Abstract
Vascular smooth muscle responsiveness to nitric oxide, as assessed by nitroglycerin-induced dilation (NID), is impaired in clinical cardiovascular disease, but its relation to adiposity is unknown. We determined the relation of NID to total and abdominal adiposity in healthy adults varying widely in adiposity. In 224 men and women [age, 18–79 years; body mass index (BMI), 16.4–42.2 kg/m2], we measured NID (brachial artery dilation to 0.4 mg sublingual nitroglycerin), total body adiposity [BMI and percent body fat (percent BF via dual-energy X-ray absorptiometry)], and indexes of abdominal adiposity [waist circumference (WC) and waist-to-hip ratio (WHR)]. In a subgroup (n = 74), we also measured total abdominal fat (TAF), abdominal visceral fat (AVF), and subcutaneous fat (ASF) using computed tomography. Based on multiple linear regression, NID was negatively related to BMI [part correlation coefficient (rpart) = −0.19, P = 0.004] and abdominal adiposity (WC, rpart = −0.22; WHR, rpart = −0.19; TAF, rpart = −0.36; AVF, rpart = −0.36; and ASF, rpart = −0.30; all P ≤ 0.009) independent of sex, but only tended to be related to total percent BF (rpart = −0.12, P = 0.07). In a subgroup of subjects with the highest compared with the lowest amount of AVF, NID was 35% lower (P = 0.003). Accounting for systolic blood pressure, HDL cholesterol, glucose, insulin resistance, adiponectin, and brachial artery diameter reduced or abolished some of the relations between NID and adiposity. In conclusion, NID is or tends to be negatively associated with measures of total adiposity (BMI and percent BF, respectively) but is consistently and more strongly negatively associated with abdominal adiposity. Adiposity may influence NID in part via other cardiovascular risk factors.
Keywords: abdominal visceral and subcutaneous fat, brachial artery, ultrasound
vascular smooth muscle function, measured as the dilation to an exogenous nitric oxide (NO) donating agent [e.g., nitroglycerin (NTG)] (18, 31), is impaired in patients with clinical disorders including coronary artery disease/atherosclerosis (18, 23), congestive heart failure (21), hypercholesterolemia (1, 33), microalbuminuria (10, 24), and type 1 (34) and type 2 (1, 9) diabetes and in smokers (1, 5). Increased adiposity and, in particular, abdominal adiposity are associated with increased risk for cardiovascular disease (29), but the underlying mechanisms are incompletely understood. It is possible that adiposity is associated with impaired responsiveness to NO in the absence of clinical disease or smoking, but this has not been established. Previous data on the relation between NTG-induced dilation and adiposity are limited and conflicting: some studies report negative (2, 38), positive (14), or no (3, 17) relation. These inconsistent findings are based on relatively small sample sizes with a limited age and/or adiposity range. In some cases, the results might have been influenced by the inclusion of patients with documented diabetes, hypertension, and coronary artery disease, thus making evaluation of the independent effect of obesity difficult. Moreover, previous studies did not measure total and abdominal fat content; instead, they were limited to the use of body mass index or waist and hip circumferences as indexes of adiposity.
Therefore, there is a clear need to determine whether increased total and abdominal adiposity are linked to impaired vascular smooth muscle function in a large cohort of both men and women free of clinical disease with a broad range of adiposity and age. Hence, in the current study we tested the hypothesis that NTG-induced dilation is inversely related to total (body mass index and percent body fat) and abdominal (waist circumference, waist-to-hip ratio, and total, visceral, and subcutaneous fat) adiposity in nonsmoking men and women without clinical disease varying widely in adiposity and age. We also sought to gain initial insight into mechanisms by which adiposity may influence NTG-induced dilation.
METHODS
Ethical Approval
The study conformed to the standards set by the Declaration of Helsinki. Procedures were approved by the Institutional Review Boards of the University of Colorado at Boulder, Texas A&M University, and Scott & White Health System. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Subjects
A total of 224 adults, 152 men and 72 women, of a wide range of age (18–79 years) and adiposity (body fat, 6.5–56.4%) were studied. All subjects were nonsmokers and were free of cardiovascular disease and other clinical disorders as assessed by medical history, physical examination, resting ECG, urinalysis, blood chemistries, and hematological evaluation. Men older than 40 years of age and women older than 50 years of age demonstrated normal ECG and blood pressure responses to incremental treadmill exercise (8) as described in Aerobic fitness and leisure-time physical activity. Premenopausal women were eumenorrheic, were not on hormonal contraceptives, and were studied during the early follicular phase. Postmenopausal women were not on hormone replacement therapy for at least 1 year before data collection.
Study Procedures
Weight and height.
Body weight was measured to the nearest 0.1 kg with an electronic scale (Tanita, Arlington Heights, IL). Subjects wore light clothing and were barefoot when weighed. Height was measured to the nearest millimeter using a stadiometer.
Resting blood pressure.
Resting blood pressure was measured over the brachial artery with a semiautomated device (Dinamap; GE, Salt Lake City, UT).
Adiposity measures.
Body mass index was determined as weight divided by height squared (kg/m2). Total body fat percentage was assessed with dual-energy X-ray absorptiometry (DPX-IQ; GE/Lunar, Salt Lake City, UT) as described previously (8). Two indexes of abdominal adiposity were used in all subjects: waist circumference and waist-to-hip ratio. With use of a nonstretchable tape, circumferences were measured in duplicate to the nearest millimeter with the subjects standing with their feet placed together. The waist measurement was performed at the narrowest part between the ribs and the iliac crest, whereas the hip measurement was performed at the widest part of the hips. In a subgroup of 74 subjects (54 men and 20 women), total, visceral, and subcutaneous fat were measured in the abdominal region (at the level of L4-L5) using a single slice computed tomography scan and the commercially available software Slice-O-Matic v4.3 (Tomovision, Magog, QC, Canada) (27).
Vascular smooth muscle responsiveness to NO donor.
