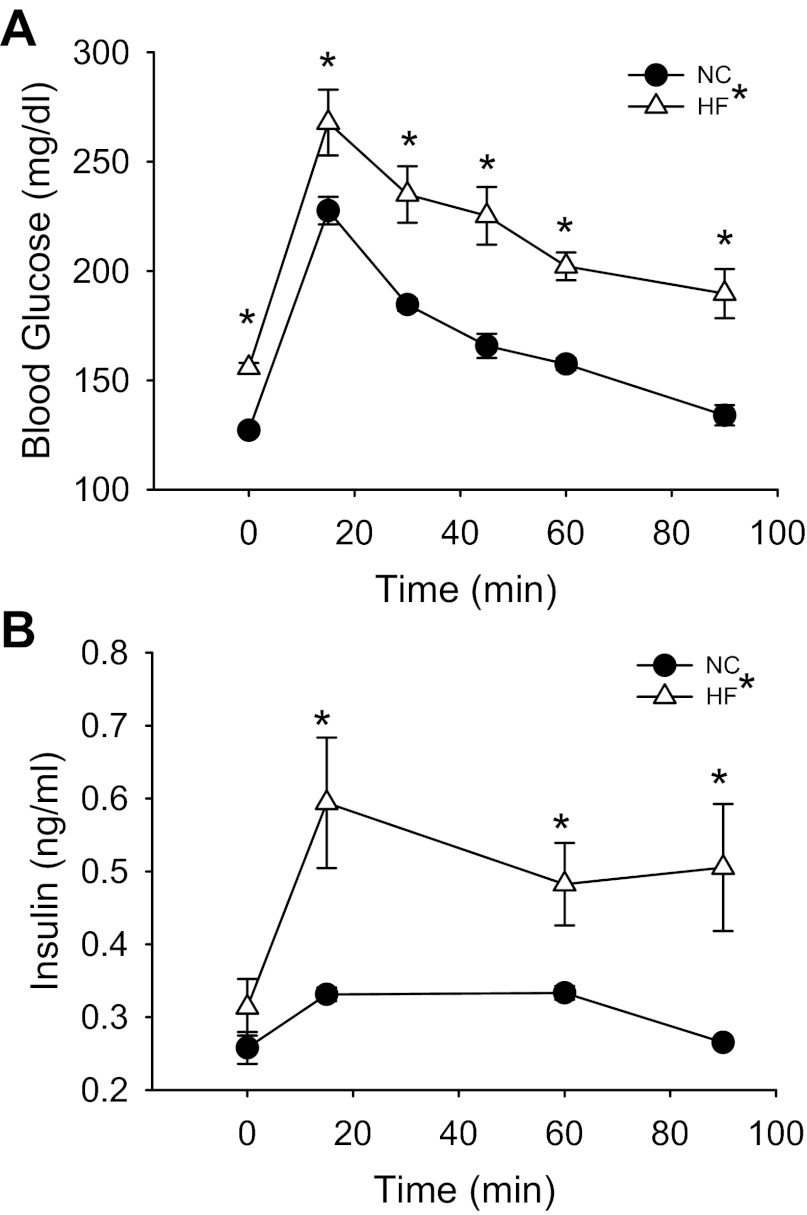
Fasted blood glucose (Fig. 1A; P < 0.01), but not plasma insulin (Fig. 1B), was higher in HF- compared with NC-fed mice. Mice were glucose intolerant after HF feeding (Fig. 1A; P < 0.01 main effect for diet) and had elevated plasma insulin concentrations throughout a GTT (Fig. 1B; P < 0.05 main effect for diet), suggestive of impaired insulin sensitivity after HF feeding. Indeed, estimating insulin sensitivity from fasted glucose and insulin concentrations with HOMA, we found insulin sensitivity (HOMA-S, 64.1 ± 4.3 vs. 85.7 ± 6.4; P = 0.05), but not β-cell function (HOMA-B, 37.8 ± 3.0 vs. 33.3 ± 5.3), to be lower in HF- compared with NC-fed mice.
Fig. 1.
Blood glucose concentrations (A) after a 2-h morning fast (time 0) and during an intraperitoneal glucose (2,000 mg/kg) tolerance test (GTT) in normal chow (NC; N = 7)- and high-fat (HF; N = 8)-fed mice. Plasma insulin concentrations (B) after 2-h fast (time 0) and during ip GTT are also shown. Significance marks in legend denote a time by group interaction or main effect for diet during repeated-measures ANOVA. Marks above curves indicate difference between groups at specific time points by 1-way ANOVA. *Differences between NC- and HF-diet groups. Data are means ± SE (P ≤ 0.05).
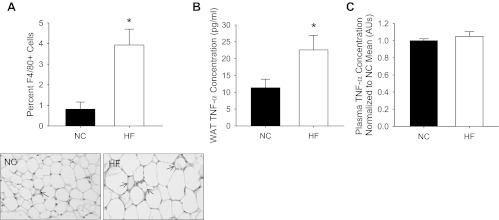
This metabolic dysfunction was accompanied by elevated tissue inflammation, but not circulating inflammation. This was demonstrated by greater macrophage infiltration (Fig. 2A; P = 0.01) and elevated TNF-α concentrations (Fig. 2B; P < 0.05) in the eWAT of HF- compared with NC-fed mice, and the absence of an increase in plasma TNF-α (Fig. 2B) after HF feeding.
