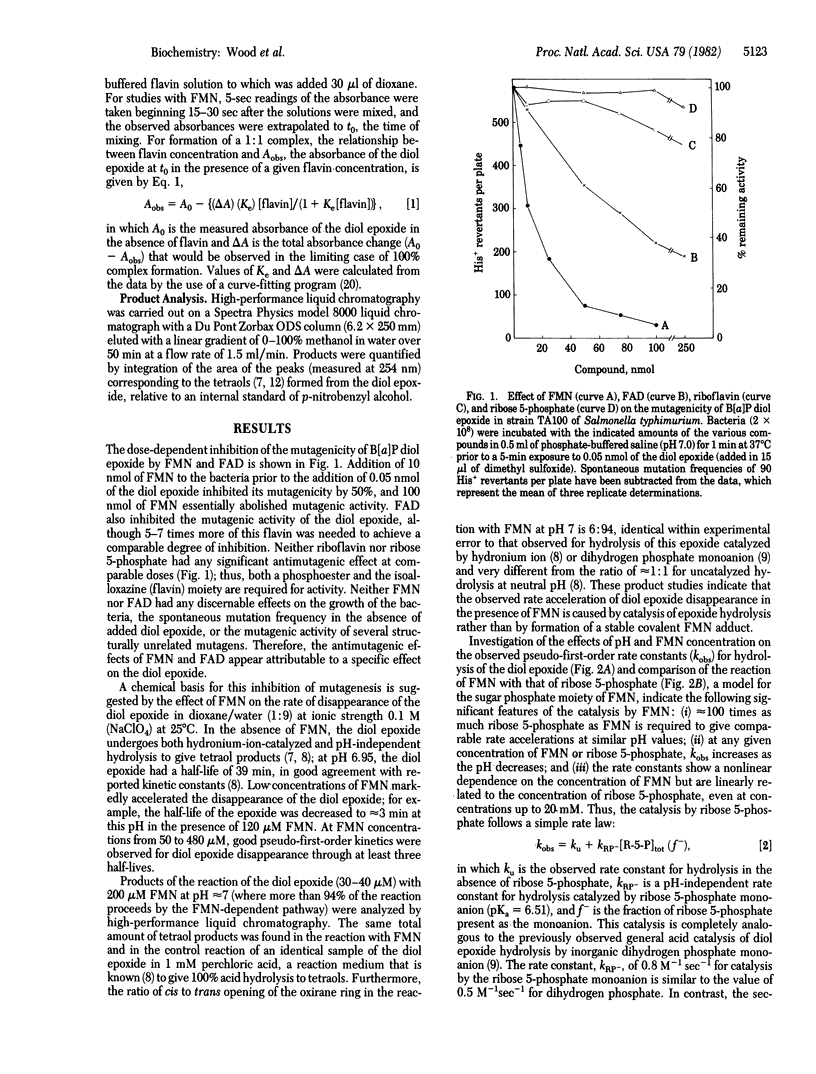
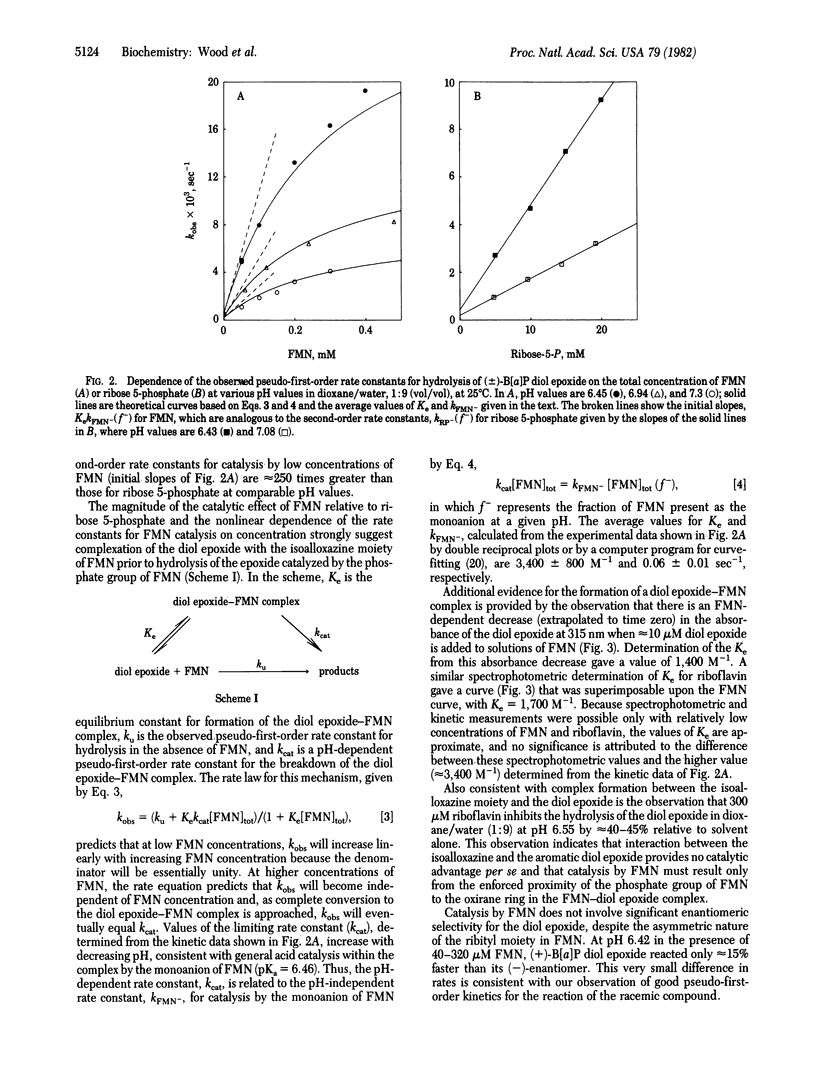
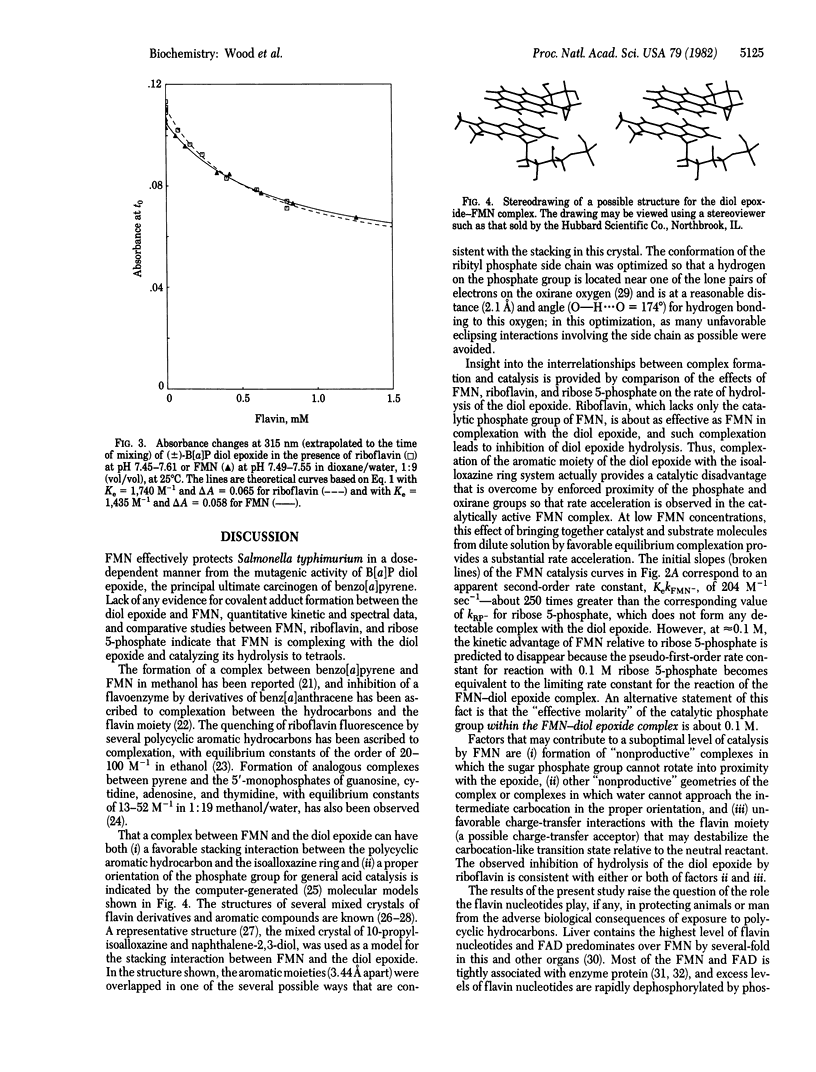
Abstract
Riboflavin 5'-phosphate (flavin mononucleotide; FMN) inhibits the mutagenicity of (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (B[a]P diol epoxide), the only known ultimate carcinogenic metabolite of benzo[a]pyrene. Coincubation of 10, 25, and 50 nmol of FMN with strain TA100 of histidine-dependent Salmonella typhimurium inhibits the mutagenicity of 0.05 nmol of the diol epoxide by 50, 70, and 90%, respectively. Ribose 5-phosphate and riboflavin show no significant effects at comparable doses. Reaction of B[a]P diol epoxide with FMN in aqueous solution at neutral pH produces only tetraols, with no evidence for covalent adducts. At pH 7 the rate of hydrolysis of B[a]P diol epoxide in dioxane/water, 1:9 (vol/vol), at 25 degrees C is increased more than 10-fold in the presence of 100 muM FMN. Spectrophotometric studies and quantitative rate data for the reaction of the diol epoxide with FMN indicate that a complex is formed between the diol epoxide and the flavin moiety of FMN (Ke = 1,400-3,400 M-1) prior to general acid-catalyzed hydrolysis of the epoxide to tetraols by the phosphate monoanion of FMN. Comparable concentrations of ribose 5-phosphate and riboflavin do not significantly increase the rate of hydrolysis, although evidence for complex formation between riboflavin and the diol epoxide is observed. General acid-catalyzed hydrolysis of bay-region polycyclic hydrocarbon diol epoxides by compounds that have a high affinity for these ultimate carcinogens represents a potentially useful way of inhibiting their carcinogenic activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CERLETTI P., IPATA P. Determination of riboflavin and its coenzymes in tissues. Biochem J. 1960 Apr;75:119–124. doi: 10.1042/bj0750119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. 