Abstract
Toll-like receptor (TLR)4 regulates inflammation and metabolism and has been linked to the pathogenesis of heart disease. TLR4 is upregulated in diabetic cardiomyocytes, and we examined the role of TLR4 in modulating cardiac fatty acid (FA) metabolism and the pathogenesis of diabetic heart disease in nonobese diabetic (NOD) mice. Both wild-type (WT) NOD and TLR4-deficient NOD animals had increased plasma triglyceride levels after the onset of diabetes. However, by comparison, TLR4-deficient NOD mouse hearts had lower triglyceride accumulation in the early stages of diabetes, which was associated with a reduction in myeloid differentiation primary response gene (88) (MyD88), phosphorylation of p38 MAPK (phospho-p38), lipoprotein lipase (LPL), and JNK levels but increased phospho-AMP-activated protein kinase (AMPK). Oleic acid treatment in H9C2 cardiomyocytes also led to cellular lipid accumulation, which was attenuated by TLR4 small interfering RNA. TLR4 deficiency in the cells decreased FA-induced augmentation of MyD88, phospho-p38, and LPL, suggesting that TLR4 may modulate FA-induced lipid metabolism in cardiomyocytes. In addition, although cardiac function was impaired in both diabetic WT NOD and TLR4-deficient NOD animals compared with control nondiabetic mice, this deficit was less in the diabetic TLR4-deficient NOD mice, which had greater ejection fraction, greater fractional shortening, and increased left ventricular developed pressure in the early stages after the development of diabetes compared with their diabetic WT NOD counterparts. Thus, we conclude that TLR4 plays a role in regulating lipid accumulation in cardiac muscle after the onset of type 1 diabetes, which may contribute to cardiac dysfunction.
Keywords: nonobese diabetic mice, Toll-like receptor 4, cardiac complications, diabetes
cardiovascular complications are a major cause of morbidity and mortality in patients with type 1 diabetes (9, 10, 14). Many patients will suffer from atherosclerotic vascular disease and associated complications. In addition, diabetic cardiomyopathy also contributes to cardiac dysfunction in the absence of coronary artery disease and hypertension (2). The risks are greater in those who have poor glycemic control and can be reduced with intensive treatment, as shown in the Diabetes Control and Complications Trial (18).
A number of metabolic abnormalities occur in diabetes that can adversely affect cardiac function, which include increased lipid uptake, increased cardiac insulin resistance, alterations in Ca2+ metabolism, direct glucotoxicity, and increased activation of the renin-angiotensin axis (5). Fatty acids (FAs) are a key energy substrate for the heart to maintain its contractile function. Under physiological conditions, cardiac FAs are, for the most part, derived from the hydrolysis of triglyceride (TG)-rich lipoproteins by lipoprotein lipase (LPL) on the endothelial surface of the coronary arteries. During diabetes, compromised glucose utilization triggers excessive FA delivery into the heart to make up the energy deficit, leading to FA supplies far beyond FA oxidation. The excess free FAs thus augment the cardiac TG pool and stimulates cell death, which is defined as “cardiac lipotoxicity” (6). Innate immune receptors, which include the Toll-like receptor (TLR) family, play a key role in pathogen recognition, and activation of innate immunity leading to inflammation is also important in the development of metabolic disorders and atherosclerosis. TLR4 has particularly been implicated in atherosclerosis (3, 8, 16) and may regulate the development of ischemic heart disease and hypertrophy. Recently, Kelly and colleagues (27) reported that TLR4 contributes to cardiac metabolic and functional abnormalities in sepsis through inhibition of peroxisome proliferator-activated receptor-γ coactivator 1α. Given the contribution of TLR4 to the development of metabolic dysfunction and heart disease, our study tested the hypothesis that TLR4 plays a role in the pathogenesis of diabetic heart disease by altering lipid metabolism. To investigate this, we examined the role of TLR4 in modulating cardiac FA metabolism and the pathogenesis of diabetic heart disease in nonobese diabetic (NOD) mice, a good animal model for the study of spontaneous autoimmune diabetes. Our data demonstrate that TLR4 deficiency reduced lipid accumulation in intact hearts in the early stages after the onset of type 1 diabetes, with a reduction in LPL (involved in the uptake of FAs) and an increase in phosphorylation of AMP-activated protein kinase (AMPK; involved in the metabolism of FAs). Our data indicate a potentially important role for TLR4 in the development of diabetic cardiomyopathy and dysfunction.
METHODS
Experimental animals.
NOD mice develop spontaneous type 1 diabetes from the age of 12 wk onward, with up to 85% of female mice developing the disease by the age of 30 wk. The autoimmune disease in these genetically susceptible mice has features that are very similar to human type 1 diabetes, having both genetic components and environmental modifiers leading to the infiltration and destruction of pancreatic insulin-producing β-cells by autoreactive T lymphocytes. NOD.TLR4-deficient mice [TLR4 knockout (TLR4KO) mice; derived from the C57BL/10ScN strain, which has a natural mutation in TLR4] backcrossed to NOD mice (33) together with wild-type (WT) NOD/Caj mice were used in these experiments. Mice were housed in specific pathogen-free conditions. Use of the animals was in accordance with National Institutes of Health (NIH) principles of laboratory animal care, and this study was approved by the Institutional Animal Care and Use Committee of Yale University.
Genetic typing of TLR4KO mice.
Mice were typed for the genetic mutation using tail biopsy genomic DNA and standard PCR conditions with 2 mM Mg2+ using the following primer pairs: TLR4KO mice, forward 5′-GTTCTCCTCAGGTCCAAGTTGCCGTTTCTTG-3′ and reverse 5′-TTCTTCAAGCTTACTAACAATTCTGTTGCTTGT-3′; and WT mice, forward 5′-GGAGAGAGAGAAAGCATGATATG-3′ and reverse 5′-CAGGGTAAACTGAAAAGCTGCTG-3′.
Diagnosis of diabetes.