Fig. 2.
Macrophage infiltration (A) of epididymal white adipose tissue (eWAT) assessed by immunohistochemistry for F4/80 in paraffin-embedded adipose tissue sections from NC (N = 5)- and HF (N = 5)-fed mice. Representative images are to the right of summary graph. Arrows indicate macrophages forming crown-like structures around adipocytes. TNF-α concentration in WAT lysates (B) from NC (N = 11)- and HF (N = 14)-fed mice is shown. Plasma TNF-α concentrations (C) in NC (N = 5)- and HF (N = 5)-fed mice are also shown. Data are presented as a ratio of NC mean. *Differences between NC- and HF-diet groups. Data are means ± SE (P ≤ 0.05). AU, arbitrary units.
There was no difference in the maximal diameter or preconstriction to phenylephrine of eWAT resistance arteries from NC- and HF-fed mice (Table 2). Incubation with the NO synthase inhibitor l-NAME did not change phenylephrine-mediated preconstriction in either the NC- or HF-fed mice (Table 2). After in vitro treatment with rTNF, preconstriction to phenylephrine was lower in eWAT resistance arteries from HF- compared with NC-fed mice, and this was true in both the absence and presence of l-NAME (both P < 0.05; Table 2). There was no effect of l-NAME on preconstriction in rTNF-treated eWAT resistance arteries from NC- or HF-fed mice (Table 2). In vitro treatment with abTNF did not affect preconstriction to phenylephrine in NC- or HF-fed mice in either the absence or presence of l-NAME (Table 2).
Table 2.
White adipose tissue artery maximal luminal diameter, percent preconstriction to PE, and sensitivity (IC50) to ACh in the absence or presence of the l-NAME and rTNF or abTNF as well as sensitivity to SNP
| Normal Chow | High-Fat Diet | |
|---|---|---|
| Maximal diameter, μm | 179 ± 9 | 183 ± 6 |
| Preconstriction (% max diameter) | ||
| PE | 47 ± 4 | 36 ± 6 |
| PE + l-NAME | 60 ± 6 | 42 ± 10 |
| PE + rTNF | 57 ± 4 | 42 ± 7* |
| PE + rTNF + l-NAME | 62 ± 8 | 42 ± 7* |
| PE + abTNF | 45 ± 5 | 40 ± 11 |
| PE + abTNF + l-NAME | 48 ± 7 | 44 ± 9 |
| IC50 (log M) | ||
| ACh | −7.66 ± 0.27 | −6.94 ± 0.43 |
| ACh + l-NAME | −6.94 ± 0.43 | −7.57 ± 0.40 |
| rTNF + ACh | −6.78 ± 0.49 | −7.65 ± 0.55 |
| rTNF + ACh + l-NAME | −7.49 ± 0.62 | −6.89 ± 0.68 |
| abTNF + ACh | −6.80 ± 0.24 | −6.87 ± 0.58 |
| abTNF + ACh + l-NAME | −6.69 ± 0.39 | −6.42 ± 0.74 |
| SNP | −8.21 ± 0.33 | −7.87 ± 0.47 |
| rTNF + SNP | −8.64 ± 0.36 | −7.59 ± 0.46 |
| abTNF + SNP | −8.04 ± 0.43 | −7.34 ± 0.14 |
Values are means ± SE. PE, phenylephrine; l-NAME, Nω-nitro-l-arginine methyl ester; rTNF, recombinant TNF-α; abTNF, neutralizing antibody against TNF-α; SNP, sodium nitroprusside.
Difference, NC vs. HF. P < 0.05.