All subjects completed a 12-h overnight fast and abstained from caffeine before measurements were taken. All measurements were performed on subjects in supine rest. Dilation to NTG was assessed using high-resolution ultrasonography as described previously (6, 28). ECG-gated ultrasound images of the brachial artery were acquired at baseline and for 10 min after sublingual NTG (0.4 mg) administration. Maximum brachial response to NTG was defined as the percent change in diameter from baseline, calculated by the maximum diameter minus baseline diameter divided by baseline diameter times 100. Digital image acquisition on a personal computer and brachial artery diameter analysis were performed using a commercially available wall-tracking software package (Vascular Analysis Tools 5.5.1; Medical Imaging Applications, LLC, Iowa City, IA).
Lipids, metabolism, and inflammation and oxidative stress markers.
Plasma lipids were analyzed using conventional assays as described previously (32). High-sensitivity C-reactive protein and fasting glucose were measured by enzymatic methods (Roche Diagnosis Systems, Indianapolis, IN). Plasma insulin was measured by radioimmunoassay (Diagnostic Systems Laboratory, Webster, TX). Insulin resistance was estimated by using the homeostasis model of insulin resistance [HOMA; HOMA = (fasting insulin μU/ml × fasting glucose mg/dl)/405]. Oxidized low-density lipoprotein was measured using ELISA (ALPCO Diagnostics, Salem, NH). Adiponectin was measured by radioimmunoassay (Linco Research).
Aerobic Fitness and Leisure-Time Physical Activity
Aerobic fitness was determined using maximal oxygen consumption (V̇o2 max) as described previously (8). Briefly, online computer-assisted open-circuit spirometry during incremental treadmill exercise was used. After a 6- to 10-min warm-up, subjects ran or walked at a comfortable speed that corresponded to 70% to 80% of their age-predicted maximal heart rate. The treadmill grade was increased 2.5% every 2 min until volitional exhaustion. Leisure-time physical activity was assessed using the Modifiable Activity Questionnaire (22).
Data Analysis
Statistical analyses were performed using SPSS (version 19.0). Statistical significance for all analyses was set at P < 0.05. To determine the relations of total and abdominal adiposity with NTG-induced dilation independent of sex, multiple linear regression analysis was performed. Separate regression models were used for each measure of adiposity: NTG-induced dilation was entered as the dependent variable, whereas a measure of adiposity and sex were entered as independent variables. Sex was included in the model to control for its effect. Part correlation coefficients derived from regression analysis were used to determine the association of adiposity with NTG-induced dilation, independent of sex. Residual analysis to test for the validity of the regression model assumptions was performed for all regression models.
To examine potential intermediary factors by which adiposity may be linked to NTG-induced dilation, bivariate Pearson product-moment correlations were performed first to identify factors that were significantly related with both adiposity and NTG-induced dilation. Each factor that met this requirement was entered in the regression models as an independent variable in addition to adiposity and sex to determine its influence on the relation between adiposity and NTG-induced dilation.
To further examine the relation between NTG-induced dilation and adiposity, we compared NTG-induced dilation in a subsample of subjects with the lowest and highest abdominal visceral fat. The subsample was chosen by selecting subjects in the lowest and highest quintiles of abdominal visceral fat to ensure the groups differed considerably. Group comparisons were examined using independent t-tests. To examine the influence of potential intermediary factors on the differences in NTG-induced dilation between the lowest and highest abdominal visceral adiposity groups, we used ANCOVA to co-vary for each potential factor.
RESULTS
Mean values and ranges for the main subject characteristics are presented in Table 1 and for the potential intermediary factors are presented in Table 2. Subjects varied widely in age, weight, total body and abdominal adiposity measures, and NTG-induced dilation.
Table 1.
Subject characteristics
| Means ± SE | Minimum-Maximum | |
|---|---|---|
| Sex (male/female) | 152/72 | |
| Age, years | 52.7 ± 1.1 | 18–79 |
| Weight, kg | 77.5 ± 1.0 | 39.8–130.7 |
| Body mass index, kg/m2 | 25.6 ± 0.3 | 16.4–42.2 |
| Body fat, % | 28.6 ± 0.7 | 6.5–56.4 |
| Waist circumference, cm | 87.1 ± 0.9 | 56.3–126.8 |
| Waist-to-hip ratio | 0.86 ± 0.01 | 0.66–1.12 |
| Abdominal fat, cm2 | ||
| Total | 426.2 ± 22.3 | 76.1–925.5 |
| Visceral | 129.4 ± 8.1 | 12.0–302.6 |
| Subcutaneous | 296.7 ± 17.3 | 64.1–717.5 |
| NTG-induced dilation, % | 23.9 ± 0.4 | 5.4–43.7 |
NTG, nitroglycerin.
Table 2.
Blood pressure, lipids, and other potential intermediary factors
| Means ± SE | Minimum-Maximum | |
|---|---|---|
| Blood pressure, mmHg | ||
| Systolic | 122 ± 1 | 88–178 |
| Diastolic | 73 ± 11 | 50–111 |
| Cholesterol, mg/dl | ||
| Total | 192 ± 2 | 107–306 |
| LDL | 116 ± 2 | 47–220 |
| HDL | 54 ± 1 | 24–115 |
| Triglycerides | 114 ± 4 | 35–566 |
| Fasting glucose, mg/dl | 91 ± 1 | 60–121 |
| Fasting insulin, μU/ml | 6.3 ± 0.4 | 1–33 |
| HOMA-IR | 1.4 ± 0.1 | 0.2–8.6 |
| Adiponectin, mg/μl | 12.8 ± 0.9 | 2.2–30.3 |
| Baseline diameter, mm | 3.8 ± 0.04 | 2.6–5.7 |
HOMA-IR, homeostasis assessment model for insulin resistance.