Mice were screened for diabetes by weekly testing for urine glycosuria (Diastix, Bayer), and diabetes was then confirmed by a blood glucose measurement of >13.9 mmol/l (250 mg/dl). Diabetic mice were treated daily with 1–2 units of Humulin N insulin subcutaneously (Lilly) given once a day to maintain the mice in a hyperglycemic state but in generally reasonable good health.
Quantitative PCR.
Total RNA were isolated from mouse heart cells using the QIAGEN RNeasy kit and reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). TLR4 cDNA was quantified by SYBR green PCR and normalized to GAPDH using the following primers: mouse TLR4, forward 5′-CAGCAAAGTCCCTGATGACA-3′ and reverse 5′-CCTGGGGAAAAACTCTGGAT-3′; and GAPDH, forward 5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Echocardiograms.
Mice were lightly anesthetized. Echocardiograms were conducted using a 15-MHz transducer and a Sonos 7500 console. Two-dimensional images were obtained using both a short-axis plane at the level of the papillary muscles and M-mode. Data were analyzed with 7500 analysis software as previously described (15).
Heart isolation and perfusion.
Mice were injected with heparin sulfate (100 units) 10 min before terminal anesthesia and perfused (4 ml/min) in Langendorff mode with modified Krebs-Henseleit buffer containing glucose (7 mmol/l), oleate (0.4 mmol/l), BSA (1%), and a low-fasting concentration of insulin (10 μU/ml) at 37°C, as previously described. A fluid-filled latex balloon connected to a solid-state pressure transducer (Millar Instruments) was inserted into the left ventricle (LV) through a left atriotomy to measure pressure. LV developed pressure (LVDP), the first derivative of LVDP (dP/dt), LV end-diastolic pressure, and heart rate were recorded using a digital-acquisition system (AD Instruments) at a balloon volume that resulted in a baseline LV end-diastolic pressure of 5 mmHg. Recordings ranging from 5 to 30 min were made (26).
Quantification of TGs and nonesterified FAs in serum.
Sera from the mice in this study were obtained at the time of termination of the experiments and analyzed in the metabolic core of the Yale Mouse Metabolic Phenotyping Center. Serum nonesterified FAs (NEFAs) were measured by the acyl-CoA synthetase-acyl-CoA oxidase enzymatic colorimetric method (Wako Pure Chemical Industries, Richmond, VA) on a Roche COBAS Mira automated chemistry analyzer (Roche Diagnostics, Indianapolis, IN). Serum TGs were measured by colorimetric enzymatic detection of glycerol after microbial lipase treatment on a Roche COBAS Mira automated chemistry analyzer (Roche Diagnostics).
Oil red O staining.
Stock solutions were prepared by adding 1 g oil red O (Fluka, St. Louis, MO) into 30 ml 2-propanol (Fisher Scientific, Pittsburgh, PA). The stock solution was then diluted with distilled water at a ratio of 6:4. Frozen tissue was embedded in OCT (Fisher Scientific), and sections of 5–8 μm were cut and stained with oil red O solution for 10 min followed by a rinse in tap water for 10 min. Sections were also stained with hematoxylin (Sigma-Aldrich, St. Louis, MO) for 1 min followed by a rinse in tap water for 10 min before being sealed (19). The area positive for lipid staining was quantified by ImageJ software (NIH).
Immunofluorescence microscopy.
Mouse hearts were fixed in paraformaldehyde lysine periodate buffer overnight followed by sucrose infusion at 4°C, embedded in OCT, and frozen. Sections of 6 μm were cut, dried at room temperature for 20 min, and subjected to proteinase-K treatment at a concentration of 3 μg/ml in PBS at room temperature for 10 min followed by three 5-min washes in PBS. Samples were blocked with 10% BSA in PBS for 1 h at 4°C. After two washes with PBS, samples were incubated in either mixed rabbit and mouse IgG or mixed rabbit anti-mouse CD36 and anti-mouse major histocompatibility (MHC) class I H-2Db antibody for 16 h at 4°C. After three washes with PBS, samples were incubated with Alexa fluor 488-conjugated goat anti-rabbit antibody (Invitrogen, Carlsbad, CA) and Alexa fluor 546-conjugated goat anti-mouse antibody (Invitrogen) at 1:400 dilutions for 1 h at 4°C. After three washes with PBS, samples were stained with 4',6-diamidino-2-phenylindole (Fluka) at a concentration of 300 nM. Images were acquired using an Axiovert 200M fluorescence microscope (magnification: ×20). The fluorescence intensity, which compared anti-CD36 with MHC class I staining (expressed on all cells), was quantified using ImageJ software.
Cell culture.
The H9C2 myocardial cell line was obtained from the American Type Culture Collection and cultured in petri dishes in DMEM with 10% FCS, 2 mM glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C and 5% CO2. Cells were harvested by trypsinization with 0.1% trypsin-EDTA before being used (29). All reagents used for cell culture were purchased from Invitrogen.
Small interfering RNA transfection.
A TLR4 small interfering (si)RNA kit (Invitrogen) was used to knockdown TLR4 gene expression when H9C2 cells were 60–70% confluent in medium without antibiotics. Nonspecific scrambled siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a control. siRNA was mixed with lipofectamine transfection reagent (Invitrogen) in serum-free medium for 20 min at room temperature. The siRNA and lipofectamine mixture was then incubated with H9C2 cells at 37°C for 24 h in serum-free medium. After this, cells were either washed with cold PBS and lysed with RIPA buffer (Santa Cruz Biotechnology) for Western blot assays to measure TLR4 expression or fixed in 90% alcohol for 10 min for histological experiments.
Oleate stimulation.
Oleate (Sigma-Aldrich) stock solution was prepared in PBS with 20% BSA. This solution was diluted to 0.4 mM oleate solution, and H9C2 cells were incubated for 12 h before Western blot analysis or oil red O staining (1).
Western blot analysis.
