Abstract
Apelin is an endogenous ligand for the angiotensin-like 1 receptor (APJ) and has beneficial effects against myocardial ischemia-reperfusion injury. Little is known about the role of apelin in the homing of vascular progenitor cells (PCs) and cardiac functional recovery postmyocardial infarction (post-MI). The present study investigated whether apelin affects PC homing to the infarcted myocardium, thereby mediating repair and functional recovery post-MI. Mice were infarcted by coronary artery ligation, and apelin-13 (1 mg·kg−1·day−1) was injected for 3 days before MI and for 14 days post-MI. Homing of vascular PCs [CD133+/c-Kit+/Sca1+, CD133+/stromal cell-derived factor (SDF)-1α+, and CD133+/CXC chemokine receptor (CXCR)-4+] into the ischemic area was examined. Myocardial Akt, endothelial nitric oxide synthase (eNOS), VEGF, jagged1, notch3, SDF-1α, and CXCR-4 expression were assessed at 24 h and 14 days post-MI. Functional analyses were performed on day 14 post-MI. Mice that received apelin-13 treatment demonstrated upregulation of SDF-1α/CXCR-4 expression and dramatically increased the number of CD133+/c-Kit+/Sca1+, CD133+/SDF-1α+, and c-Kit+/CXCR-4+ cells in infarcted hearts. Apelin-13 also significantly increased Akt and eNOS phosphorylation and upregulated VEGF, jagged1, and notch3 expression in ischemic hearts. This was accompanied by a significant reduction of myocardial apoptosis. Furthermore, treatment with apelin-13 promoted myocardial angiogenesis and attenuated cardiac fibrosis and hypertrophy together with a significant improvement of cardiac function at 14 days post-MI. Apelin-13 increases angiogenesis and improves cardiac repair post-MI by a mechanism involving the upregulation of SDF-1α/CXCR-4 and homing of vascular PCs.
Keywords: myocardial repair, c-Kit, vascular progenitor cell, stromal cell-derived factor-1α/C-X-C chemokine receptor-4, jagged1/notch3
apelin, a recently isolated bioactive peptide from bovine gastric extract, is an endogenous ligand of the human G protein-coupled receptor angiotensin-like 1 receptor (APJ) (26, 28, 39). Apelin has requisite roles for different aspects of cardiac and vascular development and has been detected in the region around presumptive blood vessels during early embryogenesis and overlapped with the expression of APJ in the cardiovascular system of Xenopus laevis and zebrafish (5, 22, 45). Furthermore, knockdown of apelin induced abnormal heart morphology and attenuated expression of tie-2, resulting in the disruption of blood vessel formation in the posterior cardinal vein as well as in intersomitic and vitelline vessels (5, 22, 45). Apelin/APJ is expressed in multiple tissues, including vascular endothelial cells and the myocardium (10, 25). Recently, the role of apelin/APJ in the pathogenesis of heart failure (HF) has received much attention. Apelin mRNA levels were increased in the left ventricle (LV) of patients with chronic HF due to coronary heart disease and dilated cardiomyopathy (10, 37). In animal models of HF, expression of apelin/APJ was upregulated, but it was downregulated in association with severe, decompensated HF (37). Myocardial infarction (MI) is a major cause of HF, with progressive worsening of cardiac performance due to structural and functional alterations. Accumulating evidence indicates that apelin has a protective role against acute ischemia and/or reperfusion-induced myocardial injury (20, 29, 35, 44). In ischemia-reperfusion injury, increased apelin has been shown to contribute to the improvement of cardiac dysfunction by suppressing myocardial apoptosis (44). Furthermore, treatment with apelin-13 (a 13-amino acid peptide), a natural peptide of apelin, reduced myocardial infarct size and limited postischemic myocardial contracture in a rat myocardial ischemia-reperfusion model (30). Although the involvement of apelin/APJ in the regulation of angiogenesis and the protection of myocardial ischemia-or reperfusion injury has been characterized, the role of apelin/APJ in ischemic HF and post-MI is less clear.
A recent study (23) reported that the apelin/APJ system is involved in the regulation of blood vessel diameter via downstream signaling of angiopoietin-1 (Ang-1)/tie-2 during angiogenesis (23). By analysis of downstream signaling after Ang-1/tie-2 activation in ischemic hearts, we found that overexpression of Ang-1 significantly increases apelin expression together with a dramatic improvement of myocardial angiogenesis and cardiac functional recovery in post-MI mice (43). However, it is unknown if the improvement in myocardial function after ischemia provided by apelin involves the upregulation of angiogenic growth factors, such as VEGF, and stimulation of angiogenesis.
Vascular progenitor cells (PCs) home to sites of ischemia and contribute to neovascularization in ischemic tissue (18). Experimental data and clinical studies have demonstrated that treatment of acute MI with vascular PCs results in a reduction in infarct size (3, 33). Stromal cell-derived factor (SDF)-1α and its receptor C-X-C chemokine receptor (CXCR)4 have been identified as the central signaling axis that regulates vascular PC homing into the injured area of myocardial ischemia and in the improvement of cardiac function post-MI (1a). In addition, SDF-1α/CXCR-4 has been shown to protect the heart post-MI (1a, 11, 15). The present study investigated whether treatment with apelin-13 promotes vascular PC homing into ischemic sites through SDF-1α/CXCR-4 signaling and whether this leads to an improvement of cardiac function in post-MI mice. Our data suggest that the apelin/APJ system plays a crucial role in the regulation of vascular PC homing, myocardial angiogenesis, and cardiac repair post-MI.
MATERIALS AND METHODS
All procedures conformed with the Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of University of Mississippi Medical Center (protocol identifier: 1280). The investigation conformed with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996).
Experimental animal model.
C57BL/6J mice (8–10 wk of age) were purchased from The Jackson Laboratories (Bar Harbor, ME). Experimental mice were anesthetized with ketamine (100 mg/kg) plus xylazine (15 mg/kg), intubated, and artificially ventilated with room air. A left thoracotomy was performed, and the left anterior descending coronary artery (LAD) was exposed. An 8-0 nylon suture was placed around the LAD. Myocardial ischemia was achieved by ligation of the LAD. Sham-operated (sham) control mice underwent surgery without the LAD ligation (4, 40).
Systemic administration of apelin-13 in experimental mice.
Experimental mice received apelin-13 intraperitoneally (1 mg·kg−1·day−1, Cayman Chemical) daily for 3 days before surgery. After surgery, mice continued to receive apelin-13 intraperitoneally for 14 days before death. A schematic diagram of the apelin-13 treatment protocol with experimental end points is shown below: where d is days and IS is ischemia.
Western blot analysis of SDF-1α, CXCR-4, VEGF, apelin, APJ, endothelial nitric oxide synthase, Akt, jagged1, and notch3 expression.
Hearts were harvested and homogenized in lysis buffer for Western blot analysis. After immunoblot analysis, membranes were immunoblotted with SDF-1α, VEGF, apelin, APJ, jagged1, and Notch3 (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA) as well as CXCR-4 (1:1,000, Sigma, St. Louis, MO) antibodies. Membranes were then washed and incubated with a secondary antibody coupled to horseradish peroxidase, and densitometric analysis was carried out using image acquisition and analysis software (TINA 2.0).
Analysis of myocardial capillary and arteriole densities.