Relations of NTG-Induced Dilation and Adiposity, Independent of Sex
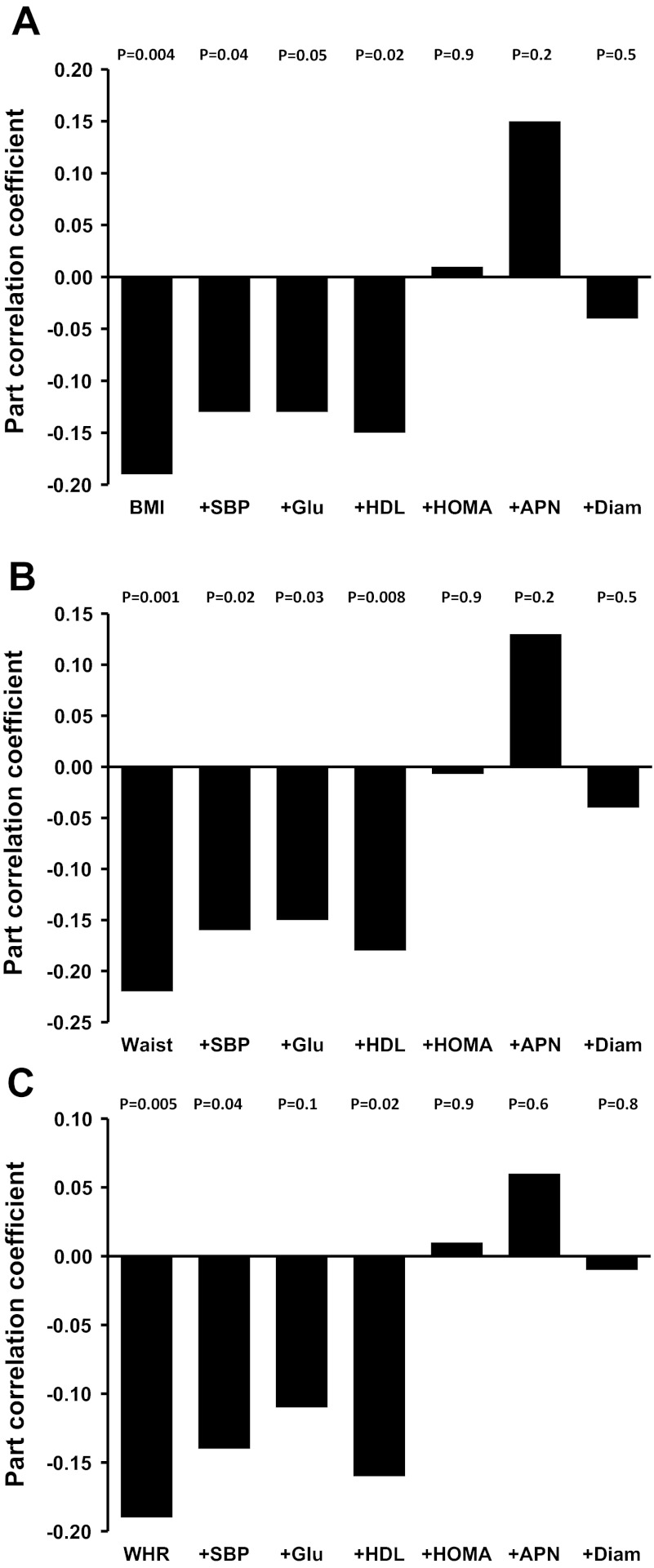
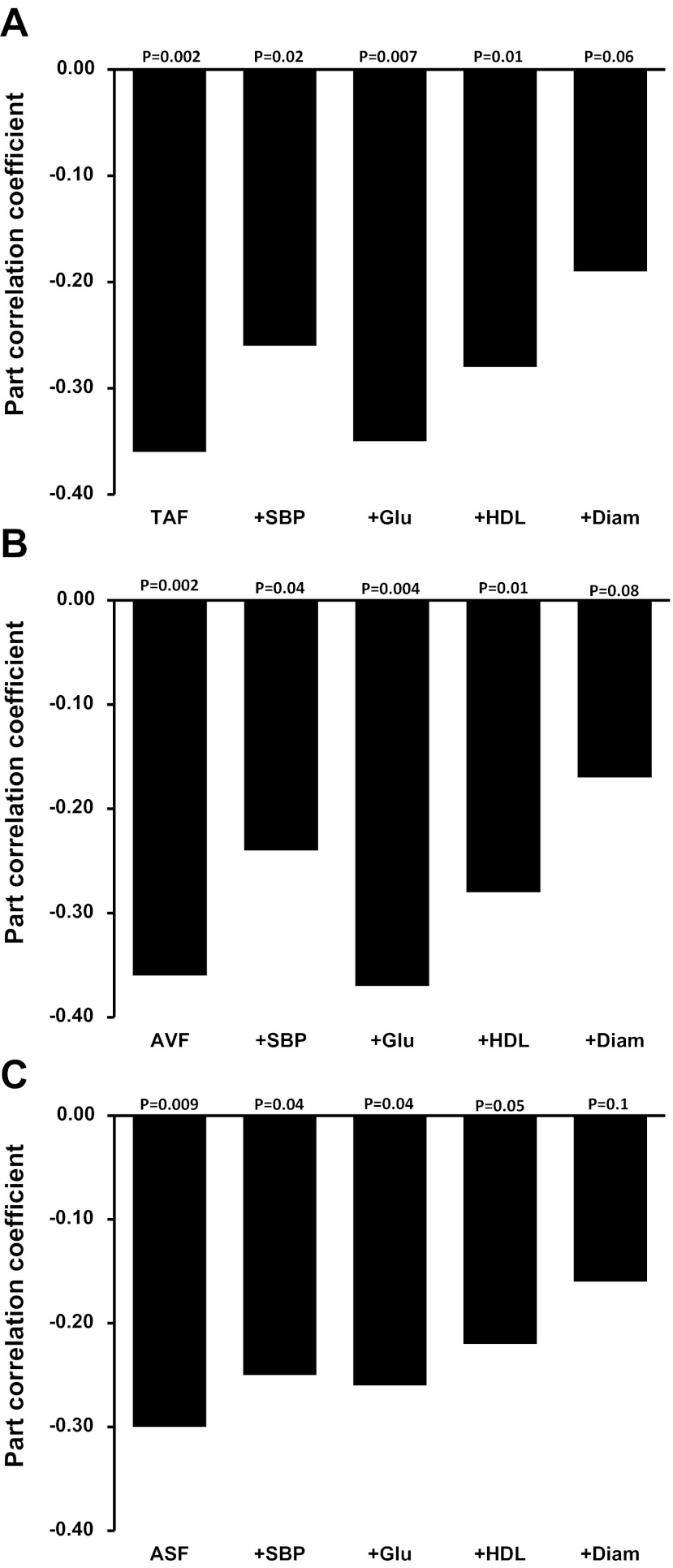
Age was not included as an independent variable to control for its effect because it did not correlate with NTG-induced dilation in our data (r = 0.04, P = 0.6). There were no significant interactions between sex and adiposity (P > 0.05), indicating that the relation between adiposity and NTG-induced dilation was not significantly different in men and women. In the entire group, NTG-induced dilation was negatively related to body mass index (P = 0.004; Fig. 1A), but only tended to be related to total percent body fat (rpart = −0.12, P = 0.07; data not shown). NTG-induced dilation was consistently negatively related with waist circumference (P = 0.001; Fig. 1B), waist-to-hip ratio (P = 0.005; Fig. 1C), total abdominal fat, and abdominal visceral and subcutaneous fat (P ≤ 0.009; Fig. 2, A–C), independent of sex.