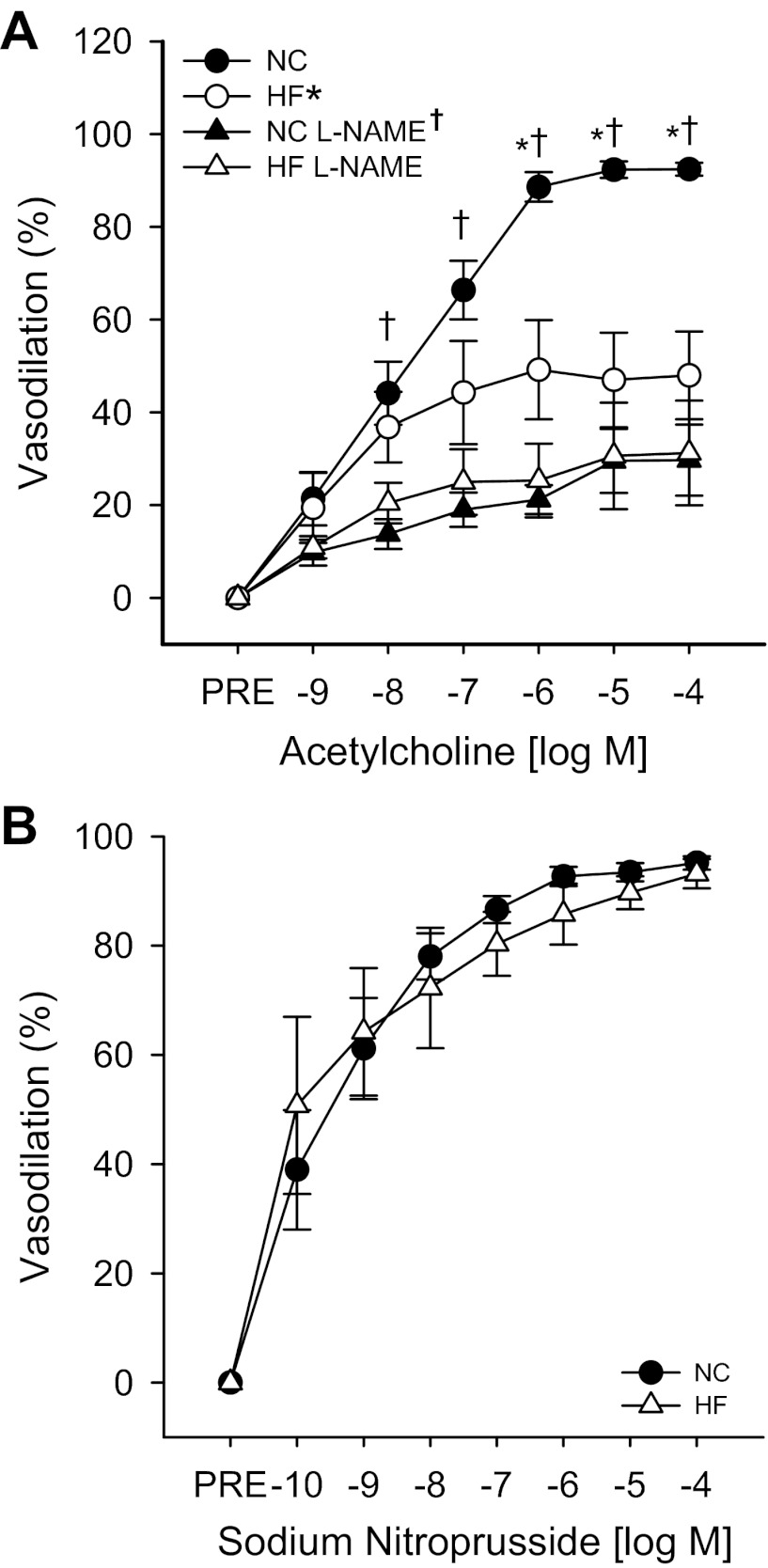
ACh-induced dilation was impaired in resistance arteries from the eWAT of HF- compared with NC-fed mice (Fig. 3A; P < 0.01). Sensitivity (IC50) to ACh did not differ between groups (Table 2). Although l-NAME reduced ACh-induced dilation in arteries from both NC- and HF-fed mice (Fig. 3A; both P < 0.01), l-NAME eliminated the differences in dilation previously observed between NC- and HF-fed mice, indicating that impaired dilation observed after HF feeding resulted from reduced NO bioavailability (Fig. 3A). Endothelium-independent dilation (Fig. 3B) and sensitivity (Table 2) to SNP did not differ between groups.
Fig. 3.
Endothelium-dependent dilation (A) to ACh in the absence or presence of the nitric oxide synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME), measured by pressure myography in resistance arteries excised from the eWAT of NC (N = 10)- and HF (N = 9)-fed mice. Endothelium-independent dilation (B) to sodium nitroprusside in resistance arteries excised from the eWAT of NC (N = 11)- and HF (N = 6)-fed mice is also shown. Significance marks in legend denote a time by group interaction or main effect for diet during repeated-measures ANOVA. Marks above curves indicate difference between groups and treatment conditions at specific time points by 1-way ANOVA with least significant difference (LSD) post hoc tests. *Differences between NC- and HF-diet groups; †differences in ACh responses in the absence and presence of l-NAME. Data are means ± SE (P ≤ 0.05).