Fourteen days after myocardial ischemia, hearts were harvested and immediately flash frozen. Sections (8 μm thick) were cut and incubated with fluorescerin-labeled Griffonia Bandeiraea simplicifolia isolectin B4 (1:200, Molecular Probes-Invitrogen, Eugene, OR) and Cy3-conjugated anti-α-smooth muscle actin (α-SMA; 1:100; Sigma). The number of capillaries (isolectin B4+ endothelial cells) was counted and expressed as capillary density per square millimeter of tissue. Myocardial arteriole (α-SMA+ smooth muscle cells located in vascular walls) density was measured using image-analysis software (ImageJ, NIH) (4).
Measurement of myocardial infarction size by 2,3,5-triphenyltetrazolium chloride staining.
Twenty-four hours after the initiation of ischemia, hearts were excised and sliced into five 1-mm-thick cross-sections below the ligature. Heart sections were incubated in 1% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma) and kept at 37°C for 30 min to stain the viable myocardium red and show the infaction as pale areas. Infarcted and total LV areas from both cut surfaces of each section were measured with Image Pro-express software (40).
Myocardial cell apoptosis.
Heart tissue sections underwent TUNEL following the manufacturer's instructions (Promega). Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Apoptosis was indexed by counting the number of TUNEL+ cells per 100 nuclei in infarcted tissue (40).
Myocardial CD133, Sca1, c-Kit, CXCR-4, and SDF-1α expression.
Heart tissue sections (8 μm thick) were incubated with CD133, CXCR-4, Sca1, c-Kit, and SDF-1α (1:200, Santa Cruz Biotechnology) antibodies overnight. CD133, Sca1, and CXCR-4 were visualized using FITC-labeled goat anti-mouse IgG antibodies; c-Kit and SDF-1α were visualized with Fluorolink Cy3-labeled goat anti-mouse IgG antibodies (1:200). Sections were counterstained with DAPI. Myocardial CD133+/c-Kit+/Sca1+, CD133+/SDF-1α+, and c-Kit+/CXCR4+ cells were assessed by counting the numbers of positive cells per 100 nuclei of ischemic tissue (43).
Cardiac function.
Experimental and sham control mice were anesthetized with ketamine (100 mg/kg) plus xylazine (15 mg/kg), intubated, and artificially ventilated with room air. A 1.4-Fr pressure-conductance catheter (SPR-839, Millar Instruments, Houston, TX) was inserted into the LV to record baseline cardiac hemodynamics of the hearts. The method was based on measuring the time-varying electrical conductance signal of two segments of blood in the LV from which total volume is calculated. Raw conductance volumes were corrected for parallel conductance by the hypertonic saline dilution method (43).
Heart weight-to-body weight ratio and fibrosis.
Cardiac hypertrophy was assessed by measuring the heart-to-body weight ratio at 14 days postmyocardial ischemia. Each heart weight was divided by the total body weight of the mouse, resulting in a ratio representative of cardiac hypertrophy. Cardiac β-myosin heavy chain (β-MHC) and atrial natriuretic pepetide (ANP) expression were examined by Western blot analysis. To determine cardiac fibrosis, sections were stained with Masson's trichrome (Sigma). Myocardial fibrosis was quantified by measuring the blue fibrotic area using NIH Image-analysis software as previously described (4).
Bone marrow-derived PC culture.
At 24 h and 14 days of myocardial ischemia, bone marrow (BM)-derived PCs (BMPCs) were obtained by flushing the tibias and femurs with 10% FBS endothelial growth medium. Immediately after isolation, 105 BM-derived mononuclear cells were plated into six-well culture plates. After 4 days of culture, nonadherent cells were removed, and adherent cells were washed three times with PBS. BMPCs were then harvested and cultured for 14 days. VEGF, apelin, and APJ levels were examined by Western blot analysis.
Statistical analysis.
Results are expressed as means ± SD. Statistical analysis was performed using one-way ANOVA followed by a multiple-comparisons test (Student-Newman-Keuls). Significance was set at P < 0.05.
RESULTS
Upregulation of apelin/APJ expression in hearts of post-MI mice.
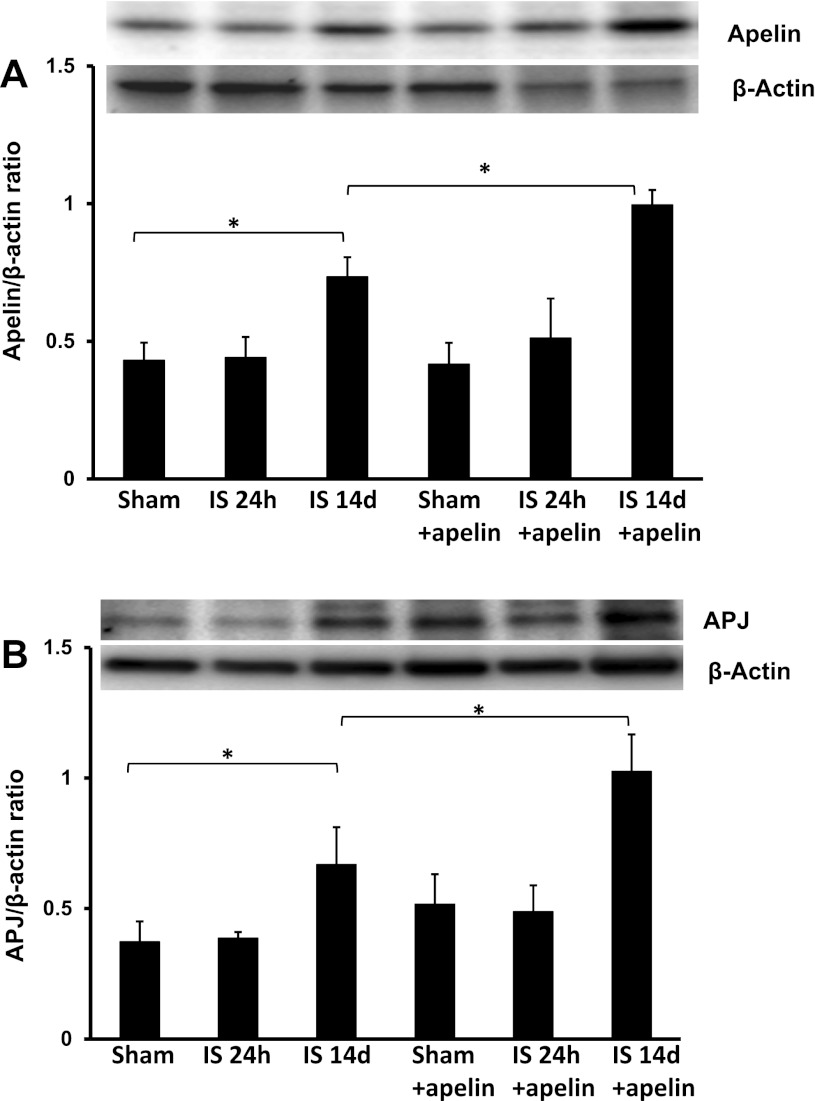
Western blot analysis demonstrated that apelin and APJ expression were significantly increased at 14 days in post-MI hearts but not at 24 h compared with sham control hearts (Fig. 1, A and B). Similarly, treatment with apelin-13 led to significant increases in apelin and APJ expression at 14 days post-MI compared with sham control hearts. Expression of apelin and APJ was significantly higher in 14-day apelin-13-treated post-MI hearts compared with 14-day untreated post-MI hearts (Fig. 1, A and B).