Fig. 1.
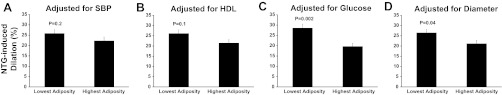
Part correlation coefficients showing the relation between body mass index (BMI; A) and indexes of abdominal adiposity [waist circumference (waist; B) and waist-to-hip ratio (WHR); C] and nitroglycerin-induced dilation (NTG)-induced dilation (expressed as percent dilation from baseline) adjusted for sex only (column at far left) and after additional adjustment for 1 of the following factors: systolic blood pressure (SBP), fasting glucose (Glu), HDL cholesterol (HDL), log homeostasis assessment model for insulin resistance (HOMA), adiponectin (APN), or brachial artery baseline diameter (Diam) (left to right).
Fig. 2.
Part correlation coefficients showing the relation between total abdominal fat (TAF; A) and abdominal visceral fat (AVF; B) and abdominal subcutaneous fat (ASF; C) and NTG-induced dilation (expressed as percent dilation from baseline) adjusted for sex only (column at far left) and after additional adjustment for 1 of the following factors: SBP, Glu, HDL, or Diam (left to right).
Comparisons Between Subjects with the Lowest and Highest Abdominal Visceral Fat
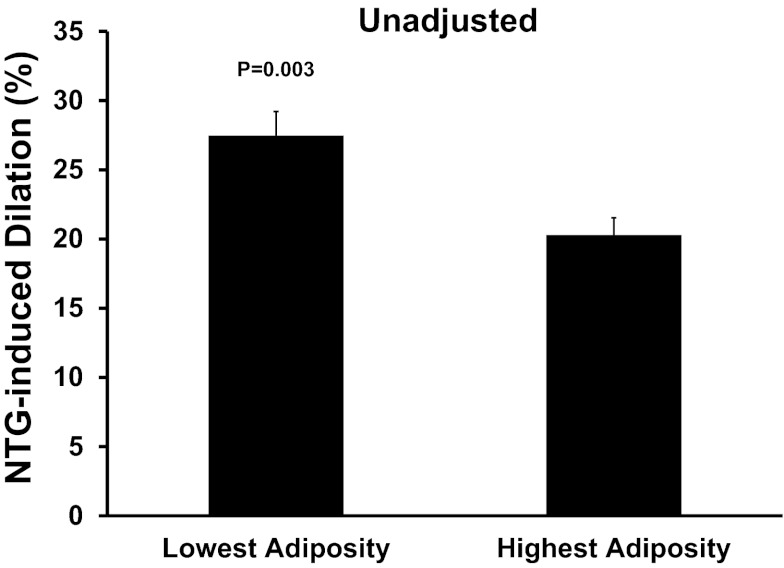
Mean values and ranges for the main characteristics of subjects with the lowest and highest abdominal visceral fat are presented in Table 3 and for potential intermediary factors are presented in Table 4. Consistent with the results of the regression analyses, NTG-induced dilation was 35% lower in subjects with the highest compared with lowest abdominal visceral fat (P = 0.003; Fig. 3).
Table 3.
Subject characteristics of a subsample of subjects with the lowest and highest abdominal visceral fat
| Lowest Adiposity | Highest Adiposity | P Values | |
|---|---|---|---|
| Sex (male/female) | 9/5 | 11/3 | 0.5 |
| Age, years | 48.1 ± 4.6 | 59.2 ± 2.3 | 0.04 |
| Weight, kg | 67.2 ± 2.8 | 102.9 ± 4.3 | <0.0001 |
| Body mass index, kg/m2 | 22.4 ± 0.7 | 32.6 ± 1.3 | <0.0001 |
| Body fat, % | 22.1 ± 3.0 | 39.6 ± 2.1 | <0.0001 |
| Waist circumference, cm | 76.4 ± 2.2 | 108.5 ± 2.7 | <0.0001 |
| Waist-to-hip ratio | 0.79 ± 0.02 | 0.95 ± 0.03 | <0.0001 |
| Abdominal fat, cm2 | |||
| Total | 205.3 ± 32.9 | 629.0 ± 41.7 | <0.0001 |
| Visceral | 38.0 ± 3.9 | 239.9 ± 12.0 | <0.0001 |
| Subcutaneous | 167.3 ± 29.8 | 389.1 ± 40.6 | <0.0001 |
Values are means ± SE.
Table 4.
Blood pressure, lipids, and other potential intermediary factors in a subsample of subjects with the lowest and highest abdominal visceral fat
| Lowest Adiposity | Highest Adiposity | P Values | |
|---|---|---|---|
| Blood pressure, mmHg | |||
| Systolic | 116 ± 3 | 138 ± 4 | <0.0001 |
| Diastolic | 73 ± 2 | 82 ± 3 | 0.02 |
| Cholesterol, mg/dl | |||
| Total | 189 ± 9 | 188 ± 7 | 0.9 |
| LDL | 112 ± 7 | 118 ± 7 | 0.5 |
| HDL | 59 ± 3 | 43 ± 2 | <0.0001 |
| Triglycerides, mg/dl | 90 ± 11 | 135 ± 27 | 0.1 |
| Fasting glucose, mg/dl | 84 ± 3 | 95 ± 2 | 0.008 |
| Baseline diameter, mm | 3.4 ± 0.1 | 4.1 ± 0.2 | 0.007 |
Values are means ± SE.