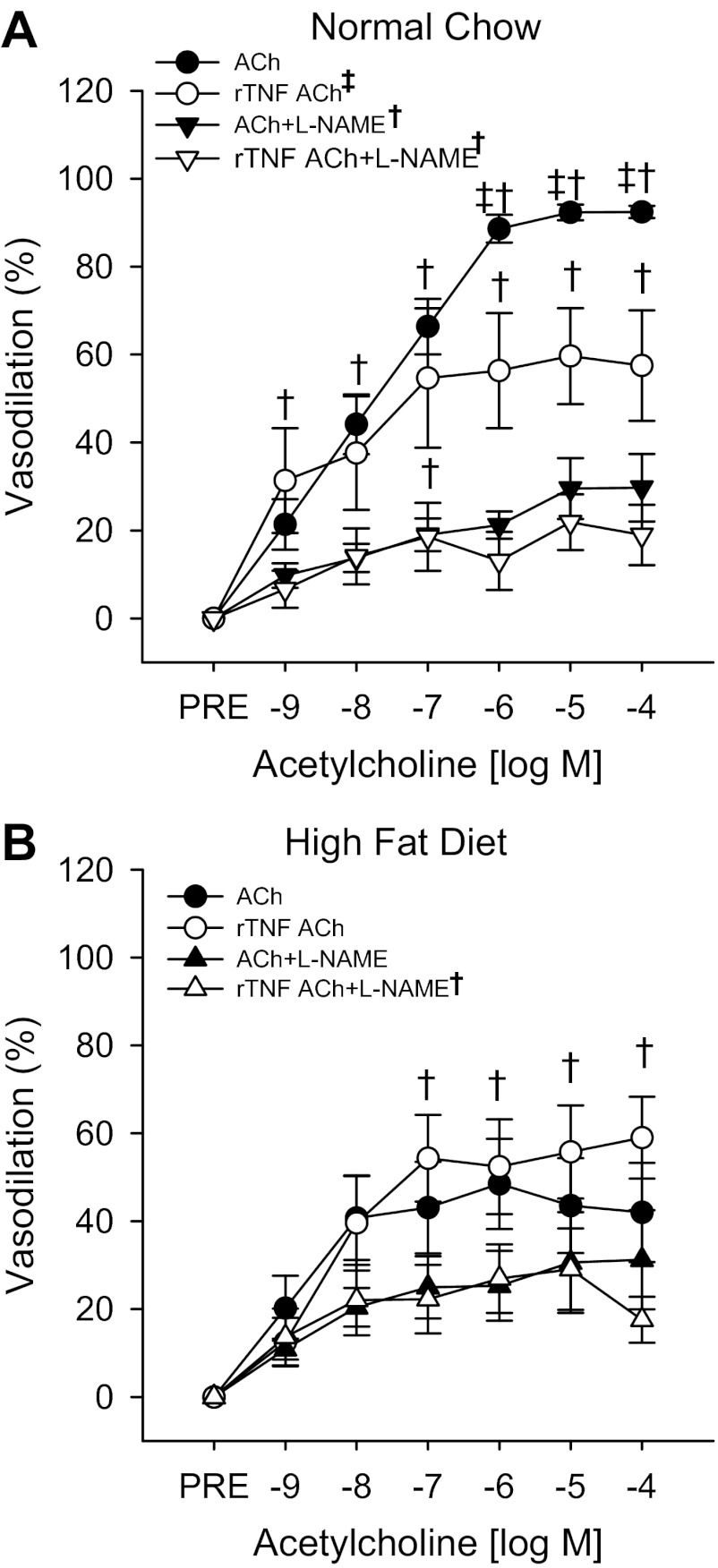
In vitro incubation of eWAT resistance arteries from NC-fed mice with rTNF resulted in an impaired dilation (Fig. 4; P = <0.01) but no change in sensitivity (Table 2) to ACh. Although incubation with l-NAME reduced dilation to ACh in rTNF-treated arteries from NC-fed mice (Fig. 4A; both P < 0.01), l-NAME eliminated the rTNF-mediated differences in dilation (Fig. 4A and Table 2). Thus impaired dilation after rTNF treatment results from a reduction in NO bioavailability, similar to what was observed after HF feeding (Fig. 4A).
Fig. 4.
Endothelium-dependent dilation (A) to ACh in the absence or presence of the NOS inhibitor l-NAME and recombinant TNF-α (rTNF) measured by pressure myography in resistance arteries excised from the eWAT of NC (N = 7)-fed mice. Endothelium-dependent dilation (B) to ACh in the absence or presence of the l-NAME and/or rTNF measured in resistance arteries excised from the eWAT of HF (N = 9)-fed mice is also shown. Significance marks in legend denote a time by group interaction or main effect for diet during repeated-measures ANOVA. Marks above curves indicate difference between groups and treatment conditions at specific time points by 1-way ANOVA with LSD post hoc tests. †Differences in ACh responses in the absence and presence of l-NAME; ‡differences in ACh responses in the absence and presence of rTNF. Data are means ± SE (P ≤ 0.05).
Vasodilation to ACh was not impaired by incubation with rTNF in eWAT arteries from HF-fed mice, and l-NAME failed to reduce ACh dilation in these arteries (Fig. 4B). No differences in sensitivity to ACh were observed in eWAT arteries from HF-fed mice after l-NAME, rTNF, or combined l-NAME and rTNF incubation (Table 2). These results suggest that the effect of rTNF to impair eWAT artery EDD and NO bioavailability is lost after HF feeding, a state during which the eWAT arteries are chronically exposed to elevations in this cytokine in vivo (Fig. 2B). rTNF did not affect endothelium-independent dilation (not shown) or sensitivity (Table 2) to SNP in arteries from NC- or HF-fed mice.