Western blot analysis was carried out as previously described (13). Perfused heart tissue was snap frozen in liquid nitrogen, and 30 mg of heart tissue were homogenized with RIPA buffer. The supernatant was collected for protein quantification with a Bradford protein assay (Bio-Rad Laboratories) after centrifugation at 13,000 rpm. Proteins were transferred onto a Nylon membrane after electrophoresis and blocked with 5% skimmed milk in Tris-buffered saline solution containing 0.1% Tween 20. Membranes were incubated with rabbit anti-TLR4 (Sigma-Aldrich), anti-myeloid differentiation primary response gene (88) (MyD88; Millipore, Billerica, MA), anti-TRIF (Imgenex, San Diego, CA), phosphorylated (p-)p38 MAPK (New England Biolabs, Beverly, MA), anti-AMPK (Cell Signaling), anti-p-JNK (Promega), and LPL (Santa Cruz Biotechnology) antibodies. Goat anti-rabbit horseradish peroxide-conjugated antibody (Abcam, Cambridge, MA) was used as a secondary antibody, and blots were developed with Amersham ECL Western Blotting Detection Reagents (GE Healthcare, Piscataway, NJ). H9C2 cells were washed three times with cold PBS and homogenized using the same protocol as for preparation of the heart tissue for Western blot analysis (36).
Statistical analysis.
GraphPad Prism software was used for statistical analysis. All values are shown as means ± SD. One-way ANOVA followed by the least significant difference calculation or Student's t-test was used to analyze differences between groups. P values of <0.05 were considered to be statistically significant.
RESULTS
Type 1 diabetes develops in NOD and NOD.TLR4-deficient mice.
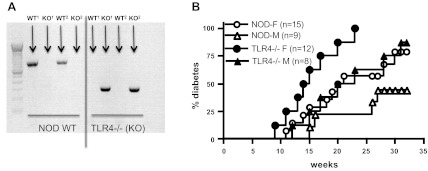
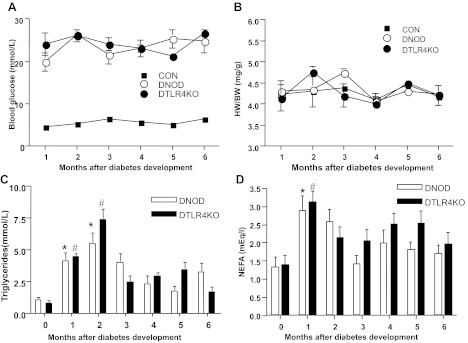
NOD.TLR4-deficient mice (33) were derived from the C57BL/10ScN strain, which has been shown to be highly resistant to entotoxin due to TLR4 deficiency (21) related to a null mutation in TLR4, as shown by the mouse genotyping in Fig. 1A. NOD.TLR4-deficient mice developed a higher incidence of diabetes compared with WT NOD/Caj mice (Fig. 1B). This was likely to be due to environmental factors as a similar strain of NOD.TLR4-deficient mice did not show a significant increase in the rate of development or overall increase in the incidence of diabetes in a different animal housing facility (33). However, once the mice were diabetic, blood glucose levels in the two groups of diabetic mice were maintained at a similar level, as shown in Fig. 2A. There were no significant differences in the ratio of heart weight-to-body weight between diabetic NOD (DNOD) and diabetic NOD.TLR4-deficient (DTLR4KO) mice (Fig. 2B). Hyperlipidemia was induced in the early stages of diabetes in both DNOD and DTLR4KO groups. Our results demonstrated a three- to fivefold increase in the concentration of TGs in the first 2 mo after the diagnosis of diabetes in both DNOD and DTLR4KO mice compared with age-matched nondiabetic control mice (Fig. 2C). TG levels peaked in the second month after the onset of diabetes, whereas NEFA levels were highest in the first month after the development of diabetes. However, there were no significant differences in TG (Fig. 2C) or NEFA (Fig. 2D) concentrations between DNOD and DTLR4KO mice.
Fig. 1.
Genotyping of nonobese diabetic (NOD) Toll-like receptor (TLR)4-deficient (TLR4−/−; NOD.TLR4-deficient) mice and the development of diabetes. A: genotyping of NOD.TLR4-deficient mice shown using wild-type (WT) primers on the left and primers for the TLR4 knockout (TLR4KO) mutation on the right. Two WT mice (WT1 and WT2) and two NOD.TLR4-deficient mice (KO1 and KO2) are shown. B: development of diabetes in WT NOD mice compared with NOD.TLR4-deficient mice. F, female; M, male.
Fig. 2.
Blood glucose concentrations, heart weight-to-body weight ratios, and triglyceride (TG) and nonesterified fatty acid (NEFA) levels in the serum of WT NOD mice compared with NOD.TLR4-deficient mice. A: the concentration of blood glucose remained above 16.5 mmol/l at all times after the diagnosis of diabetes in mice. Blood glucose in nondiabetic mice was <14 mmol/l. B: heart weight-to-body weight ratio measured in control nondiabetic (CON), diabetic NOD (DNOD), and diabetic TLR4KO (DTLR4KO) mice at different time points after the development of diabetes. C: serum TG concentrations in DNOD and DTLR4KO mice at different time points after the diagnosis of diabetes. TG concentrations were significantly higher in both DNOD (*P < 0.05) and DTLR4KO (#P < 0.05) mice in the first 2 mo after the diagnosis of diabetes compared with newly diagnosed mice (0 mo). D: serum NEFA concentrations in DNOD and DTLR4KO mice at different time points. NEFA concentrations were significantly higher in both DNOD (*P < 0.05) and DTLR4KO (#P < 0.05) mice in the first month after the diagnosis of diabetes compared with newly diagnosed mice (n = 6). Diabetic mice were 12–14 wk of age at the time of diagnosis, and control mice were age matched.
TLR4 regulates cardiac TG augmentation after early but not long-term diabetes.