Fig. 3.
Comparison of NTG-induced dilation (expressed as percent dilation from baseline) between subjects with lowest and highest abdominal visceral fat (unadjusted).
Potential Intermediary Factors
We sought to examine the association between NTG-induced dilation and factors that were related with adiposity to determine whether any of these factors could potentially explain the relation between NTG-induced dilation and adiposity. The results of these bivariate correlations are presented in Table 5. Accounting for each of the intermediary factors that were related with NTG-induced dilation (i.e., systolic blood pressure, glucose, HDL cholesterol, HOMA, adiponectin, and brachial artery baseline diameter) reduced the sex-independent relations between adiposity and NTG-induced dilation and in many instances rendered them nonsignificant (P > 0.05; Figs. 1, A–C, and 2, A–C). The role of adiponectin, oxidized LDL, and HOMA as potential intermediary factors could not be evaluated for total, visceral, and subcutaneous abdominal fat because these factors were not measured in all of the subjects for whom computed tomography was performed.
Table 5.
Bivariate relations between NTG-induced dilation and potential intermediary factors
| r | P Values | |
|---|---|---|
| V̇o2max, ml·kg−1·min−1 | −0.004 | 0.9 |
| Leisure-time physical activity | 0.05 | 0.4 |
| Blood pressure | ||
| Systolic | −0.17 | 0.01 |
| Diastolic | 0.04 | 0.6 |
| Fasting glucose | −0.18 | 0.009 |
| Fasting insulin | −0.11 | 0.2 |
| Log (HOMA-IR) | −0.22 | 0.01 |
| Cholesterol | ||
| Total | 0.04 | 0.6 |
| LDL | −0.02 | 0.8 |
| HDL | 0.21 | 0.002 |
| Triglycerides | −0.05 | 0.5 |
| Log(C-reactive protein) | −0.09 | 0.3 |
| Oxidized LDL | −0.05 | 0.5 |
| Adiponectin | 0.27 | 0.02 |
| Baseline diameter | −0.49 | <0.0001 |
V̇o2max, maximal oxygen consumption.
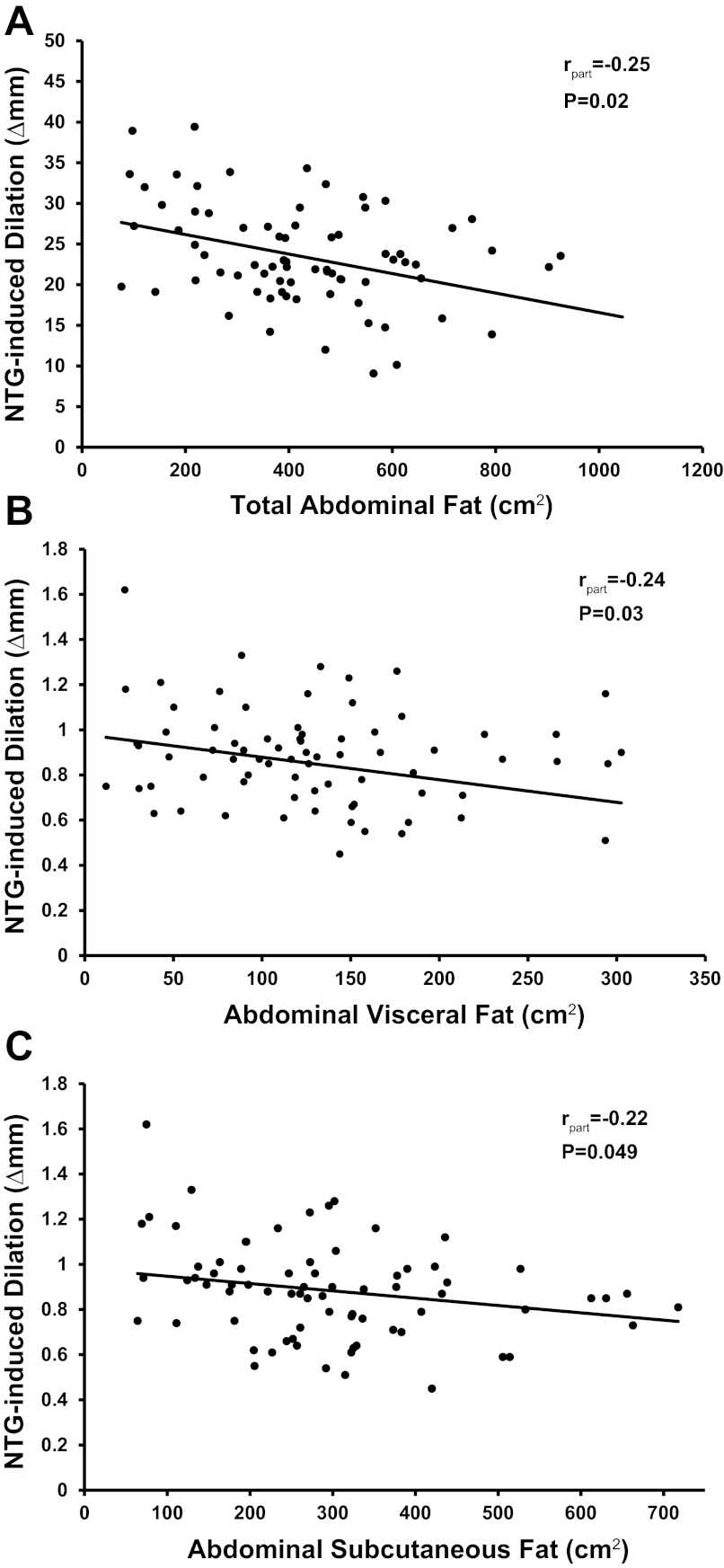
To further evaluate the influence of baseline diameter on the relation between adiposity and NTG-induced dilation, we expressed NTG-induced dilation as absolute change in diameter from baseline (in Δmm), an expression not influenced by baseline diameter (r = 0.09, P = 0.2; data not shown). NTG-induced dilation (in Δmm) was negatively related with total abdominal fat (rpart = −0.25, P = 0.02; Fig. 4A), abdominal visceral fat (rpart = −0.24, P = 0.03; Fig. 4B), and abdominal subcutaneous fat (rpart = −0.22, P = 0.049; Fig. 4C), independent of sex, but was not related with body mass index, percent body fat, waist circumference, and waist-to-hip ratio (rpart = −0.06 to −0.1, P ≥ 0.1; data not shown).