Fig. 1.
Apelin-13 increases apelin and angiotensin-like 1 receptor (APJ) expression in ischemic hearts. A: results from Western blot analysis demonstrating that exposure to ischemia (IS) for 14 days leads to a significant increase in apelin expression compared with sham-operated (sham) control mice. Treatment with apelin-13 further upregulated apelin expression in ischemic mouse hearts at 14 days. These changes were not seen at 24 h (n = 4 mice/group). *P < 0.05. B: results from Western blot analysis demonstrating that exposure to ischemia for 14 days leads to a significant increase in APJ expression compared with sham control mice. Treatment with apelin-13 further increased APJ expression in ischemic mouse hearts at 14 days. These changes were not apparent at 24 h (n = 4 mice/group). *P < 0.05.
Treatment with apelin-13 promotes vascular PC homing to infarcted hearts via upregulation of SDF-1α/CXCR-4 expression.
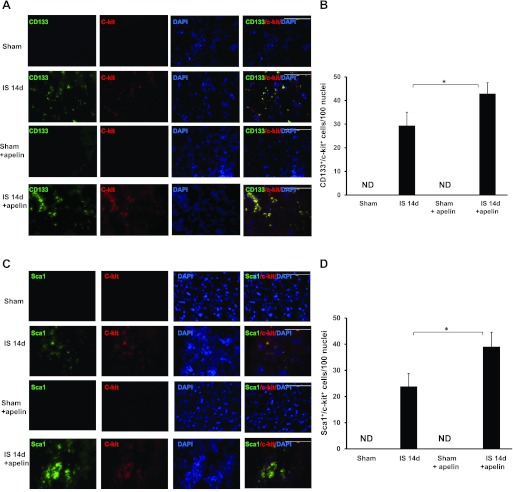
Next, we investigated whether treatment with apelin-13 affected homing of PCs into infarcted mouse hearts. CD133+/Sca1+/c-Kit+ cells were examined in the border zone of infarcted hearts at 24 h and 14 days after myocardial ischemia. Numbers of CD133+/Sca1+/c-Kit+ cells were increased in infarcted hearts at day 14 post-MI (Fig. 2, A–D). Treatment with apelin-13 resulted in a significant increase in CD133+/Sca1+/c-Kit+ cells compared with controls at day 14 post-MI. No CD133+/Sca1+/c-Kit+ cells were observed in sham control and apelin-13-treated sham control mouse hearts on day 14 (Fig. 2, A–D). Surprisingly, no CD133+/Sca1+/c-Kit+ cells were detected at 24 h of ischemia in the experimental groups (data not shown).
Fig. 2.
Apelin-13 promotes vascular progenitor cell (PC) homing into ischemic hearts. A: representative images showing the colocalization of CD133 with c-Kit in the border zone of infarcted mouse hearts at 14 days postmyocardial infaction (post-MI). CD133+ cells (green), c-Kit+ cells (red), and nuclei stained by 4′,6-diamidino-2-phenylindole (DAPI; blue) are shown. Magnification: ×40. CD133+ and c-Kit+ cells were recruited into the ischemic area of mouse hearts at day 14 post-MI. Merged images show that CD133+ cells colocalized with c-Kit+ cells (yellow) in post-MI animals and that the increase was more striking in post-MI animals that received apelin-13. No specific staining was observed in the sham control groups. B: results from quantitative analysis of CD133+/c-Kit+ cells demonstrating that the number of CD133+/c-Kit+ cells was significantly increased in apelin-13-treated mice subjected to MI compared with control ischemic mice. CD133+/c-Kit+ cells are expressed as total numbers per 100 nuclei. ND, not detected. All data are means ± SD; n = 4 mice/group. *P < 0.05. C: immunofluorescence images showing the colocalization of Sca1 and c-Kit in the border zone of ischemic mouse hearts. Sca1 was stained with mouse Sca1 antibody (green), c-Kit was stained with c-Kit antibody (red), and nuclei were stained with DAPI (blue). Magnification: ×40. Merged images showed that Sca1 colocalized with c-Kit (yellow) in hearts from post-MI mice and that the increase was more dramatic in post-MI animals that received apelin-13. No specific staining was observed in the sham control groups. D: results from quantitative analysis of Sca1+/c-Kit+ cells demonstrating that the number of Sca1+/c-Kit+ cells was significantly increased in apelin-13-treated mice subjected to MI compared with control ischemic mice. Sca1+/c-Kit+ cells are expressed as total numbers per 100 nuclei. All data are means ± SD; n = 4 mice/group. *P < 0.05.
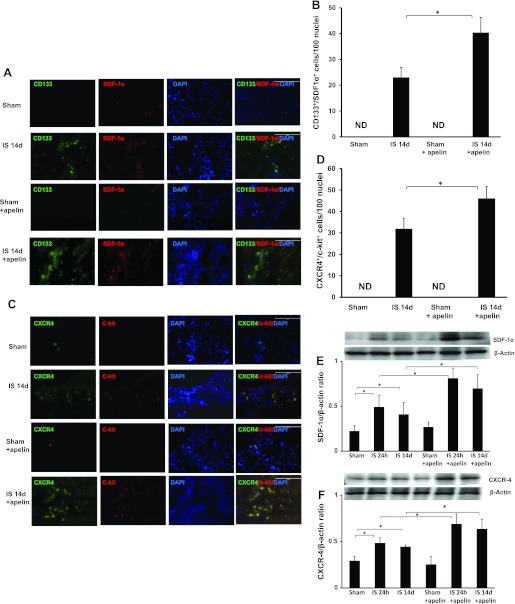
To examine the potential role of SDF-1α/CXCR-4 in apelin-13-induced vascular PC homing into infarcted mouse hearts, we examined the numbers of CD133+/SDF-1α and CXCR-4+/c-Kit+ cells and SDF-1α/CXCR-4 protein expression after 14 days of MI. As shown in Fig. 3, A–D, treatment with apelin-13 led to a significant increase in the numbers of CD133+/SDF-1α+ and CXCR-4+/c-Kit+ cells in the border zone of infarcted hearts compared with post-MI mice that did not receive apelin-13. No CD133+/SDF-1α+ and CXCR-4+/c-Kit+ cells were found in either sham control or apelin-13-treated sham control mouse hearts. Western blot analysis revealed that CXCR-4 and SDF-1α expression were significantly increased at 24 h and 14 days of ischemia. Furthermore, treatment with apelin-13 resulted in significant increases in CXCR-4 and SDF-1α expression compared with control ischemic hearts at both time points (Fig. 3, E and F).
Fig. 3.