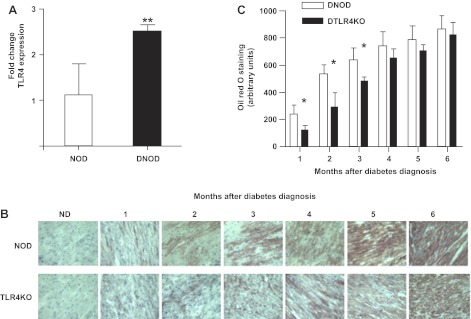
TLR4 is upregulated in cardiomyocytes after the onset of diabetes in NOD mice (Fig. 3A). Thus, to test whether TLR4 is involved in the toxic lipid effects seen in diabetes, we assessed lipid accumulation in the hearts of DNOD and DTLR4KO mice with oil red O staining (Fig. 3B). TG accumulation in both DNOD and DTLR4KO hearts was considerably increased after the onset of diabetes in a time-dependent manner. Despite similar levels of lipid in the circulation, there was higher lipid deposition in DNOD hearts compared with DTLR4KO hearts in the first 3 mo after the development of diabetes. This difference was not statistically significant at later time points (Fig. 3C). Our data suggest that TLR4 may regulate cardiac FA metabolism in early diabetes.
Fig. 3.
TLR4 is increased and lipid accumulates in the cardiac muscle of diabetic mice. A: quantitative PCR was carried out in age-matched nondiabetic (ND) and recent-onset (within 3 wk) DNOD mice (16–18 wk of age) for the expression of TLR4 in cardiomyocytes. **P < 0.05. B: lipid accumulation in the ventricular muscle of diabetic mice over time after the diagnosis of diabetes, as shown by oil red O staining (brown). C: lipid staining of heart muscle shown in the frozen tissue sections in A was quantified, and the results are shown in arbitrary units. *P < 0.05, DNOD mice vs. DTLR4KO mice in the first 3 mo after the diagnosis of diabetes. Diabetic mice were 12–14 wk of age at the time of diagnosis, and control ND mice were age matched.
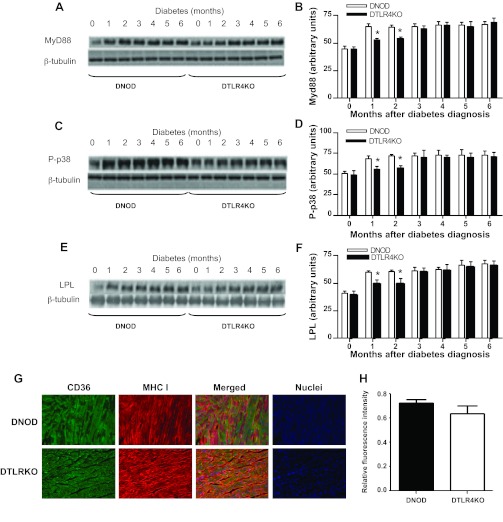
TLR4 deficiency is associated with lower levels of MyD88, p-p38, and LPL in the heart after diabetes.
LPL, synthesized by cardiomyocytes, is a major modulator of lipid metabolism in the heart (23). The phosphorylation and activation of p38 increase LPL activity in cardiomyocytes (12). TLR4 modulates MAPK (p38) activation through interactions with MyD88 and TRIF (34). Thus, we tested whether TLR4 regulates cardiac lipid accumulation through p38 and LPL. We measured MyD88 and TRIF in both WT and TLR4KO hearts and found that there was decreased protein expression of MyD88 in TLR4-deficient hearts after the onset of diabetes compared with WT mice (Fig. 4, A and B). However, there was no difference in the expression of TRIF between these two groups (data not shown). Concordant with the results seen for lipid accumulation in cardiac muscle (Fig. 3), p-p38 (Fig. 4, C and D) and cardiac LPL (Fig. 4, E and F) were significantly lower in DTLR4KO mice compared with DNOD mice in the first 2 mo after the development of diabetes, but the difference was not significant at later time points. To further test parameters associated with lipid transport into tissues, we also examined CD36, which acts as a FA translocase and also influences the uptake of free FAs. However, unlike the changes seen with LPL, although there was a suggestion that CD36 expression may be reduced in DTLR4KO heart tissue, there was no significant difference in CD36 expression between DTLR4KO and DNOD mice (Fig. 4, G and H).
Fig. 4.
Expression of myeloid differentiation primary response gene (88) (MyD88), phosphorylated (p-)p38 MAPK, and lipoprotein lipase (LPL) in the ventricular muscle of DNOD mice compared with DTLR4KO mice. A: Western blot analysis of MyD88 expression in ventricular muscle samples taken at different time points after the diagnosis of diabetes. B: densitometry readings comparing the samples shown in A, normalized to β-tubulin, from three separate experiments. C: Western blot analysis for p-p38 expression in ventricular muscle samples taken at different times after the diagnosis of diabetes. D: densitometry readings of the results shown in C, normalized to β-tubulin, from three separate experiments. E: Western blot analysis for LPL expression in ventricular muscle samples taken at different times after the diagnosis of diabetes. F: densitometry readings of the results shown in E, normalized to β-tubulin, from three separate experiments. *P < 0.05, DNOD mice vs. DTLR4KO mice. G: immunofluorescence microscopy of CD36 and major histocompatibility class I (MHC I) in ventricular muscle sections from DNOD (top) and DTLR4KO (bottom) mice within 2 mo after the onset of diabetes. Sections were stained with anti-CD36-Alexa fluor 488 (green; left) and anti-MHC I H-2Db-Alexa fluor 546 (red; middle left). Merged images are shown on the middle right, and 4′,6-diamidino-2-phenylindole staining (nuclei) is shown on the right. Images were acquired using an Axiovert 200M fluorescence microscope (magnification: ×20) with staining of both CD36 and MHC I shown in representative tissue sections. H: CD36 green fluorescence intensity was normalized to MHC I red fluorescence intensity using ImageJ software. n = 3 mice/group. Student's t-test showed no significant differences between the groups (P = 0.087). Diabetic mice were 12–14 wk of age at the time of diagnosis of diabetes, and control mice were age matched.