Fig. 4.
The relation between NTG-induced dilation (expressed as absolute change in diameter from baseline; Δmm) and abdominal fat (A–C) adjusted for sex is represented by the regression line. The rpart indicates the part correlation coefficient derived from multiple linear regression analysis.
Consistent with the results of the regression analyses, the difference in NTG-induced dilation between subjects with the lowest and highest abdominal visceral fat was abolished after accounting for systolic blood pressure and HDL cholesterol (P ≥ 0.1; Fig. 5, A and B) but was not changed after co-varying for glucose (P = 0.002; Fig. 5C) or brachial artery baseline diameter (P = 0.04; Fig. 5D). Co-varying for age did not influence the difference in NTG-induced dilation between the groups (P = 0.003; data not shown). Similarly, the difference in NTG-induced dilation remained unchanged after adjustment for physical activity levels (P = 0.006; data not shown).
Fig. 5.
Comparison of NTG-induced dilation (expressed as percent dilation from baseline) between subjects with lowest and highest abdominal visceral fat after co-varying for SBP (A), HDL (B), fasting glucose (C), and brachial artery baseline diameter (D).
Finally, to address the potential concern that obese subjects might have been exposed to lower levels of NTG, we expressed the dose (0.4 mg) relative to lean body weight (LBW; NTG-to-LBW ratio) following the dose scaling recommendations for obese patients (16, 19) and examined if this factor altered the relation between NTG-induced dilation and adiposity. The relations between NTG-induced dilation and adiposity (waist, waist-to-hip ratio, and total, visceral, and subcutaneous abdominal fat) remained significant (P ≤ 0.03) except for body mass index, which approached significance (P = 0.055). NTG-to-LBW ratio was not a significant predictor of NTG-induced dilation in all of the regression models (P = 0.07 to 0.2) with the exception of one (waist-to-hip ratio model; P = 0.04). Likewise, the difference in NTG-induced dilation between subjects with the lowest and highest abdominal visceral fat remained unchanged (P = 0.007; data not shown) after adjusting for NTG-to-LBW ratio, and the latter was not a significant covariate (P = 0.8) in the ANCOVA model.
DISCUSSION
We measured NTG-induced dilation and total and abdominal adiposity in 224 carefully screened men and women. We are not aware of any previous studies that comprehensively examined the relation of NTG-induced dilation to adiposity in a large group of men and women free of clinical disease. The key novel finding of the present study is that NTG-induced dilation is or tends to be negatively associated with measures of total adiposity (body mass index and total body fat, respectively), but is consistently and more strongly negatively associated with abdominal adiposity among healthy adults. Moreover, our findings suggest that blood pressure, HDL cholesterol, fasting glucose, insulin resistance, adiponectin, and brachial artery diameter may be among the intermediary mechanisms linking elevated adiposity to impaired NTG-induced dilation.
NTG-Induced Dilation and Adiposity
Vascular smooth muscle relaxation to NTG is achieved via a complex cascade of events involving the bioconversion of NTG to NO and the NO signaling pathways: activation of soluble guanylate cyclase, synthesis of cyclic GMP, activation of PKG, and reduction in cytosolic calcium leading to smooth muscle relaxation. The molecular mechanisms that may contribute to obesity-related impairments in NTG-induced dilation in humans are not clear. In vitro studies in vascular smooth muscle cells from obese and lean Zucker rats showed that obesity is associated with multiple defects in the NO/cyclic GMP/PKG pathways: 1) a defect in the NO-induced activation of soluble guanylate cyclase, 2) impaired ability of NO and cyclic GMP to activate PKG leading to decreased phosphorylation of vasodilator-stimulated phosphoprotein (VASP), and 3) increased oxidative stress leading to reduced ability of NO to activate the cyclic GMP/PKG/VASP cascade (30). Antioxidants restore these defects in vitro in vascular smooth muscle cells from obese Zucker rats but have no effect on lean Zucker rats (30), consistent with the observation that antioxidants improve vasodilator responses to a NO donor in vivo in obese but not lean Zucker rats (13). No data are available concerning obesity-related defects in bioconversion of NTG to NO or in mechanisms regulating vascular tone (e.g., calcium regulatory mechanisms).
Our data show that abdominal visceral and subcutaneous fat are similarly predictive of vascular smooth muscle responsiveness to NTG. This is consistent with previous data showing a significant relation between abdominal subcutaneous fat and cardiovascular risk factors such as dyslipidemia, hypertension, and insulin resistance (12). In agreement with our findings, Parikh et al. (26) found that both abdominal visceral and subcutaneous fat were negatively related with vascular endothelial function. However, in their study after adjustment for clinical covariates, only abdominal visceral remained significantly associated with endothelial function, whereas in our study co-varying for cardiovascular risk factors influenced similarly the relations of abdominal visceral and subcutaneous adiposity with NTG-induced dilation.
The intermediary factors through which adiposity may be influencing NTG-induced dilation include blood pressure, HDL cholesterol, fasting glucose, insulin resistance, adiponectin, and brachial artery diameter. We found that adjusting for systolic blood pressure reduced the relation of NTG-induced dilation and adiposity in the entire group, and completely abrogated the difference in NTG-induced dilation between subjects with lowest and highest amounts of abdominal visceral fat. In support of our findings, Olsen et al. (25) have shown that NTG-induced dilation is negatively associated with systolic blood pressure in hypertension. They suggest that impairment in the vasodilatory response to NTG occurs either secondarily or in parallel to vascular structural changes induced by high blood pressure, since NTG-induced dilation also was negatively associated with carotid wall thickness and arterial stiffness.
We found that HDL cholesterol is another intermediary factor that modulates the relation between NTG-induced dilation and adiposity, consistent with previous observations (2, 24). The mechanism underlying the association is unclear, but it has been suggested that the antioxidant/anti-inflammatory properties of HDL cholesterol could be involved (2, 24). Indeed, some studies have reported that markers of inflammation and oxidative stress are negatively associated with NTG-induced dilation in humans (2, 20). However, in our study there was no association between NTG-induced dilation, oxidized LDL, and high-sensitivity C-reactive protein.