3. Flavoproteins. Biochim Biophys Acta. 1971 Mar 2;226(2):269–284. doi: 10.1016/0005-2728(71)90094-6. [DOI] [PubMed] [Google Scholar]
- Feldman R. J., Bacon C. R., Cohen J. S. Versatile interactive graphics display system for molecular modelling by computer. Nature. 1973 Jul 13;244(5411):113–115. doi: 10.1038/244113a0. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Levin W., Conney A. H., Yagi H., Jerina D. M. Benzo[a]pyrene 7,8-dihydrodiol is more carcinogenic than benzo[a]pyrene in newborn mice. Nature. 1977 Mar 24;266(5600):378–380. doi: 10.1038/266378a0. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Wislocki P. G., Levin W., Yagi H., Jerina D. M., Conney A. H. Tumorigenicity studies with diol-epoxides of benzo(a)pyrene which indicate that (+/-)-trans-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene is an ultimate carcinogen in newborn mice. Cancer Res. 1978 Feb;38(2):354–358. [PubMed] [Google Scholar]
- Knott G. D. Mlab--a mathematical modeling tool. Comput Programs Biomed. 1979 Dec;10(3):271–280. doi: 10.1016/0010-468x(79)90075-8. [DOI] [PubMed] [Google Scholar]
- LIAO S., DULANEY J. T., WILLIAMS-ASHMAN H. G. Purification and properties of a flavoprotein catalyzing the oxidation of reduced ribosyl nicotinamide. J Biol Chem. 1962 Sep;237:2981–2987. [PubMed] [Google Scholar]
- Singer T. P., Edmondson D. E. 8 alpha-substituted flavins of biological importance. FEBS Lett. 1974 May 15;42(1):1–14. doi: 10.1016/0014-5793(74)80266-8. [DOI] [PubMed] [Google Scholar]