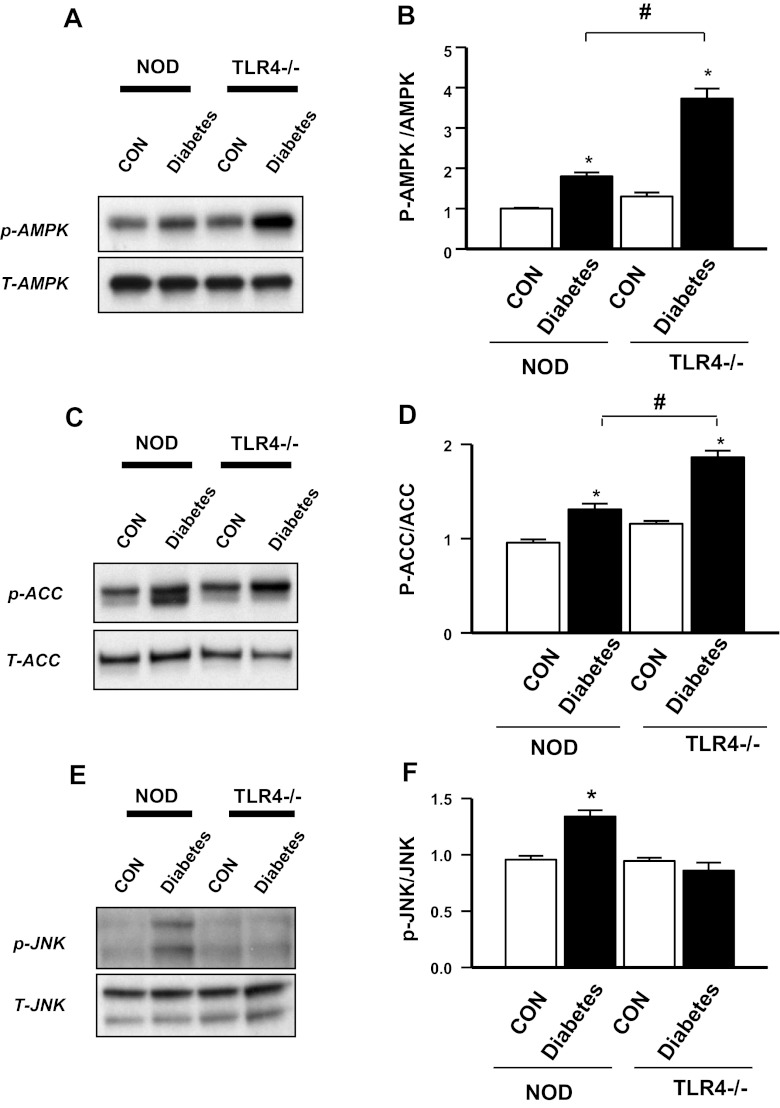
TLR4 deficiency increases AMPK and reduces JNK activation.
To investigate other mechanisms for the lower lipid accumulation in cardiomyocytes in the early stages after the onset of diabetes, we examined AMPK, as this protein has been recognized to regulate cardiac metabolism after diabetes. The data indicated that AMPK was significantly increased after the onset of diabetes in both NOD and NOD.TLR4-deficient mice. However, DTLR4KO hearts had a greater enhancement in AMPK phosphorylation (Fig. 5, A and B) and its downstream target acetyl-CoA carboxylase (Fig. 5, C and D) compared with DNOD hearts. Given the important role of AMPK in FA metabolism, we speculate that TLR4 deficiency may facilitate the upregulation of cardiac FA metabolism through AMPK activation after diabetes.
Fig. 5.
Expression of AMP-activated protein kinase (AMPK), acetyl-CoA carboxylase (ACC), and JNK in cardiomyocytes of DNOD mice compared with DTLR4KO mice. A: Western blot analysis for p-AMPK and total (T-)AMPK in ventricular muscle samples taken at 2–3 mo after the diagnosis of diabetes showing control nondiabetic mice compared with diabetic mice for both WT and TLR4KO strains. B: densitometry readings comparing p-AMPK with total AMPK from the samples shown in A. n = 3 mice/group. *P < 0.05, control mice vs. diabetic mice; #P < 0.05, DNOD mice vs. DTLR4KO mice. C: Western blot analysis for p-ACC and total ACC in ventricular muscle samples taken at 2–3 mo after the diagnosis of diabetes showing control nondiabetic mice compared with diabetic mice for both WT and TLR4KO strains. D: densitometry readings comparing p-ACC with total ACC from the samples shown in C. n = 3 mice/group. *P < 0.05, control mice vs. diabetic mice; #P < 0.05, DNOD mice vs. DTLR4KO mice. E: Western blot analysis for p-JNK and total JNK in ventricular muscle samples taken at 2–3 mo after the diagnosis of diabetes showing control nondiabetic mice compared with diabetic mice for both WT and TLR4KO strains. F: densitometry readings comparing p-JNK with total JNK from the samples shown in E. n = 3 mice/group. *P < 0.05, DNOD mice vs. other groups. Diabetic mice were 12–14 wk of age at the time of diagnosis of diabetes, and control mice were age matched.
In concert with this upregulation of AMPK, we also tested the activation of JNK as a downstream mediator of cardiomyocyte damage. As shown in Fig. 5, E and F, there was reduced p-JNK in DTLR4KO cardiomyocytes compared with DNOD cardiomyocytes.
TLR4 knockdown in H9C2 cardiomyocytes decreases FA-induced lipid accumulation.
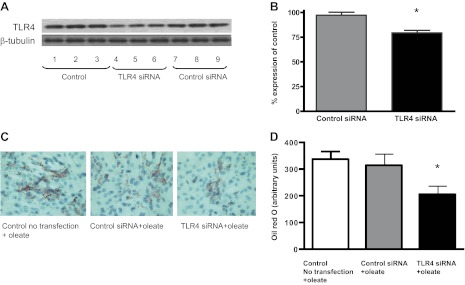
To confirm whether TLR4 regulates lipid accumulation in cardiomyocytes, TLR4 siRNA was used to knockdown TLR4 gene expression in H9C2 cells. After oleate treatment, TLR4-deficient cells showed a statistically significant reduction in lipid accumulation compared with control cells with oil red O staining, supporting the observation that TLR4 plays a role in facilitating FA uptake into cardiomyocytes (Fig. 6, A–D).
Fig. 6.