Adjusting for fasting glucose also reduced and in some cases abolished the relation between NTG-induced dilation and adiposity. Hyperglycemia has previously been shown to impair NTG-induced dilation (34) by accumulation of advanced glycation end products in the subendothelial space (4). However, our subjects did not have diabetes and the majority of them had clinically normal fasting blood glucose. Despite this, fasting glucose still appeared to have a moderate modulatory influence on NTG-induced dilation in our regression models. In contrast with systolic blood pressure and HDL cholesterol, glucose did not modulate the differences in NTG-induced dilation among adults with lowest and highest adiposity (Fig. 3D). Moreover, in the present study adjusting for insulin resistance or adiponectin abolished the relation between NTG-induced dilation and total (body mass index) and abdominal (waist and waist-to-hip ratio) adiposity. The underlying mechanisms are unclear, but an association between NTG-induced dilation and insulin resistance (35) and adiponectin (11) has been reported previously.
In our study, accounting for brachial artery baseline diameter rendered the relations nonsignificant between NTG-induced dilation expressed as percent dilation from baseline and adiposity, suggesting that maladaptive arterial remodeling (enlarged vessels) in obesity (15) might be partly responsible for impaired NTG-induced dilation. An association between NTG-induced dilation (percent change from baseline) and baseline diameter has been observed previously (1), but the mechanism responsible is not known. In contrast, NTG-induced dilation expressed as absolute change was negatively associated with abdominal adiposity and not associated with baseline diameter. In addition, co-varying for baseline diameter did not influence the difference in NTG-induced dilation (in percent change from baseline) among adults with the lowest and highest adiposity. Taken together, these results indicate that impaired vascular smooth muscle responsiveness to NO is associated with higher abdominal adiposity, independent of baseline diameter.
Adjusting for the dose of the NTG-to-LBW ratio did not alter the relation between adiposity and NTG-induced dilation or the difference in NTG-induced dilation between subjects with the highest and lowest abdominal adiposity in our data. The pharmacodynamics of NTG, including NTG plasma concentration, do not appear to be affected by obesity (37). A dose of 0.4 mg is the standard dose for testing vascular smooth muscle function and is expected to produce maximal dilation regardless of body size. Indeed, Ayer et al. (2) demonstrated in severely obese subjects that brachial artery dilation was similar to a dose of 0.4 mg compared with 0.5 mg of NTG, and the dose-response curves of the lean and obese subjects remained separated across the entire range of NTG doses (0.05 to 0.5 mg). Taken together, these findings demonstrate that the reduced vascular smooth muscle responsiveness to NTG in obesity is not likely to be due to lower levels of NTG in the obese subjects.
One might expect that physical activity and aerobic fitness influence vascular smooth muscle responsiveness to NTG because they are inversely related to adiposity and cardiovascular risk factors. Interestingly, neither physical activity levels nor aerobic fitness was related to NTG-induced dilation. Our results are in agreement with a previous report of no differences in NTG-induced dilation between athletes and sedentary adults (36).
Study Limitations
First, we recognize that our data are based on associations and do not examine directly the mechanisms linking increased adiposity to impaired NTG-induced dilation. However, our results are an important first step to definitively establish that a significant association exists and provide initial insight into the intermediary mechanisms that may be involved. Second, although age did not relate to NTG-induced dilation in our study and the study of others in healthy adults (7), advancing age is associated with increased number of cardiovascular disease risk factors. Our study sought to account statistically for the effect of cardiovascular risk factors known to be related to adiposity and NTG-induced dilation, but our findings are limited to the specific risk factors we examined. Third, our study used a cross-sectional design. To gain optimal insight into the pathophysiology behind obesity-related impairments in NTG-induced dilation, adequately powered interventions designed specifically to examine the influence of weight loss or weight gain on NTG-induced dilation are needed. However, these studies are highly time- and labor intensive, so it is important to provide preliminary evidence to support such a trial. A previous study from our laboratory (albeit focused primarily on endothelial function) demonstrated that NTG-induced dilation was not altered after 12 wk of dietary restriction-induced weight loss that was associated with significant decreases in systolic blood pressure (27). That fasting glucose and HDL cholesterol were not altered significantly after the weight loss suggests perhaps that a combination of these factors play a significant role in NTG-induced dilation. Finally, we recognize that because our subjects were free of clinical disease, our results are restricted to this population.
Clinical Significance
Reduced vasodilator response to nitrodilators is associated with a significant increase in cardiovascular events including myocardial infarction, ischemic stroke, coronary angioplasty, coronary or peripheral bypass operation, and death from cardiovascular causes (18, 31). Thus understanding the factors that influence vascular responsiveness to NO donors may be clinically important. Furthermore, nitrodilators are clinically used to treat conditions such as angina pectoris, myocardial infarction, and congestive heart failure; therefore, understanding whether increased total body and particularly abdominal fatness may influence the efficacy of the NTG treatment may have important clinical implications.
Conclusions
The present findings are the first to establish that NTG-induced dilation is negatively associated with total and, particularly, abdominal adiposity in a large sample of healthy adults. Our results also indicate that some conventional risk factors for cardiovascular disease may partly explain these relations. As such, these observations provide insight into a novel mechanism (i.e., impaired NTG-induced dilation) by which adiposity may contribute to cardiovascular risk.
GRANTS
This work was supported by National Institute on Aging Grant AG-032067 and American Heart Association Grant 0865117F (to D. D. Christou) and National Institutes of Health Grants AG015897, AG006537, and AG013038 (to D. R. Seals) and UL1-RR025780.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.D.C., G.L.P., A.E.W., and D.R.S. conception and design of research; D.D.C., G.L.P., A.E.W., M.-H.H., J.-K.Y., M.J.L., T.H.M., and M.E. performed experiments; D.D.C., G.L.P., A.E.W., M.-H.H., J.-K.Y., and M.J.L. analyzed data; D.D.C., G.L.P., and D.R.S. interpreted results of experiments; D.D.C. prepared figures; D.D.C. drafted manuscript; D.D.C., G.L.P., A.E.W., M.-H.H., J.-K.Y., M.J.L., T.H.M., M.E., and D.R.S. edited and revised manuscript; D.D.C., G.L.P., A.E.W., M.-H.H., J.-K.Y., M.J.L., T.H.M., M.E., and D.R.S. approved final version of manuscript.