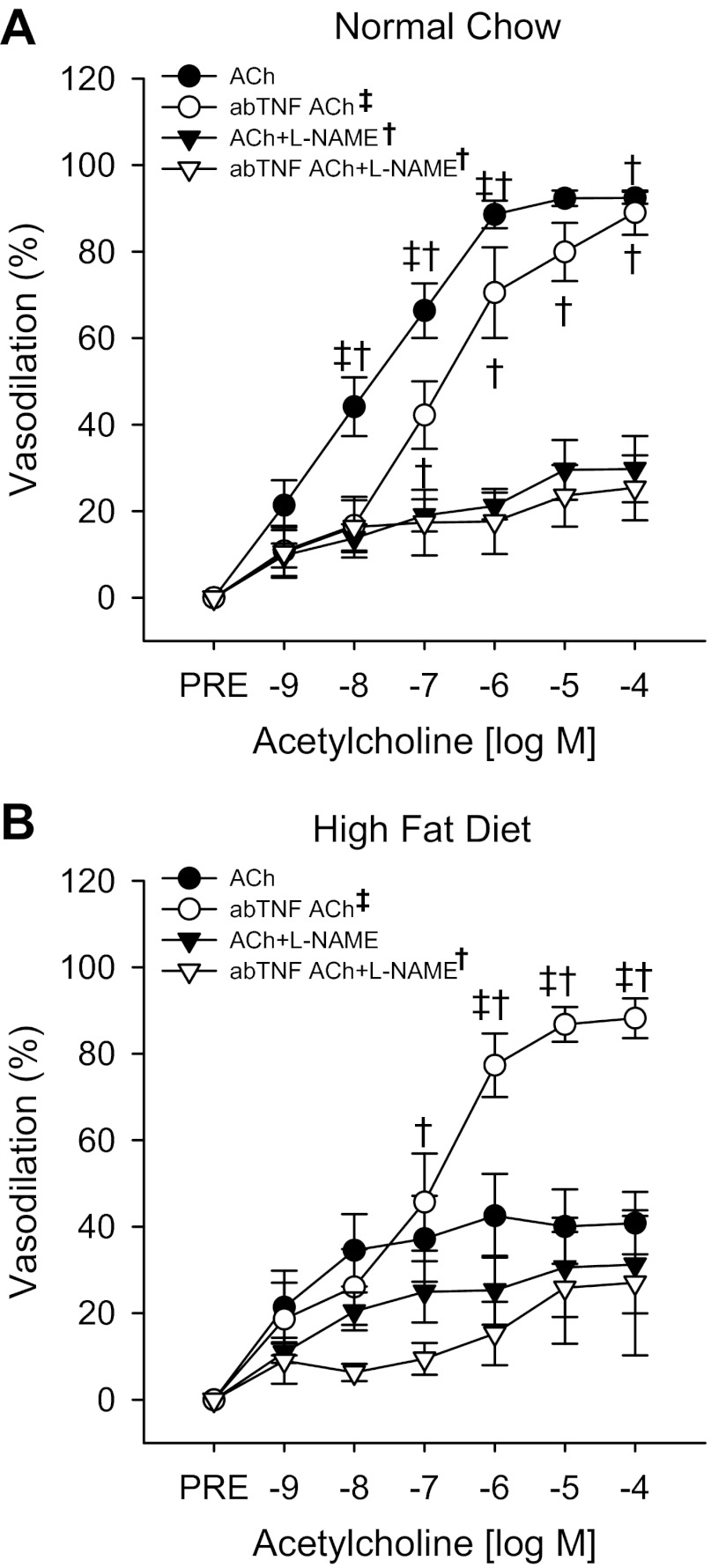
Although not reduced at maximal dilation, the dose response to ACh in eWAT resistance arteries from NC-fed mice was reduced (shifted rightward) after in vitro treatment with abTNF (Fig. 5A; P < 0.05). However, this rightward shift was insufficient to reduce sensitivity to ACh (Table 2). l-NAME reduced dilation (Fig. 5A; P < 0.01) but not sensitivity (Table 2) to ACh in abTNF-treated arteries from NC-fed mice.
Fig. 5.
Endothelium-dependent dilation (A) to ACh in the absence or presence of the NOS inhibitor l-NAME and a neutralizing TNF-α antibody (abTNF) measured by pressure myography in resistance arteries excised from the eWAT of NC (N = 6)-fed mice. Endothelium-dependent dilation (B) to ACh in the absence or presence of l-NAME and/or abTNF in resistance arteries excised from the eWAT of HF (N = 5)-fed mice is also shown. Significance marks in legend denote a time by group interaction or main effect for diet during repeated-measures ANOVA. Marks above curves indicate difference between groups and treatment conditions at specific time points by 1-way ANOVA with LSD post hoc tests. †Differences in ACh responses in the absence and presence of l-NAME; ‡denotes differences in ACh responses in the absence and presence of abTNF. Data are means ± SE (P ≤ 0.05).