Effects of in vitro TLR4 knockdown on cellular lipid accumulation. A: small interfering (si)RNA was used to knockdown TLR4 expression in the H9C2 cell line. Western blots for TLR4 of triplicate untreated control cell samples, triplicate samples treated with TLR4 siRNA, and triplicate samples treated with control siRNA compared with β-tubulin are shown. B: densitometry of the Western blots shown in A, normalized to β-tubulin, shown as the percent expression of control. *P < 0.05 compared with samples treated with control siRNA. C: oil red O staining of untreated control H9C2 cells treated with oleate, H9C2 cells treated with control siRNA + oleate, and H9C2 cells treated with TLR4 siRNA + oleate. D: quantification of oil red O staining of the H9C2 cell samples shown in C. *P < 0.05, TLR4 siRNA + oleate-treated samples compared with control siRNA + oleate-treated samples.
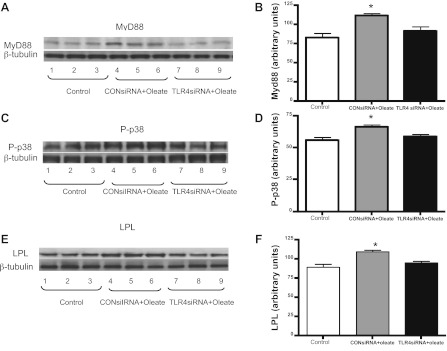
TLR4 knockdown alters MyD88 and p38 phosphorylation and LPL in H9C2 cells after FA treatment.
To further test whether TLR4-regulated FA uptake occurs through p-38 and LPL pathways, we examined the expression of Myd88 and LPL as well as the phosphorylation of p38 in H9C2 cells. Oleate treatment increased MyD88 expression, which was attenuated by the inhibition of TLR4 (Fig. 7, A and B). In addition, the expression of LPL and the phosphorylation of p38 were upregulated by FA, and TLR4 knockdown reversed this effect (Fig. 7, C–F). These data, combined with the data shown in Fig. 4, suggest that TLR4 plays a role in regulating p38 and LPL pathways, and this may contribute to FA accumulation in cells.
Fig. 7.
Effects of in vitro TLR4 knockdown on Myd88, p-p38 MAPK, and LPL expression in cardiac muscle. siRNA was used to knockdown TLR4 expression in the H9C2 cell line (Fig. 6). A and B: Western blots (A) and quantification of Western blot Myd88 expression (B) in triplicate untreated control cell samples, triplicate samples treated with control siRNA, and triplicate samples treated with TLR4 siRNA normalized to β-tubulin. C and D: Western blots (C) and quantification of Western blot p-p38 expression (D) in triplicate untreated control cell samples, triplicate samples treated with control siRNA, and triplicate samples treated with TLR4 siRNA normalized to β-tubulin. E and F: Western blots (E) and quantification of Western blot LPL expression (F) in triplicate untreated control cell samples, triplicate samples treated with control siRNA, and triplicate samples treated with TLR4 siRNA normalized to β-tubulin. *P < 0.05, control siRNA + oleate-treated samples compared with TLR4 siRNA + oleate-treated samples.
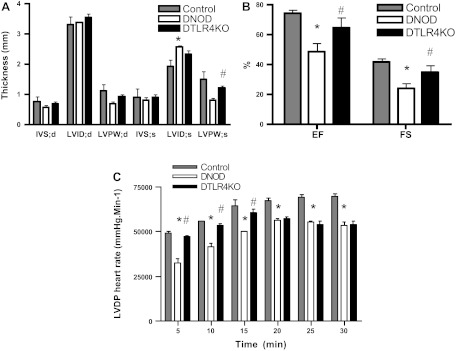
TLR4 regulates diabetic cardiac dysfunction.
In animal models of lipotoxicity, the accumulation of lipids within cardiomyocytes is associated with contractile dysfunction (28). To measure the functional changes associated with diabetes in cardiomyocytes, we performed echocardiograms in diabetic mice 2–3 mo after the development of diabetes. We also studied perfused hearts 3 mo after the development of diabetes. LV internal dimensions in systole, as a measure of systolic contraction, were significantly increased in DNOD mice compared with nondiabetic control mice (Fig. 8A). In addition, both ejection fraction (in %) and fractional shortening (in %) decreased significantly in DNOD mice compared with nondiabetic control mice (Fig. 8B). However, in DTLR4KO mice, the LV internal dimensions in systole was smaller and ejection fraction and fractional shortening were both greater compared with WT DNOD mice, indicating reduced impairment in the absence of TLR4, at an early time point after the onset of diabetes. When Langendorff-perfused heart preparations from diabetic mice were examined, we found that the rate-pressure product (LVDP × heart rate) was decreased in hearts from both DNOD and DTLR4KO mice. This decrease was greater in WT DNOD mice at 5, 10, and 15 min compared with DTLR4KO mice (Fig. 8C).
Fig. 8.
Cardiac functional abnormalities were attenuated in DTLR4KO mice. A and B: echocardiograms were performed on nondiabetic control (n = 3), DNOD (n = 6), and DTLR4KO (n = 7) mice 2–3 mo after the diagnosis of diabetes. A: interventricular septum at diastole (IVS:d), interventricular septum at systole (IVS:s), left ventricular (LV) posterior wall at diastole (LVPW:d), LV posterior wall at systole (LVPW:s), LV internal dimension at diastole (LVID:d), and LV internal dimension at systole (LVID:s). B: ejection fraction (EF; in %) and fractional shortening (FS; in %). C: hearts were isolated and perfused for 30 min, and LV developed pressure (LVDP) multiplied by heart rate (mmHg·beats·min−1) was measured. *P < 0.05, DNOD mice vs. control mice; #P < 0.05, DTLR4KO mice vs. DNOD mice. Diabetic mice were 12–14 wk of age at the time of diagnosis of diabetes, and control mice were age matched at the time of measurement.