Apelin-13 upregulates stromal cell-derived factor (SDF)-1α and C-X-C chemokine receptor (CXCR)-4 expression in ischemic hearts. A: immunofluorescence images showing the colocalization of CD133 with SDF-1α in the border zone of infarcted mouse hearts. CD133+ cells (green), SDF-1α+ cells (red), and nuclei stained by DAPI (blue) are shown. Magnification: ×40. Merged images show that CD133+ cells colocalized with SDF-1α+ in ischemic control and apelin-13-treated ischemic mice. No specific staining was observed in the sham control groups. B: results from quantitative analysis of CD133+/SDF-1α+ cells demonstrating that the number of CD133+/SDF-1α+ cells was significantly increased in apelin-13-treated mice subjected to MI compared with control ischemic mice. CD133+/SDF-1α+ cells are expressed as total numbers per 100 nuclei. All data are means ± SD; n = 4 mice/group. *P < 0.05. C: immunofluorescence images showing colocalization of c-Kit with CXCR-4 in the border zone of infarcted mouse hearts. CXCR-4+ cells (green), c-Kit+ cells (red), and nuclei stained by DAPI (blue) are shown. Magnification: ×40. Merged images show that CXCR-4+ cells colocalized with c-Kit+ in ischemic control and apelin-13-treated ischemic mice. No specific staining was observed in the sham control groups. D: results from quantitative analysis of CXCR-4+/c-Kit+ cells demonstrating that the number of CXCR-4+/c-Kit+ cells was significantly increased in apelin-13-treated mice subjected to MI compared with control ischemic mice. CXCR-4+/c-Kit+ cells are expressed as total numbers per 100 nuclei. All data are means ± SD; n = 4 mice/group. *P < 0.05. E: results from Western blot and densitometric analyses demonstrating that SDF-1α expression was significantly increased in mouse hearts subjected to ischemia for 24 h and 14 days compared with sham control hearts. Treatment with apelin-13 significantly increased SDF-1α expression in ischemic hearts compared with ischemic control harts. All data are means ± SD; n = 4 mice/group. *P < 0.05. F: results from Western blot and densitometric analyses of myocardial CXCR-4 expression showing that expression was significantly increased in mouse hearts subjected to ischemia for 24 h and 14 days compared with sham control hearts. Treatment with apelin-13 significantly increased CXCR-4 expression in ischemic hearts compared with ischemic control hearts. All data are means ± SD; n = 4 mice/group. *P < 0.05.
Treatment with apelin-13 attenuates myocardial infarct size and apoptosis.
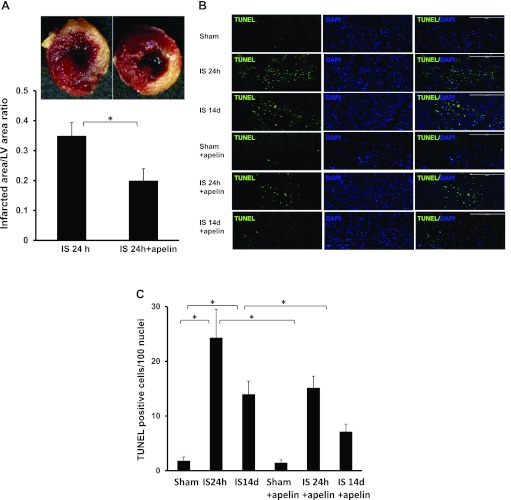
To determine whether treatment with apelin-13 minimizes the area of MI and apoptosis, we performed TTC and TUNEL staining analyses at 24 h and 14 days post-MI. Treatment with apelin-13 led to a significant decrease in the area of MI, as assessed by the ratio of the infarcted area to LV area at 24 h (Fig. 4A). TUNEL staining further revealed that mouse hearts subjected to 24 h and 14 days of myocardial ischemia significantly increased numbers of TUNEL+ cells in the infarcted area of the LV compared with sham control hearts. Treatment with apelin-13 resulted in a significant reduction of TUNEL+ cells in the infarcted area of the LV at both time points (Fig. 4, B and C).
Fig. 4.
Apelin-13 attenuates myocardial apoptosis and reduces infarct size. A: representative images and quantification of infarct size in the left ventricle of control post-MI and apelin-13-treated mice after 24 h of ischemia. The noninfarcted area appears red and the infarct area appears white after 2,3,5-triphenyltetrazolium chloride (TTC) staining. The myocardial infarct area was significantly reduced in apelin-13-treated mice compared with control post-MI mice (n = 3 mice/group). *P < 0.05. B: representative images of TUNEL-stained heart sections of the infarcted area from sham control and apelin-13-treated mice 24 h and 14 days post-MI. Apoptotic cells in the infarcted area of the left ventricle were identified by TUNEL staining (green) and total nuclei by DAPI counterstaining (blue). Magnification: ×10. TUNEL staining was seen post-MI at 24 h and 14 days, but in apelin-treated mice, fewer TUNEL+ cells were found at each time point. C: results from quantitative analysis of apoptotic cells in the infarcted area of control and apelin-13-treated mouse hearts 24 h and 14 days after sham surgery or ischemia. TUNEL+ cells are expressed as total numbers per 100 nuclei in the infracted area. Apoptotic cells were significantly increased post-MI at 24 and 14 days. Significantly fewer TUNEL+ cells were seen in apelin-13-treated mice at both time points (n = 4 mice/group). *P < 0.05.
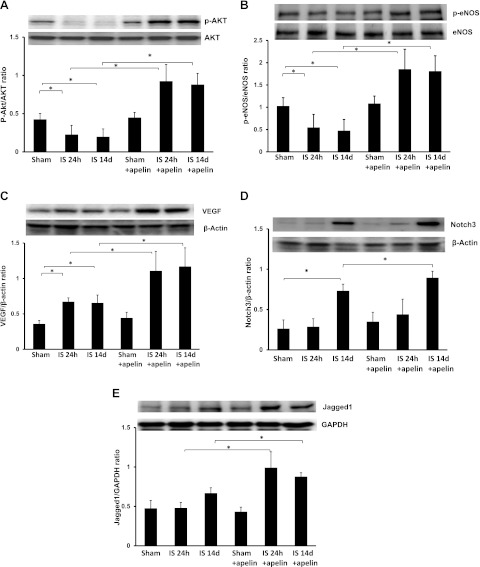
Apelin-13 increases Akt and eNOS phosphorylation and upregulates angiogenic growth factor expression.
To explore the potential intracellular molecular mechanism by which apelin-13 attenuates myocardial apoptosis, myocardial expression of prosurvival signaling Akt/eNOS was examined. Western blot analysis showed that phosphorylation of Akt and eNOS was significantly decreased in the border zone of mouse hearts subjected to ischemia for 24 h and 14 days compared with sham control hearts, whereas total eNOS and Akt expression showed little change. Treatment with apelin-13 led to significant increases in Akt and eNOS phosphorylation at 24 h and 14 days after myocardial ischemia, whereas total levels of Akt and eNOS were unchanged (Fig. 5, A and B).
Fig. 5.