In vitro incubation of eWAT resistance arteries from HF-fed mice with abTNF resulted in an improved dilation (Fig. 5B; P = 0.05) to ACh. Incubation with l-NAME reduced dilation to ACh in abTNF-treated arteries from HF-fed mice (Fig. 5B; P < 0.01). Thus impaired dilation and reduced NO bioavailability observed with HF feeding in eWAT resistance arteries can be restored by abTNF treatment. There were no differences in sensitivity to ACh with or without l-NAME treatment in abTNF-treated arteries (Table 2). Neither dilation (not shown) nor sensitivity (Table 2) to SNP differed between NC- and HF-fed mice after abTNF treatment.
DISCUSSION
The novel findings of the present study are that HF diet-induced glucose intolerance and insulin resistance is concomitant with endothelial dysfunction and reduced NO bioavailability in visceral eWAT resistance arteries. We extend these observations by demonstrating that exogenous administration of TNF-α can impair function in eWAT arteries from NC-, but not HF-fed, mice and that the chronic in vivo exposure of eWAT arteries to elevated TNF-α resulting from HF feeding underlies the observed endothelial dysfunction. Although the causal role of adipose tissue dysfunction and inflammation in obesity-associated metabolic and cardiovascular disease risk is well established (3, 15, 24, 32), there is surprisingly little information regarding the role of vascular function per se in the overall function/phenotype of the adipose tissue (30), or how this vascular function is affected by obesity (12). Here, we provide direct evidence for a HF-diet-mediated, TNF-α-induced, impairment in adipose tissue arterial function that may have important implications for tissue-specific and systemic metabolic and vascular health.
The findings presented here extend our knowledge of the consequences of obesity on vascular function. Our results suggest that endothelial dysfunction in the resistance vasculature of the adipose tissue per se may 1) underlie the previously reported impairments in adipose tissue blood flow and 2) contribute to the adipose tissue inflammatory phenotype by enhancing adipose artery adhesion molecule expression that then contributes to macrophage infiltration (40). Although, we cannot provide direct evidence for a causal effect of endothelial dysfunction on impairments in adipose tissue blood flow in obese humans (1, 18), future studies should directly examine the effect of obesity on visceral adipose blood flow, as well as determine whether a putative impairment in visceral adipose tissue blood flow is the consequence of a reduction in vasodilator capacity of its resistance vasculature.
Previous studies have demonstrated that obesity is associated with arterial dysfunction in various vascular beds such as the skeletal muscle (11, 22), mesenteric, (6, 34) and coronary circulations (14, 29), and this dysfunction has been attributed, at least in part, to the effects of adipose-derived factors (29, 43). Although subcutaneous adipose tissue blood flow has been shown to be impaired in obese humans in response to feeding or β-adrenergic stimulation (1, 17, 18, 38), little is known about how vascular function is modified by obesity in the visceral adipose stores.
A recent study by Farb et al. (12) demonstrated a reduced dilation in the resistance arteries from the visceral compared with subcutaneous adipose tissue of obese humans. However, this study lacked samples from lean patients, and thus cannot exclude the possibility that the reduced visceral adipose artery dilation is simply the result of tissue specificity, as has been observed in resistance arteries from other closely related tissues like white and red skeletal muscle (26). The authors demonstrated that the reduced dilation in the visceral adipose tissue arteries is associated with a more proinflammatory adipose tissue phenotype relative to the subcutaneous adipose, suggesting a role for the adipose tissue microenvironment in the reduced dilation observed in the visceral adipose arteries. Because visceral, rather than subcutaneous, adiposity is associated with elevated disease risk and impaired endothelium-dependent dilation (3, 16, 28), elucidation of the mechanisms of impaired vasodilator capacity and blood flow in the visceral adipose after HF feeding/obesity is of critical importance. Here, we demonstrate that HF feeding impairs EDD and NO bioavailability in the visceral WAT stores and provide initial evidence that this is mediated by increases in inflammation.
Here, we provide evidence for a critical role of TNF-α in adipose artery endothelial dysfunction, occurring as a consequence of diet-induced obesity. Similar to our results, impairments in EDD have also been seen in mesenteric arterioles after long-term (14 h), but not short-term (30 min), in vitro exposure to IL-1β (42). However, in the present study, we demonstrate that adipose tissue arteries from NC- but not HF-fed mice are sensitive to TNF-α exposure in vitro, resulting in impaired EDD and reduced NO bioavailability in these “naïve” arteries. With HF feeding, adipose arteries are chronically exposed to a proinflammatory microenvironment, characterized by increased macrophage infiltration and increased adipose tissue TNF-α. Under these conditions, the in vitro effect of TNF-α to impair endothelial function was lost in the already dysfunctional HF-fed mouse arteries. To establish a causal role for TNF-α in the HF diet-induced endothelial dysfunction observed in the eWAT arteries, we examined whether inhibition of TNF-α could restore EDD and NO bioavailability in arteries from HF-fed mice. Here we found that incubation with a neutralizing antibody against TNF-α restored EDD and NO bioavailability in eWAT arteries from HF-fed mice and was without effect in arteries from NC-fed mice. These results suggest that although other proinflammatory mediators such as IL-1β may be elevated in obesity and have the capacity to impair endothelial function (19, 42), in the adipose tissue circulation, TNF-α is a critical mediator of impaired function after HF feeding.