- Thakker D. R., Yagi H., Akagi H., Koreeda M., Lu A. H., Levin W., Wood A. W., Conney A. H., Jerina D. M. Metabolism of benzo[a]pyrene. VI. Stereoselective metabolism of benzo[a]pyrene and benzo[a]pyrene 7,8-dihydrodiol to diol epoxides. Chem Biol Interact. 1977 Mar;16(3):281–300. doi: 10.1016/0009-2797(77)90108-9. [DOI] [PubMed] [Google Scholar]
- Thakker D. R., Yagi H., Lu A. Y., Levin W., Conney A. H. Metabolism of benzo[a]pyrene: conversion of (+/-)-trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene to highly mutagenic 7,8-diol-9,10-epoxides. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3381–3385. doi: 10.1073/pnas.73.10.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILK M. [On the mechanism of chemical carcinogenesis by 3,4-benzopyrene]. Biochem Z. 1960;333:166–174. [PubMed] [Google Scholar]
- Wood A. W., Chang R. L., Levin W., Lehr R. E., Schaefer-Ridder M., Karle J. M., Jerina D. M., Conney A. H. Mutagenicity and cytotoxicity of benz[alpha]anthracene diol epoxides and tetrahydro-epoxides: exceptional activity of the bay region 1,2-epoxides. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2746–2750. doi: 10.1073/pnas.74.7.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. W., Chang R. L., Levin W., Ryan D. E., Thomas P. E., Lehr R. E., Kumar S., Sardella D. J., Boger E., Yagi H. Mutagenicity of the bay-region diol-epoxides and other benzo-ring derivatives of dibenzo(a,h)pyrene and dibenzo(a,i)pyrene. Cancer Res. 1981 Jul;41(7):2589–2597. [PubMed] [Google Scholar]
- Wood A. W., Wislocki P. G., Chang R. L., Levin W., Lu A. Y., Yagi J., Hernandez O., Herina D. M., Conney A. H. Mutagenicity and cytotoxicity of benzo(a)pyrene benzo-ring epoxides. Cancer Res. 1976 Sep;36(9 PT1):3358–3366. [PubMed] [Google Scholar]