Apelin-13 increases survival and angiogenic growth factor expression in ischemic hearts. A: representative results from Western blot and densitometric analyses of myocardial Akt phosphorylation. Phosphorylated (p-)Akt was significantly reduced in mouse hearts subjected to ischemia for 24 h and 14 days compared with sham control hearts. Treatment with apelin-13 significantly prevented ischemia-induced downregulation of Akt phosphorylation and increased levels over sham control values at both time points (n = 4 mice/group). *P < 0.05. B: representative results from Western blot and densitometric analyses of myocardial endothelial nitric oxide synthase (eNOS) phosphorylation. p-eNOS was significantly reduced in mouse hearts subjected to ischemia for 24 h and 14 days compared with sham control hearts. Treatment with apelin-13 significantly prevented and increased levels over sham control levels of ischemia-induced downregulation of eNOS phosphorylation. (n = 4 mice/group). *P < 0.05. C: representative results from Western blot and densitometry analyses of myocardial VEGF protein expression at 24 h and 14 days after myocardial ischemia. VEGF expression was significantly increased in ischemic control mice compared with sham control mice at 24 h and 14 days. Treatment with apelin-13 resulted in a significant increase in VEGF expression compared with control mice subjected to myocardial ischemia for 24 h and 14 days and increased levels over sham controls (n = 4 mice/group). *P < 0.05. D: representative results from Western blot and densitometric analyses of Notch3 expression. Notch3 expression was significantly increased in ischemic control mice compared with sham control mice at 14 days. Treatment with apelin-13 resulted in significant increases in Notch3 expression compared with ischemic control mice at 14 days (n = 4 mice/group). *P < 0.05. E: representative results from Western blot and densitometric analyses of jagged1 expression. Jagged1 expression was similar in sham control mice at 24 h and 14 days post-MI. Treatment with apelin-13 resulted in a significant increase in jagged1 expression compared with ischemic control mice at 24 h and 14 days post-MI (n = 4 mice/group). *P < 0.05.
Since apelin has been shown to be involved in the regulation of neovascularization after ischemia, we next examined the expression of VEGF, jagged1, and notch3. As shown in Fig. 5C, VEGF expression was significantly upregulated in the border zone of infarcted hearts after 24 h and 14 days of ischemia compared with sham control hearts at both 24 h and 14 days. The expression of VEGF was significantly enhanced in apelin-13-treated ischemic hearts compared with ischemic control hearts at both time points. Intriguingly, notch3 expression was only increased at 14 days post-MI compared with sham control hearts, whereas jagged1 expression was unaffected (Fig. 5, D and E). Treatment with apelin-13 further enhanced notch3 expression at 14 days post-MI (Fig. 5D). Surprisingly, jagged1 expression was significantly upregulated at both time points in apelin-13-treated mouse hearts (Fig. 5E).
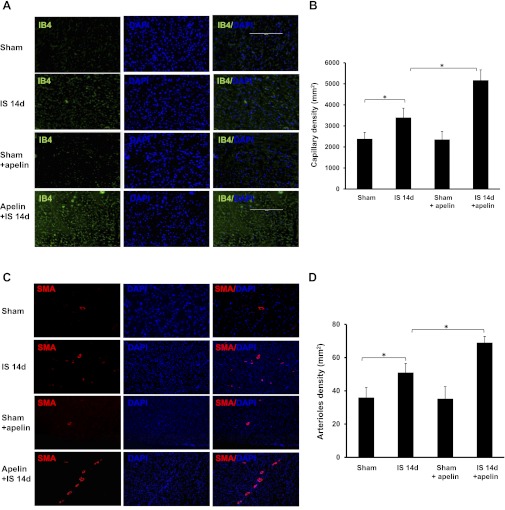
Apelin-13 increases myocardial capillary and arteriole density.
To investigate whether apelin-13 improves myocardial angiogenesis in vivo, myocardial capillary density and arteriole formation were examined at 14 days post-MI. Hearts subjected to ischemia showed a significant increase in myocardial capillary density in the border zone at 14 days post-MI compared with sham control hearts. Treatment with apelin-13 led to a further significant increase in myocardial capillary density at 14 days post-MI (Fig. 6, A and B). Furthermore, the number of arterioles in the border zone was significantly increased in apelin-13-treated mouse hearts compared control mouse hearts at 14 days post-MI (Fig. 6, C and D).
Fig. 6.
Apelin-13 enhances myocardial neovascularization in ischemic hearts. A and B: representative images (A) and quantitative analysis (B) showing that myocardial ischemia significantly increased myocardial capillary density by isolectin B4 (IB4) staining at 14 days. Treatment of ischemic mice with apelin-13 significantly increased capillary formation compared with ischemic control mice (n = 5 mice/group). *P < 0.05. C and D: representative images (C) and quantitative analysis (D) of α-smooth muscle actin (SMA) staining showing that myocardial ischemia significantly increased myocardial arteriole density in ischemic control mice compared with sham control mice at 14 days. Treatment of ischemic mice with apelin-13 caused a significant increase in arteriole formation compared with ischemic control mice (n = 6 mice/group). *P < 0.05.
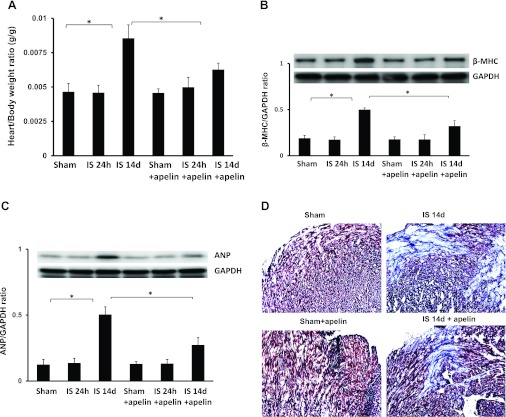
Apelin-13 prevents cardiac hypertrophy and improves cardiac functional recovery in post-MI mice.
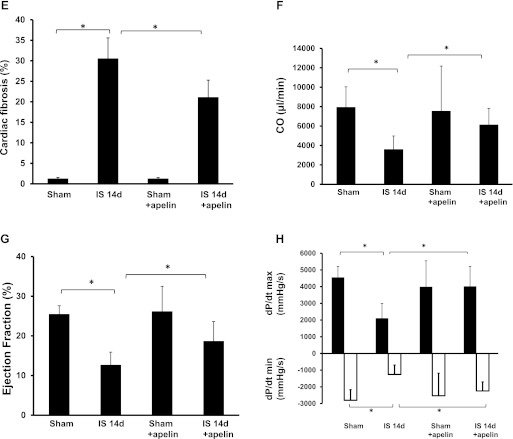
The heart weight-to-body weight ratio and myocardial fibrosis were evaluated to further investigate the consequences of apelin-13-induced homing of vascular PCs on cardiac remodeling. As shown in Fig. 7A, the heart weight-to-body weight ratio was significantly increased in post-MI mice compared with sham control mice. Treatment with apelin-13 led to a significant decrease in the heart weight-to-body weight ratio in post-MI mice compared with post-MI mice alone (Fig. 7A). Furthermore, expression of the hypertrophic genes β-MHC and ANP was significantly increased in mice 14 days post-MI. Treatment with apelin-13 led to significant decreases in β-MHC and ANP expression in mice 14 days post-MI (Fig. 7, B and C). Myocardial fibrosis in the infarcted area was also significantly increased in post-MI mice compared with sham control mice (Fig. 7, D and E). Treatment with apelin-13 resulted in a significant decrease in myocardial fibrosis compared with control mice at 14 days post-MI (Fig. 7, D and E).
Fig. 7.