Although the mechanism by which TNF-α leads to impaired EDD and reduced NO likely involves either a direct inhibition of NOS activation (20) or increases in oxidative stress (8, 45), these possibilities require direct examination. It is also possible that the lack of effect of rTNF in HF mouse arteries is the result of receptor desensitization/downregulation following chronic in vivo TNF-α exposure; however, these possibilities also require further examination.
In the present study, we observed an increase in soleus muscle mass after HF feeding. Although the mechanism for the observed increase in soleus muscle mass is unclear, it may be related to an increase in lipid accumulation. To explore this possibility we measured lipid content in quadriceps muscle from NC- and HF-fed mice. It should be noted that ∼100 mg of tissue is used for digestion and triglyceride determination, and thus the small mass of the soleus precluded us from making these measures directly in the soleus muscle. Although the triglyceride content of the quadriceps muscle was significantly increased after HF feeding (Table 1), if one assumes a similar content of lipid between the quadriceps and soleus muscles, lipid accumulation cannot completely explain the increase in soleus mass since this concentration of lipid would account for less than a milligram of the total muscle mass in the soleus after HF feeding. Hypertrophy of the muscle in response to increased body mass may explain the remaining differences in soleus mass between NC- and HF-fed mice.
Limitations.
We acknowledge that the concentration of TNF-α used in the bath is an order of magnitude greater than what was measured in the eWAT. Although this is a limitation of the present study, we felt that due to the acute (60 min) in vitro exposure of the arteries, the higher concentration was warranted and necessary to evaluate the effects of TNF-α on vascular function. Indeed, this dose of TNF-α (1 ng/ml) has previously been demonstrated to induce endothelial dysfunction in the coronary arteries (44). Our studies cannot rule out other inflammatory mediators in the development of obesity-associated adipose tissue endothelial dysfunction, nor can we delineate the specific cellular source of the TNF-α, e.g., vascular or immune cell derived (8, 45). These are questions that require further study. Finally, there have been concerns raised over the use of SNP as an endothelium-independent vasodilator, since its long-term and/or high dose usage can lead to cyanide toxicity. However, the reaction has a long time course and requires the binding of the iron group in SNP to a sulfhydryl group present in vivo in erythrocytes (41) and are not present in our preparation. Thus we believe that SNP is appropriate in the present investigation.
In conclusion, HF feeding results in a decreased EDD in visceral adipose tissue arteries and appears to be mediated by their proinflammatory tissue environment. This adipose tissue arterial dysfunction may impact adipose tissue blood flow and contribute to the adipose inflammation that results in the metabolic derangements observed; however, this possibility requires direct examination. By demonstrating a HF-diet induced reduction in eWAT resistance artery vasodilator capacity, these studies raise the possibility that inflammation-mediated vascular dysfunction within the adipose tissue may be an important factor in adipose tissue dysfunction and subsequent systemic metabolic dysfunction resulting from diet-induced obesity.
GRANTS
This work was supported by awards from the National Institute on Aging and the National Heart, Lung, and Blood Institute (K01-AG033196, R21-AG033755, T35-HL007744, R01-AG040297 and T32-HL007576).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.J.D. and L.A.L. conception and design of research; A.J.D., G.D.H., R.G.M., R.A.E., A.E.W., and L.A.L. performed experiments; A.J.D., G.D.H., R.G.M., R.A.E., A.E.W., and L.A.L. edited and revised manuscript; A.J.D., G.D.H., R.G.M., R.A.E., A.E.W., and L.A.L. approved final version of manuscript; L.A.L. analyzed data; L.A.L. interpreted results of experiments; L.A.L. prepared figures; L.A.L. drafted manuscript.
ACKNOWLEDGMENTS
All experiments were performed in the Translational Vascular Physiology Laboratory at the University of Utah and VAMC-SLC Geriatrics Research Education and Clinical Center.