REFERENCES
- 1. Adams MR, Robinson J, McCredie R, Seale JP, Sorensen KE, Deanfield JE, Celermajer DS. Smooth muscle dysfunction occurs independently of impaired endothelium-dependent dilation in adults at risk of atherosclerosis. J Am Coll Cardiol 32: 123–127, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Ayer JG, Harmer JA, David C, KSS, Seale JP, Celermajer DS. Severe obesity is associated with impaired arterial smooth muscle function in young adults. Obesity (Silver Spring) 19: 54–60, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol 88: 1264–1269, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest 87: 432–438, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 88: 2149–2155, 1993 [DOI] [PubMed] [Google Scholar]
- 6. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Christou DD, Gentile CL, DeSouza CA, Seals DR, Gates PE. Fatness is a better predictor of cardiovascular disease risk factor profile than aerobic fitness in healthy men. Circulation 111: 1904–1914, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Clarkson P, Celermajer DS, Donald AE, Sampson M, Sorensen KE, Adams M, Yue DK, Betteridge DJ, Deanfield JE. Impaired vascular reactivity in insulin-dependent diabetes mellitus is related to disease duration and low density lipoprotein cholesterol levels. J Am Coll Cardiol 28: 573–579, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Clausen P, Jensen JS, Jensen G, Borch-Johnsen K, Feldt-Rasmussen B. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation 103: 1869–1874, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Fernandez-Real JM, Castro A, Vazquez G, Casamitjana R, Lopez-Bermejo A, Penarroja G, Ricart W. Adiponectin is associated with vascular function independent of insulin sensitivity. Diabetes Care 27: 739–745, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116: 39–48, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Frisbee JC, Stepp DW. Impaired NO-dependent dilation of skeletal muscle arterioles in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol 281: H1304–H1311, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Fulop T, Jebelovszki E, Erdei N, Szerafin T, Forster T, Edes I, Koller A, Bagi Z. Adaptation of vasomotor function of human coronary arterioles to the simultaneous presence of obesity and hypertension. Arterioscler Thromb Vasc Biol 27: 2348–2354, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Hamburg NM, Mott MM, Bigornia SJ, Duess MA, Kluge MA, Hess DT, Apovian CM, Vita JA, Gokce N. Maladaptive enlargement of the brachial artery in severe obesity is reversed with weight loss. Vasc Med 15: 215–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han PY, Duffull SB, Kirkpatrick CM, Green B. Dosing in obesity: a simple solution to a big problem. Clin Pharmacol Ther 82: 505–508, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Hashimoto M, Akishita M, Eto M, Kozaki K, Ako J, Sugimoto N, Yoshizumi M, Toba K, Ouchi Y. The impairment of flow-mediated vasodilatation in obese men with visceral fat accumulation. Int J Obes Relat Metab Disord 22: 477–484, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Ingrande J, Lemmens HJ. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth 105, Suppl 1: i16–i23, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Jarvisalo MJ, Lehtimaki T, Raitakari OT. Determinants of arterial nitrate-mediated dilatation in children: role of oxidized low-density lipoprotein, endothelial function, and carotid intima-media thickness. Circulation 109: 2885–2889, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, Solomon S, Nikolic SD, Forman R, LeJemtel TH. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol 19: 918–925, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Bennett PH, Kuller LH. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care 13: 401–411, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Liao JK, Bettmann MA, Sandor T, Tucker JI, Coleman SM, Creager MA. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circ Res 68: 1027–1034, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Meeking DR, Cummings MH, Thorne S, Donald A, Clarkson P, Crook JR, Watts GF, Shaw KM. Endothelial dysfunction in type 2 diabetic subjects with and without microalbuminuria. Diabet Med 16: 841–847, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Olsen MH, Wachtell K, Aalkjaer C, Dige-Petersen H, Rokkedal J, Ibsen H. Vasodilatory capacity and vascular structure in long-standing hypertension: a LIFE substudy. Losartan Intervention For Endpoint-Reduction in Hypertension. Am J Hypertens 15: 398–404, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Parikh NI, Keyes MJ, Larson MG, Pou KM, Hamburg NM, Vita JA, O'Donnell CJ, Vasan RS, Mitchell GF, Hoffmann U, Fox CS, Benjamin EJ. Visceral and subcutaneous adiposity and brachial artery vasodilator function. Obesity (Silver Spring) 17: 2054–2059, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 52: 72–79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-κB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 119: 1284–1292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113: 898–918, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Russo I, Del Mese P, Doronzo G, Mattiello L, Viretto M, Bosia A, Anfossi G, Trovati M. Resistance to the nitric oxide/cyclic guanosine 5′-monophosphate/protein kinase G pathway in vascular smooth muscle cells from the obese Zucker rat, a classical animal model of insulin resistance: role of oxidative stress. Endocrinology 149: 1480–1489, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101: 1899–1906, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation 115: 627–637, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest 93: 50–55, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sorensen VR, Mathiesen ER, Clausen P, Flyvbjerg A, Feldt-Rasmussen B. Impaired vascular function during short-term poor glycaemic control in Type 1 diabetic patients. Diabet Med 22: 871–876, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Suzuki M, Takamisawa I, Suzuki K, Hiuge A, Horio T, Yoshimasa Y, Harano Y. Close association of endothelial dysfunction with insulin resistance and carotid wall thickening in hypertension. Am J Hypertens 17: 228–232, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Wang EQ, Fung HL. Effects of obesity on the pharmacodynamics of nitroglycerin in conscious rats. AAPS PharmSci 4: E28, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams IL, Chowienczyk PJ, Wheatcroft SB, Patel A, Sherwood R, Momin A, Shah AM, Kearney MT. Effect of fat distribution on endothelial-dependent and endothelial-independent vasodilatation in healthy humans. Diabetes Obes Metab 8: 296–301, 2006 [DOI] [PubMed] [Google Scholar]