- Wynder E. L., Chan P. C. The possible role of riboflavin deficiency in epithelial neoplasia. II. Effect of skin tumor development. Cancer. 1970 Dec;26(6):1221–1224. doi: 10.1002/1097-0142(197012)26:6<1221::aid-cncr2820260607>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Yagi H., Akagi H., Thakker D. R., Mah H. D., Koreeda M., Jerina D. M. Absolute sterochemistry of the highly mutagenic 7,8-diol 9,10-epoxides derived from the potent carcinogen trans-7,8-dihydroxy-7,8-dihydrobenzol[a]pyrene. J Am Chem Soc. 1977 Mar 30;99(7):2358–2359. doi: 10.1021/ja00449a066. [DOI] [PubMed] [Google Scholar]
- Yagi H., Hernandez O., Jerina D. M. Letter: Synthesis of (+/-)-7 beta,8alpha-dihydroxy-9 beta,10beta-epoxy-7,8,-9,10-tetrahydrobenzo(a)pyrene, a potential metabolite of the carcinogen benzo(a)pyrene with stereochemistry related to the antileukemic triptolides. J Am Chem Soc. 1975 Nov 12;97(23):6881–6883. doi: 10.1021/ja00856a057. [DOI] [PubMed] [Google Scholar]
- Yagi H., Thakker D. R., Hernandez O., Koreeda M., Jerina D. M. Synthesis and reactions of the highly mutagenic 7,8-diol 9,10-epoxides of the carcinogen benzo[a]pyrene. J Am Chem Soc. 1977 Mar 2;99(5):1604–1611. doi: 10.1021/ja00447a053. [DOI] [PubMed] [Google Scholar]
- Yang S. K., McCourt D. W., Roller P. P., Gelboin H. V. Enzymatic conversion of benzo(a)pyrene leading predominantly to the diol-epoxide r-7,t-8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene through a single enantiomer of r-7, t-8-dihydroxy-7,8-dihydrobenzo(a)pyrene. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2594–2598. doi: 10.1073/pnas.73.8.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]