DISCUSSION
Diabetic heart disease is not only a consequence of vascular abnormalities related to atherosclerosis, but its development is also linked to metabolic dysfunction in the heart. TLR4 is expressed in the heart (4) and is linked to atherosclerosis (3) and ischemia-induced cardiac dysfunction (35). It has been reported to play a key role in metabolic dysfunction and heart disease (17). Thus, our study aimed to investigate the role of TLR4 in regulating cardiac metabolism, especially FA metabolism and heart disease after the development of autoimmune diabetes in NOD mice. Both WT NOD and NOD.TLR4-deficient mice had considerably increased plasma TG levels after the onset of diabetes. However, compared with WT NOD mouse hearts, NOD.TLR4-deficient mouse hearts had lower TG accumulation in the early stage of diabetes. This reduced fat accumulation was associated with reductions of MyD88, p-p38 MAPK, LPL, and p-JNK. Conversely, we found increased activation of the AMPK pathway. Oleic acid treatment in H9C2 cardiomyocytes also led to cellular lipid accumulation, which was attenuated by knockdown of TLR4 using TLR4 siRNA. The induced TLR4 deficiency in H9C2 cells decreased FA-induced augmentation of MyD88, p-p38, and LPL, suggesting that TLR4 may modulate FA-induced lipid metabolism in cardiomyocytes. In addition, although cardiac function was impaired in both WT NOD and NOD.TLR4-deficient animals after the development of diabetes compared with DNOD mice, this deficit was less in DTLR4KO mice in the early stages of diabetes. Thus, we conclude that TLR4 plays a hitherto unreported role in regulating cardiac metabolism and heart disease in the early stages after the onset of type 1 diabetes.
In the present study, although lipid levels rose in all the diabetic mice, NOD.TLR4-deficient hearts demonstrated an initial lower level of lipid accumulation after the development of diabetes compared with hearts from WT NOD mice, suggesting that TLR4 may play a role in lipid uptake and metabolism in the heart after acute glucose stress. We examined a number of key proteins involved in the uptake of FAs and metabolism in cardiomyocytes. LPL is central to the metabolism of TG-rich lipoproteins and is a major enzyme in the regulation of the FA supply to cardiac muscle for energy generation. Cardiomyocytes synthesize LPL, which is transported to the cardiomyocyte cell surface onto heparan sulfate proteoglycan-binding sites and ultimately to heparan sulfate proteoglycan sites on the capillary endothelium, where TG-rich lipoproteins are hydrolyzed (32). Many factors, including glucocorticoids (25), diabetes, and insulin resistance (22), regulate LPL protein expression and activity (12). Here, TLR4 deficiency was associated with a lower level of LPL in the early stages of diabetes with the highest levels of plasma FAs and TGs, suggesting that the absence of TLR4 may initially limit FA uptake in part through inhibition of LPL in the heart. TLR4 signaling activates MAPK family members, including p38 and JNK, through its downstream protein MyD88 (20), and p38 MAPK has been shown to be an important means by which LPL is transported to the cardiomyocyte surface (31). TLR4 deficiency, as might be expected, led to reductions of MyD88 protein, p-p38, and p-JNK. The decrease in p-p38 MAPK together with LPL in our DTLR4KO mice suggests that signaling through TLR4 contributes to the regulation of LPL synthesis and translocation into its functional position, the endothelial surface, contributing to the accumulation of lipids in cardiac muscle. As JNK activation has been implicated in cardiac ischemic damage (24), the reduction in p-JNK may also contribute to the delay in the development of cardiac dysfunction. A recent study (11) has indicated that saturated FAs are TLR4 ligands that can modulate insulin resistance through the augmentation of ceramide generation, although there is no previous evidence to suggest that unsaturated FAs are TLR4 ligands. However, whether the high levels of NEFAs after the onset of diabetes contain FA ligands of TLR4 that regulate LPL in the heart was an unanswered question. To examine this, we exposed H9C2 cardiomyocytes to high levels of oleate treatment, as one component of raised lipids in the serum of diabetic mice. We showed that oleate treatment increased p-p38 MAPK and augmented LPL protein. This effect of oleate was reduced after knockdown of TLR4, which reinforced the observation that TLR4 may be important for the increased lipid deposition in these cells.
Although there is an effect on LPL that increases uptake of lipids in the heart, this is not the only pathway of transport of lipids into cardiomyocytes. However, we did not observe a clear difference in the expression of CD36, which also has an important role as a FA translocase (7). CD36 interacts with TLR4 and TLR6 to form a heterotrimeric complex through which atherogenic lipids may cause damage (30). In our experimental system, although TLR4 is deficient, TLR6 is still intact, and this may contribute to the fact that we did not observe clear differences in the expression of CD36.
In addition to changes in the uptake of FAs, TLR4 may regulate the FA utilization pathway. AMPK has been recognized to play a key role in modulating FA metabolism after diabetes. A previous report (35) has indicated that TLR4 is negatively related to AMPK activation in the heart after ischemia-reperfusion. In the present study, we found that, although diabetes increased AMPK phosphorylation in both DNOD and DTLR4KO hearts, NOD.TLR4KO-deficient hearts demonstrated a higher level of AMPK and acetyl-CoA carboxylase phosphorylation compared with NOD hearts after early diabetes. Thus, we believe that TLR4 deficiency not only reduces FA delivery into cells after diabetes but also increases FA utilization. Both of these mechanisms would contribute to the decreased FA accumulation and lipotoxicity in cardiomyocytes and prevent cells from the development of cardiomyopathy in the early stages. It is not clear why these protective changes are only seen in the early stages, but we speculate that this may be related to later saturation of the receptors, and, thus, the protective effects are no longer seen.
Finally, in parallel with the noted alterations in lipid metabolism, the myocardial dysfunction seen in DTLR4KO mice was attenuated up to 3 mo after the diagnosis of diabetes. The abnormalities of systolic dysfunction were greater in WT DNOD mice compared with those found in DTLR4KO mice, as measured by the reduced ejection fraction, fractional shortening, and LVDP and increased LV internal dimensions in systole. As expected, there was no myocardial dysfunction in nondiabetic mice that were TLR4 sufficient or deficient. Although there were no measurable differences in serum lipid levels between TLR4-sufficient or -deficient DNOD mice, the myocardial dysfunction seen in our study indicates that the raised lipid levels have different consequences and may be disposed of differently in the presence or absence of TLR4. It is not clear, at this stage, whether lipids can directly alter myocardial function through TLR4.