Apelin-13 reduces cardiac hypertrophy/fibrosis and improves cardiac functional recovery in post-MI mice. A: heart weight-to-body weight ratio in sham control, ischemic, and apelin-13-treated mouse hearts at 24 h and 14 days post-MI. A significant increase in cardiac hypertrophy was observed in mouse hearts after 14 days of ischemia. Apelin-13 treatment significantly suppressed cardiac hypertrophy compared with mice treated with saline (n = 4–6 mice/group). *P < 0.05. B: results from Western blot and densitometric analyses demonstrating that β-myosin heavy chain (β-MHC) expression was significantly increased in 14-day post-MI mice compared with sham control mice. Treatment with apelin-13 significantly decreased β-MHC expression in 14-day post-MI mice compared with ischemic control mice. All data are means ± SD; n = 4 mice/group. *P < 0.05. C: results from Western blot and densitometric analyses demonstrating that atrial natriuretic peptide (ANP) expression was significantly increased in 14-day post-MI mice compared with sham control mice. Treatment with apelin-13 significantly attenuated ANP expression in 14-day post-MI mice compared with ischemic control mice. All data are means ± SD; n = 4 mice/group. *P < 0.05. D and E: representative images of cardiac fibrosis in the infarction zone (D) and quantitative analysis of the fibrotic area (E) in mice treated with saline or apelin-13 (Masson's trichrome stain). Apelin-13 significantly reduced the area of cardiac fibrosis (blue) in ischemic mice compared with saline-treated ischemic mice. All data are means ± SD; n = 5 mice/group. *P < 0.05. F: cardiac output (CO) was significantly reduced in 14-day post-MI mice (n = 6) compared with sham control mice (n = 5). Apelin-13 treatment (n = 6) significantly increased CO compared with ischemic control mice. All data are means ± SD; *P < 0.05. G: myocardial ejection fraction (EF; in %) was significantly decreased in 14-day post-MI mice (n = 6) compared with sham control mice (n = 5). Apelin-13 treatment (n = 6) significantly increased EF compared with ischemic control mice. All data are means ± SD. *P < 0.05. H: left ventricular function was significantly improved in ischemic mice treated with apelin-13 compared with saline-treated ischemic mice, as shown by the higher +dP/dtmax and lower −dP/dtmin at 14 days of ischemia. All data are means ± SD; n = 6 apelin-13-treated ischemic mice, 6 saline-treated ischemic mice, and 5 sham control mice. *P < 0.05.
To further investigate whether treatment with apelin-13 improves cardiac functional recovery post-MI, cardiac function was measured in sham control, post-MI, or apelin-13-treated post-MI mice at 14 days. Load-dependent hemodynamic parameters were assessed with the catheter in the LV (Table 1). As shown in Table 1, by 14 days post-MI, there was a decrease in cardiac contractility, as reflected by decreases in stroke work, stroke volume, end-systolic pressure, and the end-systolic pressure-volume relationship. Treatment with apelin-13 significantly improved cardiac contractility in 14 days post-MI mice. Treatment with apelin-13 alone had little effect on cardiac contractility but significantly increased end-diastolic and end-diastolic pressure-volume relationships (Table 1). Post-MI mice also showed significant reductions in cardiac function, as reflected by decreased cardiac output and ejection fraction, lower +dP/dtmax, and higher −dP/dtmin compared with sham control mice. Treatment with apelin-13 caused significant increases in each of these variables compared control mouse hearts at 14 days post-MI (Fig. 7, F–H).
Table 1.
Hemodynamic parameters of cardiac function in mice treated with apelin-13 for 14 days after myocardial infaction
| Parameter | Control | 14-Day Apelin | 14-Day Ischemia | 14-Day Ischemia + Apelin |
|---|---|---|---|---|
| Number of mice | 5 | 8 | 6 | 6 |
| Stroke work, mmHg/μl | 2,056 ± 969 | 2,184 ± 1046 | 840 ± 674* | 2,384 ± 551† |
| Stroke volume, μl | 29.6 ± 8.3 | 33.1 ± 13.8 | 20.0 ± 7.4 | 36.4 ± 8.6† |
| End-systolic volume, μl | 92.3 ± 16.6 | 96.3 ± 17.2 | 143.4 ± 25.0* | 119.2 ± 11.3 |
| End-diastolic volume, μl | 113.1 ± 21.9 | 121.9 ± 26.1 | 155.9 ± 30.9* | 148.3 ± 16.9 |
| End-systolic pressure, mmHg | 91.1 ± 20.7 | 121.2 ± 44.9 | 61.2 ± 18.4* | 92.6 ± 7.9† |
| End-diastolic pressure, mmHg | 7.7 ± 3.8 | 26.0 ± 9.0* | 6.8 ± 8.4 | 13.7 ± 7.3 |
| Heart rate, beats/min | 283 ± 91 | 237 ± 119 | 184 ± 64 | 171 ± 26 |
| Arterial elastance, mmHg/μl | 3.2 ± 1.0 | 4.9 ± 4.0 | 3.4 ± 1.2 | 2.9 ± 1.0 |
| End-systolic pressure-volume relationship, mmHg/μl | 1.0 ± 0.24 | 1.33 ± 0.65 | 0.43 ± 0.11* | 0.79 ± 0.13† |
| End-diastolic pressure-volume relationship, mmHg/μl | 0.069 ± 0.036 | 0.23 ± 0.12* | 0.043 ± 0.043 | 0.089 ± 0.042 |
Values are means ± SD.
P < 0.05 vs. the control group;
P < 0.05 vs. the 14-day ischemic group.
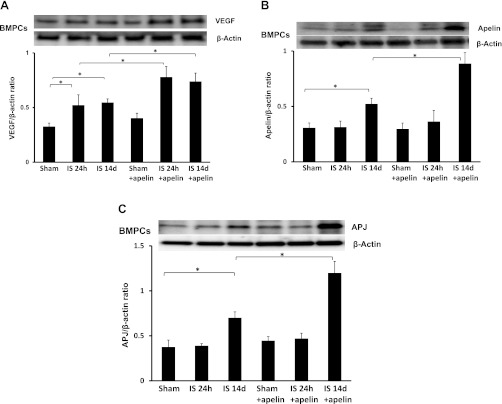
Apelin-13 increases VEGF and apelin/APJ expression in BMPCs from post-MI mice.
Recent studies have indicated that BMPCs stimulate myocardial angiogenesis and promote ischemic functional recovery through a paracrine mechanism (6, 19). To investigate whether apelin-13 increases angiogenic factors in BMPCs, the expression of VEGF and apelin/APJ was examined. As shown in Fig. 8A, VEGF expression was significantly increased in BMPCs of apelin-13-treated post-MI mice. Fourteen days of MI resulted in increased expression of apelin and APJ expression in BMPCs compared with sham control mice. Treatment with apelin-13 resulted in further increases in apelin and APJ expression in BMPCs of post-MI mice at 14 days compared with MI alone (Fig. 8, B and C).
Fig. 8.
Treatment with apelin-13 increases VEGF and apelin/APJ expression in bone marrow (BM)-derived PCs (BMPCs). A: results from Western blot analyses of VEGF expression in BMPCs. VEGF expression was significantly upregulated in BMPCs of apelin-13-treated ischemic mice compared with saline-treated mice at 24 h and 14 days post-MI. B and C: results from Western blot analyses of apelin (B) and APJ (C) expression in BMPCs. Apelin and APJ expression were upregulated in BMPCs of 14-day post-MI mice compared with sham control mice. Treatment with apelin-13 for 14 days further increased apelin and APJ expression in BMPCs of post-MI mice.