REFERENCES
- 1. Ardilouze JL, Fielding BA, Currie JM, Frayn KN, Karpe F. Nitric oxide and beta-adrenergic stimulation are major regulators of preprandial and postprandial subcutaneous adipose tissue blood flow in humans. Circulation 109: 47–52, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Baron AD, Clark MG. Role of blood flow in the regulation of muscle glucose uptake. Annu Rev Nutr 17: 487–499, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96: 939–949, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bisset WI, Butler AR, Glidewell C, Reglinski J. Sodium nitroprusside and cyanide release: reasons for re-appraisal. Br J Anaesth 53: 1015–1018, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Blaak EE, van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, Saris WH. Beta-adrenergic stimulation and abdominal subcutaneous fat blood flow in lean, obese, and reduced-obese subjects. Metabolism 44: 183–187, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Bouvet C, Belin de Chantemele E, Guihot AL, Vessieres E, Bocquet A, Dumont O, Jardel A, Loufrani L, Moreau P, Henrion D. Flow-induced remodeling in resistance arteries from obese Zucker rats is associated with endothelial dysfunction. Hypertension 50: 248–254, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 295: E732–E750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol 170: 388–398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erdei N, Toth A, Pasztor ET, Papp Z, Edes I, Koller A, Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol 291: H2107–H2115, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Farb MG, Ganley-Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, Bigornia SJ, Fiscale AJ, Apovian CM, Carmine B, Hess DT, Vita JA, Gokce N. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol 32: 467–473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord 27: 875–888, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation 115: 245–254, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav 94: 206–218, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11: 11–18, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Karpe F, Fielding BA, Ardilouze JL, Ilic V, Macdonald IA, Frayn KN. Effects of insulin on adipose tissue blood flow in man. J Physiol 540: 1087–1093, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karpe F, Fielding BA, Ilic V, Macdonald IA, Summers LK, Frayn KN. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes 51: 2467–2473, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Kessler P, Popp R, Busse R, Schini-Kerth VB. Proinflammatory mediators chronically downregulate the formation of the endothelium-derived hyperpolarizing factor in arteries via a nitric oxide/cyclic GMP-dependent mechanism. Circulation 99: 1878–1884, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Kim F, Gallis B, Corson MA. TNF-α inhibits flow and insulin signaling leading to NO production in aortic endothelial cells. Am J Physiol Cell Physiol 280: C1057–C1065, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci 64: 9–20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lesniewski LA, Donato AJ, Behnke BJ, Woodman CR, Laughlin MH, Ray CA, Delp MD. Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. Am J Physiol Heart Circ Physiol 294: H1840–H1850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301: H1025–H1032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lesniewski LA, Hosch SE, Neels JG, de Luca C, Pashmforoush M, Lumeng CN, Chiang SH, Scadeng M, Saltiel AR, Olefsky JM. Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nat Med 13: 455–462, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21: 2191–2192, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Organization WH. World Health Organization Statistics 2011, 2011
- 28. Parikh NI, Keyes MJ, Larson MG, Pou KM, Hamburg NM, Vita JA, O′Donnell CJ, Vasan RS, Mitchell GF, Hoffmann U, Fox CS, Benjamin EJ. Visceral and subcutaneous adiposity and brachial artery vasodilator function. Obesity (Silver Spring) 17: 2054–2059, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol 30: 1711–1717, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol 292: H93–H100, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res 99: 69–77, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Red Eagle A, Chawla A. In obesity and weight loss, all roads lead to the mighty macrophage. J Clin Invest 120: 3437–3440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodriguez-Sureda V, Peinado-Onsurbe J. A procedure for measuring triacylglyceride and cholesterol content using a small amount of tissue. Anal Biochem 343: 277–282, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Romanko OP, Stepp DW. Reduced constrictor reactivity balances impaired vasodilation in the mesenteric circulation of the obese Zucker rat. Am J Physiol Heart Circ Physiol 289: H2097–H2102, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Samra JS, Simpson EJ, Clark ML, Forster CD, Humphreys SM, Macdonald IA, Frayn KN. Effects of adrenaline infusion on the interstitial environment of subcutaneous adipose tissue as studied by microdialysis. Clin Sci (Lond) 91: 425–430, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Samra JS, Simpson EJ, Clark ML, Forster CD, Humphreys SM, Macdonald IA, Frayn KN. Effects of epinephrine infusion on adipose tissue: interactions between blood flow and lipid metabolism. Am J Physiol Endocrinol Metab 271: E834–E839, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Summers LK, Samra JS, Humphreys SM, Morris RJ, Frayn KN. Subcutaneous abdominal adipose tissue blood flow: variation within and between subjects and relationship to obesity. Clin Sci (Lond) 91: 679–683, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Tobin L, Simonsen L, Bulow J. The dynamics of the microcirculation in the subcutaneous adipose tissue is impaired in the postprandial state in type 2 diabetes. Clin Physiol Funct Imaging 31: 458–463, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Turner RC, Holman RR, Matthews D, Hockaday TD, Peto J. Insulin deficiency and insulin resistance interaction in diabetes: estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrations. Metabolism 28: 1086–1096, 1979 [DOI] [PubMed] [Google Scholar]
- 40. Vallbracht KB, Schwimmbeck PL, Seeberg B, Kuhl U, Schultheiss HP. Endothelial dysfunction of peripheral arteries in patients with immunohistologically confirmed myocardial inflammation correlates with endothelial expression of human leukocyte antigens and adhesion molecules in myocardial biopsies. J Am Coll Cardiol 40: 515–520, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Verner IR. Sodium nitroprusside: theory and practice. Postgrad Med J 50: 576–581, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vila E, Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol Heart Circ Physiol 288: H1016–H1021, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Xiang L, Hester RL. Adipocyte-derived factor reduces vasodilatory capability in ob-/ob- mice. Am J Physiol Heart Circ Physiol 297: H689–H695, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang DX, Yi FX, Zou AP, Li PL. Role of ceramide in TNF-α-induced impairment of endothelium-dependent vasorelaxation in coronary arteries. Am J Physiol Heart Circ Physiol 283: H1785–H1794, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 116: 219–230, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]