In conclusion, we have demonstrated that, in the presence of TLR4, DNOD mice develop more accelerated cardiac abnormalities both in terms of earlier fat infiltration of cardiac muscle as well as earlier manifestation of cardiac dysfunction compared with the delayed onset seen in DTLR4KO mice. This implies that signaling pathways through TLR4 contribute to the damage inflicted on cardiac muscle in diabetes. We postulate that modulation of TLR4 may help to reduce the severity of cardiac complications in diabetes.
GRANTS
This work was funded by Innovative Partnership Grant 19-2006-1075 from the Juvenile Diabetes Research Foundation (to L. Wen and F. S. Wong) and was supported by the Diabetes Animal Core of Yale Diabetes Endocrine Research Center through National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-06-014 (to L. Wen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.D., D.Q., L.Y., Y.H., X.X., and N.T. performed experiments; B.D., D.Q., L.Y., Y.H., X.X., N.T., L.W., and F.S.W. analyzed data; B.D., D.Q., Y.H., L.W., and F.S.W. interpreted results of experiments; B.D. and F.S.W. prepared figures; B.D., D.Q., L.Y., Y.H., X.X., N.T., L.W., and F.S.W. approved final version of manuscript; D.Q., L.W., and F.S.W. conception and design of research; D.Q., L.W., and F.S.W. edited and revised manuscript; F.S.W. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Jian Peng for technical assistance. The authors thank Gary Cline at the Yale Analytic Core for lipid measurements and Alexander Chervonsky of the University of Chicago for providing the initial TLR4KO breeders and primer sequences for genotyping.
REFERENCES
- 1. Anderson LG, Carroll R, Ewart HS, Acharya A, Severson DL. Fatty acids reduce heparin-releasable LPL activity in cultured cardiomyocytes from rat heart. Am J Physiol Endocrinol Metab 273: E759–E767, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 121: 748–757, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bjorkbacka H. Multiple roles of Toll-like receptor signaling in atherosclerosis. Curr Opin Lipidol 17: 527–533, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-κB dependent inflammatory response. Cardiovasc Res 72: 384–393, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech 2: 454–466, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Chavez JA, Summers SA. Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim Biophys Acta 1801: 252–265, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 275: 32523–32529, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57: 3090–3098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dorman JS, Laporte RE, Kuller LH, Cruickshanks KJ, Orchard TJ, Wagener DK, et al. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes 33: 271–276, 1984 [DOI] [PubMed] [Google Scholar]
- 10. Green A, Hougaard P. Epidemiological studies of diabetes mellitus in Denmark: 5. Mortality and causes of death among insulin-treated diabetic patients. Diabetologia 26: 190–194, 1984 [DOI] [PubMed] [Google Scholar]
- 11. Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 121: 1858–1870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim MS, Kewalramani G, Puthanveetil P, Lee V, Kumar U, An D, et al. Acute diabetes moderates trafficking of cardiac lipoprotein lipase through p38 mitogen-activated protein kinase-dependent actin cytoskeleton organization. Diabetes 57: 64–76, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem 278: 39422–39427, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 46: 760–765, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Lei L, Mason S, Liu D, Huang Y, Marks C, Hickey R, et al. Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol Cell Biol 28: 3790–3803, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA 101: 10679–10684, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem 278: 1561–1568, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353: 2643–2653, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nunnari JJ, Zand T, Joris I, Majno G. Quantitation of oil red O staining of the aorta in hypercholesterolemic rats. Exp Mol Pathol 51: 1–8, 1989 [DOI] [PubMed] [Google Scholar]
- 20. O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7: 353–364, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Pulinilkunnil T, Qi D, Ghosh S, Cheung C, Yip P, Varghese J, et al. Circulating triglyceride lipolysis facilitates lipoprotein lipase translocation from cardiomyocyte to myocardial endothelial lining. Cardiovasc Res 59: 788–797, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Pulinilkunnil T, Rodrigues B. Cardiac lipoprotein lipase: metabolic basis for diabetic heart disease. Cardiovasc Res 69: 329–340, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Qi D, Hu X, Wu X, Merk M, Leng L, Bucala R, et al. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J Clin Invest 119: 3807–3816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qi D, Pulinilkunnil T, An D, Ghosh S, Abrahani A, Pospisilik JA, et al. Single-dose dexamethasone induces whole-body insulin resistance and alters both cardiac fatty acid and carbohydrate metabolism. Diabetes 53: 1790–1797, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Rodrigues B, Cam MC, Jian K, Lim F, Sambandam N, Shepherd G. Differential effects of streptozotocin-induced diabetes on cardiac lipoprotein lipase activity. Diabetes 46: 1346–1353, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Schilling J, Lai L, Sambandam N, Dey CE, Leone TC, Kelly DP. Toll-like receptor-mediated inflammatory signaling reprograms cardiac energy metabolism by repressing peroxisome proliferator-activated receptor γ coactivator-1 signaling. Circ Heart Fail 4: 474–482, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 18: 1692–1700, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Simunek T, Boer C, Bouwman RA, Vlasblom R, Versteilen AM, Sterba M, et al. SIH–a novel lipophilic iron chelator–protects H9c2 cardiomyoblasts from oxidative stress-induced mitochondrial injury and cell death. J Mol Cell Cardiol 39: 345–354, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11: 155–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277: 15107–15112, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 297: E271–E288, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455: 1109–1113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301: 640–643, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Zhao P, Wang J, He L, Ma H, Zhang X, Zhu X, Dolence EK, Ren J, Li J. Deficiency in TLR4 signal transduction ameliorates cardiac injury and cardiomyocyte contractile dysfunction during ischemia. J Cell Mol Med 13: 1513–1525, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zordoky BN, El-Kadi AO. H9c2 cell line is a valuable in vitro model to study the drug metabolizing enzymes in the heart. J Pharmacol Toxicol Methods 56: 317–322, 2007 [DOI] [PubMed] [Google Scholar]