DISCUSSION
Our data demonstrate that treatment with apelin-13 leads to a significant increase in homing of CD133+/c-Kit+/Sca1+ to ischemic areas after experimental MI in mice. This is accompanied by significant increases in myocardial capillary density and arteriole formation. Apelin-13 treatment also results in a significant improvement of cardiac function post-MI. Our study also reveals that apelin-13 upregulates SDF-1α and CXCR-4 expression in the infarcted heart. These results suggest that apelin-13 promotes cardiac repair and functional recovery post-MI by promoting vascular PC homing via a mechanism involving SDF-1α/CXCR-4 signaling.
Apelin-13 has been previously shown to alleviate myocardial ischemic injury in animal models (24, 30, 35, 44); however, the underlying mechanisms by which apelin-13 promotes cardiac repair and functional recovery are not completely understood. Accumulating evidence reveals that BMPCs are involved in healing the ischemic myocardium and may be important in functional recovery post-MI (2, 17, 42). Gao and colleagues (12) showed that transplantation of BM led to a significant increase in apelin levels and an improvement of cardiac dysfunction in patients with severe HF. Furthermore, they demonstrated that expression of the apelin-APJ pathway during the differentiation of BM into cardiomyogenic cells contributed to myocardial regeneration and functional recovery after BM transplantation (13). Previous studies also revealed that loss of apelin and APJ function affect endothelial, hematopoietic, and cardiac PC differentiation (16, 45). APJ has also been shown to regulate the migration of cells fated to form myocardial PCs (34). Most recently, apelin and APJ were found as important promoters of mammalian cardiomyogenesis (7). To our knowledge, the present study is the first demonstration that treatment with apelin-13 significantly increases the number of CD133+/c-Kit+/Sca1+ cells homing to myocardial ischemic areas. The increased number of CD133+/c-Kit+ cells colocalize with CXCR-4+ and SDF-1α + cells in the ischemic area, suggesting that activation of SDF-1α/CXCR-4 signaling may contribute to apelin-13-induced homing of vascular PCs into the ischemic heart. Our data also show that treatment with apelin-13 significantly increases myocardial VEGF, apelin and APJ expression, and Akt and eNOS phosphorylation at 14 days post-MI. Most intriguingly, treatment with apelin-13 leads to significant increases in VEGF and apelin/APJ expression in BMPCs of post-MI mice. Based on these findings, we postulate that apelin-13 promotes vascular PC homing to ischemic areas, leading to the secretion of VEGF and apelin as well as activation of the Akt/eNOS signaling pathway, which attenuates myocardial apoptosis and improves cardiac function post-MI. Our present study showed that CXCR-4 and SDF-1α levels were elevated after 24 h of ischemia; surprisingly, homing of CD133+/c-Kit+ cells was not apparent in the ischemia area. A previous study has revealed that the numbers of PCs in circulation were significantly decreased on day 1 post-MI but markedly increased on days 3 and 5 post-MI (38). Interestingly, CXCR4+ cells from both the circulation and BM of post-MI mice were significantly increased compared with those of noninfarcted mice (38). The discrepancy of CXCR-4/SDF-1α expression and homing of PCs seen in our present study may be due to the decreased numbers of PCs mobilizing from the BM to the circulation on day 1 post-MI. In addition, our study reveals that treatment with apelin-13 upregulates prosurvival signaling VEGF expression and Akt/eNOS phosphorylation at 24 h of ischemia. Treatment with apelin-13 also reduces myocardial apoptosis and infarction size at 24 h of ischemia, suggesting a direct action of apelin-13 on ischemic hearts. This direct protection by apelin-13 against myocardial ischemic injury may also contribute, at least in part, to the improvement of cardiac functional recovery in post-MI mice. We also found that treatment with apelin-13 for 14 days alone led to a significant increase in the end-diastolic pressure-volume relationship, but whether treatment with apelin-13 alone causes cardiac hypertrophy, which leads to diastolic dysfunction, needs further investigation.
Increased angiogenesis and the formation of neovessels are critical processes in the restoration of coronary blood flow and in the repair of ischemic injury post-MI. Myocardial function is significantly improved by promoting angiogenesis in ischemic areas (32). Our present data also show that treatment with apelin-13 increases myocardial capillary density and promotes mature and large arteriole formation in infarcted hearts. This was accompanied by a significant improvement of cardiac function post-MI. Our data further demonstrate that treatment with apelin-13 results in a significant upregulation of jagged1 and notch3 expression in post-MI mouse hearts. The notch ligand jagged1 is essential for vascular remodeling and has been linked to congenital HF in humans (9, 36, 41). The notch ligand jagged1 plays a critical role in the regulation of vascular smooth muscle cell differentiation and vessel development via notch3 during early embryonic development (14). Deficiency of notch3 has been shown to disrupt vascular smooth muscle cell differentiation and to increased infarct size in ischemic stroke (1, 8, 21). A mutation in notch3 leads to a reduction in the diameter of cerebral arteries and capillary density (21) as well as increased risk of MI (27). The increased expression of jagged1/notch3 may contribute to the larger and matured arteriole formation seen in infarcted hearts after apelin-13 treatment. Taken together, our data implicate the apelin/APJ system in the regulation of angiogenesis by a mechanism involving the activation of jagged1/notch3 pathways. Further studies are needed to elucidate the molecular mechanisms and interactions between jagged1/notch3 and apelin/APJ pathways in the regulation of cardiac angiogenesis and cardiac repair post-MI.
The present study demonstrates that treatment with apelin-13 promotes vascular PC homing and a dramatic improvement of cardiac function in mice post-MI. These changes are associated with a significant upregulation of SDF-1α/CXCR-4 expression and improvement of myocardial angiogenesis. These results provide a novel mechanistic explanation for how apelin-13 might improve cardiac function in patients with MI and HF. The results of the present study also suggest that apelin-13 or pharmacological agonists of the APJ receptor could act as novel therapies for the treatment of patients with coronary artery disease.
GRANTS
This work was supported National Heart, Lung, and Blood Institute Grant HL-102042 (to J.-X. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.L. and H.Z. performed experiments; L.L. and H.Z. analyzed data; L.L. interpreted results of experiments; L.L. prepared figures; J.-X.C. conception and design of research; J.-X.C. drafted manuscript; J.-X.C. edited and revised manuscript; J.-X.C. approved final version of manuscript.
REFERENCES
- 1. Arboleda-Velasquez JF, Zhou Z, Shin HK, Louvi A, Kim HH, Savitz SI, Liao JK, Salomone S, Ayata C, Moskowitz MA, Artavanis-Tsakonas S. Linking notch signaling to ischemic stroke. Proc Natl Acad Sci USA 105: 4856–4861, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a. Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362: 697–703, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Barcelos LS, Duplaa C, Krankel N, Graiani G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica M, Simm A, Campagnolo P, Mangialardi G, Stevanato L, Alessandri G, Emanueli C, Madeddu P. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res 104: 1095–1102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation 108: 2212–2218, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Chen JX, Stinnett A. Ang-1 gene therapy inhibits hypoxia-inducible factor-1α (HIF-1α)-prolyl-4-hydroxylase-2, stabilizes HIF-1α expression, and normalizes immature vasculature in db/db mice. Diabetes 57: 3335–3343, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cox CM, D'Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol 296: 177–189, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Crisostomo PR, Abarbanell AM, Wang M, Lahm T, Wang Y, Meldrum DR. Embryonic stem cells attenuate myocardial dysfunction and inflammation after surgical global ischemia via paracrine actions. Am J Physiol Heart Circ Physiol 295: H1726–H1735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D'Aniello C, Lonardo E, Iaconis S, Guardiola O, Liguoro AM, Liguori GL, Autiero M, Carmeliet P, Minchiotti G. G protein-coupled receptor APJ and its ligand apelin act downstream of Cripto to specify embryonic stem cells toward the cardiac lineage through extracellular signal-regulated kinase/p70S6 kinase signaling pathway. Circ Res 105: 231–238, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Dichgans M. Genetics of ischaemic stroke. Lancet Neurol 6: 149–161, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev 18: 2730–2735, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foldes G, Horkay F, Szokodi I, Vuolteenaho O, Ilves M, Lindstedt KA, Mayranpaa M, Sarman B, Seres L, Skoumal R, Lako-Futo Z, deChatel R, Ruskoaho H, Toth M. Circulating and cardiac levels of apelin, the novel ligand of the orphan receptor APJ, in patients with heart failure. Biochem Biophys Res Commun 308: 480–485, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Frederick JR, Fitzpatrick JR, III, McCormick RC, Harris DA, Kim AY, Muenzer JR, Marotta N, Smith MJ, Cohen JE, Hiesinger W, Atluri P, Woo YJ. Stromal cell-derived factor-1α activation of tissue-engineered endothelial progenitor cell matrix enhances ventricular function after myocardial infarction by inducing neovasculogenesis. Circulation 122: S107–S117, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao LR, Xu RY, Zhang NK, Chen Y, Wang ZG, Zhu ZM, Fei YX, Cao Y, Xu HT, Yang Y. Increased apelin following bone marrow mononuclear cell transplantation contributes to the improvement of cardiac function in patients with severe heart failure. Cell Transplant 18: 1311–1318, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Gao LR, Zhang NK, Bai J, Ding QA, Wang ZG, Zhu ZM, Fei YX, Yang Y, Xu RY, Chen Y. The apelin-APJ pathway exists in cardiomyogenic cells derived from mesenchymal stem cells in vitro and in vivo. Cell Transplant 19: 949–958, 2010 [DOI] [PubMed] [Google Scholar]
- 14. High FA, Jain R, Stoller JZ, Antonucci NB, Lu MM, Loomes KM, Kaestner KH, Pear WS, Epstein JA. Murine jagged1/notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest 119: 1986–1996, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1α confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1α-CXCR4 axis. Circulation 116: 654–663, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inui M, Fukui A, Ito Y, Asashima M. Xapelin and Xmsr are required for cardiovascular development in Xenopus laevis. Dev Biol 298: 188–200, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Invernici G, Madeddu P, Emanueli C, Parati EA, Alessandri G. Human fetal aorta-derived vascular progenitor cells: identification and potential application in ischemic diseases. Cytotechnology 58: 43–47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest 103: 1231–1236, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jameel MN, Zhang J. Stem cell therapy for ischemic heart disease. Antioxid Redox Signal 13: 1879–1897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Japp AG, Newby DE. The apelin-APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem Pharmacol 75: 1882–1892, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, Lemaire-Carrette B, Domenga V, Schedl A, Lacombe P, Hubner N. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest 120: 433–445, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalin RE, Kretz MP, Meyer AM, Kispert A, Heppner FL, Brandli AW. Paracrine and autocrine mechanisms of apelin signaling govern embryonic and tumor angiogenesis. Dev Biol 305: 599–614, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kidoya H, Ueno M, Yamada Y, Mochizuki N, Nakata M, Yano T, Fujii R, Takakura N. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J 27: 522–534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kleinz MJ, Baxter GF. Apelin reduces myocardial reperfusion injury independently of PI3K/Akt and P70S6 kinase. Regul Pept 146: 271–277, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept 118: 119–125, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O'Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem 74: 34–41, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Lesnik Oberstein SA, Jukema JW, Van Duinen SG, Macfarlane PW, van Houwelingen HC, Breuning MH, Ferrari MD, Haan J. Myocardial infarction in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Medicine (Baltimore) 82: 251–256, 2003 [DOI] [PubMed] [Google Scholar]
- 28. O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 136: 355–360, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Quazi R, Palaniswamy C, Frishman WH. The emerging role of apelin in cardiovascular disease and health. Cardiol Rev 17: 283–286, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Rastaldo R, Cappello S, Folino A, Berta GN, Sprio AE, Losano G, Samaja M, Pagliaro P. Apelin-13 limits infarct size and improves cardiac postischemic mechanical recovery only if given after ischemia. Am J Physiol Heart Circ Physiol 300: H2308–H2315, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Sasaki H, Fukuda S, Otani H, Zhu L, Yamaura G, Engelman RM, Das DK, Maulik N. Hypoxic preconditioning triggers myocardial angiogenesis: a novel approach to enhance contractile functional reserve in rat with myocardial infarction. J Mol Cell Cardiol 34: 335–348, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI trial. J Am Coll Cardiol 44: 1690–1699, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Scott IC, Masri B, D'Amico LA, Jin SW, Jungblut B, Wehman AM, Baier H, Audigier Y, Stainier DY. The g protein-coupled receptor agtrl1b regulates early development of myocardial progenitors. Dev Cell 12: 403–413, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Simpkin JC, Yellon DM, Davidson SM, Lim SY, Wynne AM, Smith CC. Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemia-reperfusion injury. Basic Res Cardiol 102: 518–528, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Suchting S, Freitas C, le NF, Benedito R, Breant C, Duarte A, Eichmann A. Negative regulators of vessel patterning. Novartis Found Symp 283: 77–80, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Szokodi I, Tavi P, Foldes G, Voutilainen-Myllyla S, Ilves M, Tokola H, Pikkarainen S, Piuhola J, Rysa J, Toth M, Ruskoaho H. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res 91: 434–440, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Tano N, Kim HW, Ashraf M. microRNA-150 regulates mobilization and migration of bone marrow-derived mononuclear cells by targeting Cxcr4. PLos One 6: e23114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251: 471–476, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Tuo QH, Zeng H, Stinnett A, Yu H, Aschner JL, Liao DF, Chen JX. Critical role of angiopoietins/Tie-2 in hyperglycemic exacerbation of myocardial infarction and impaired angiogenesis. Am J Physiol Heart Circ Physiol 294: H2547–H2557, 2008 [DOI] [PubMed] [Google Scholar]
- 41. van den Akker NM, Caolo V, Wisse LJ, Peters PP, Poelmann RE, Carmeliet P, Molin DG, Gittenberger-de Groot AC. Developmental coronary maturation is disturbed by aberrant cardiac vascular endothelial growth factor expression and notch signalling. Cardiovasc Res 78: 366–375, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Voo S, Dunaeva M, Eggermann J, Stadler N, Waltenberger J. Diabetes mellitus impairs CD133+ progenitor cell function after myocardial infarction. J Intern Med 265: 238–249, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Zeng H, Li L, Chen JX. Overexpression of angiopoietin-1 increases CD133+/c-kit+ cells and reduces myocardial apoptosis in db/db mouse infarcted hearts. PLos One 7: e35905, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeng XJ, Zhang LK, Wang HX, Lu LQ, Ma LQ, Tang CS. Apelin protects heart against ischemia/reperfusion injury in rat. Peptides 30: 1144–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Zeng XX, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell 12: 391–402, 2007 [DOI] [PubMed] [Google Scholar]