Abstract
Cardiac dysfunction is a primary cause of patient mortality in Duchenne muscular dystrophy, potentially related to elevated cytosolic calcium. However, the regional versus global functional consequences of cellular calcium mishandling have not been defined in the whole heart. Here we sought for the first time to elucidate potential regional dependencies between calcium mishandling and myocardial fiber/sheet function as a manifestation of dystrophin-deficient (mdx) cardiomyopathy. Isolated-perfused hearts from 16-mo-old mdx (N = 10) and wild-type (WT; N = 10) were arrested sequentially in diastole and systole for diffusion tensor MRI quantification of myocardial sheet architecture and function. When compared with WT hearts, mdx hearts exhibited normal systolic sheet architecture but a lower diastolic sheet angle magnitude (|β|) in the basal region. The regional diastolic sheet dysfunction was normalized by reducing perfusate calcium concentrations. Optical mapping of calcium transients in isolated hearts (3 mdx and 4 WT) revealed a stretch-inducible regional defect of intracellular calcium reuptake, reflected by a 25% increase of decay times (T50) and decay constants, at the base of mdx hearts. The basal region of mdx hearts also exhibited greater fibrosis than did the apex, which matched the regional sheet dysfunction. We conclude that myocardial diastolic sheet dysfunction is observed initially in basal segments along with calcium mishandling, ultimately culminating in increased fibrosis. The preservation of relatively normal calcium reuptake and diastolic/systolic sheet mechanics throughout the rest of the heart, together with the rapid reversibility of functional defects by reducing cytosolic calcium, points to the significance of regional mechanical factors in the progression of the disease.
Keywords: diffusion tensor magnetic resonance imaging, myocardial sheet, Duchenne muscular dystrophy, calcium transients
duchenne muscular dystrophy (DMD) is a fatal X-linked genetic disease that affects 1:3,500 of new-born boys who manifest unremitting deterioration of all muscle groups over time. Cardiac dysfunction now accounts for 10∼20% of mortality in DMD, making it the second leading cause of patient death following respiratory failure (32), because of advances in respiratory support and patient care that have allowed many patients to survive into their late 20s and 30s (1, 38, 73). Accordingly, cardiac complications now are commanding closer scrutiny in the management of advanced disease in patients with DMD (24, 34, 46, 56).
The clinical DMD phenotype reflects the absence of normal dystrophin as a consequence of mutations of the dystrophin-encoding gene on the X chromosome. Dystrophin is a key component of the transmembrane dystrophin-associated glycoprotein complex that bridges intracellular actin and the extracellular matrix to facilitate mechanical coupling and signal transduction and to stabilize the sarcolemma membrane (25, 56, 66). Our previous work using magnetic resonance (MR) tagging uncovered occult abnormal wall strains occurring regionally at the base of heart in DMD patients (3). A subsequent report by Li et al. (44) also suggested that cardiac mechanical strain abnormalities are more prominent in the basal segments of hearts in muscular dytrophy (mdx) mice. These observed regional dependencies for DMD cardiac mechanical dysfunction remain to be explained, as the mutation is present homogeneously in all cardiomyocytes (5, 11, 70).
The heart comprises a syncytium of cardiomyocytes that are organized in unique three-dimensional (3-D) fiber-sheet architecture that is critical for optimizing a coordinated sequence of excitation, contraction, and relaxation (15, 62). Many reports have shown that sheet function produces a sliding motion that is accompanied by dynamic sheet reorientation in systole, which contributes ∼50% of focal wall thickening (14, 16, 41, 42). Because one role of dystrophin is to anchor cardiomyocytes to the extracellular matrix to coordinate contraction-relaxation coupling, we propose that dystrophin deficiency could adversely affect sheet function. Given that ventricular wall strain/stress vary regionally, and in light of the expected vulnerability of dystrophin deficient cardiomyocytes to stretch-induced membrane injury, we sought to examine the role of sheet function in the regional mechanical defects reported in the dystrophin-deficient cardiomyopathy (3, 44).
Diffusion tensor MRI (DTI) is an established method for rapid 3-D reconstruction of myocardial microarchitecture that can depict structural changes across the heart cycle in the intact heart. (14, 61). The technique involves a pixel-by-pixel estimation of the eigenvectors of water diffusion tensor from seven or more MR images (6, 7). The primary eigenvector, or the eigenvector corresponding to the largest eigenvalue, is considered to be aligned with the local orientation of cardiac myofibers, and thus defines the fiber angle (α) (27, 35, 36, 60). The secondary eigenvector, which corresponds to the second largest eigenvalue, is aligned parallel to sheet surfaces (68) and thus defines the sheet angle (β). This definition system is based on the fact that eigenvalues of a diffusion tensor are also the apparent diffusion coefficients describing microscopic diffusion movements, which is largest in the fiber direction and second largest in the sheet direction (13, 60). In addition to the orientation information, the diffusion tensor also provides shape information within the microdiffusion environment based on fractional anisotropy (FA) (22).
With respect to pathophysiological features of dystrophin deficiency potentially responsible for mechanical dysfunction, several recent studies in isolated cardiomyocytes of mdx mouse have suggested that intracellular Ca2+ concentration ([Ca2+]i) mishandling is a key causative factor (2, 23, 75). However, how calcium mishandling influences cardiac mechanical dysfunction in whole heart preparations has not been clarified, especially taking into account cellular and mechanical events that are spatio-temporally distributed (e.g., excitation-contraction coupling) and regionally heterogeneous (e.g., stress and strain) (4, 17). Given the recognized abnormal and regionally heterogeneous wall strains (3) and fibrosis (3, 76, 77) in DMD hearts and the similarly heterogeneous wall strains (44) and fibrosis (44, 63) in mdx mouse heart, the present work is focused on the hypothesis that the functional consequences of dystrophin deficiency play out regionally in DMD hearts over time, despite the ubiquitous genetic abnormality in all cardiomyocytes.
Accordingly, we sought to elucidate such regional mechanical consequences of the disease on local myocardial fiber-sheet function with the use of DTI, in concert with whole heart optical calcium mapping. The complete global 3-D information set acquired in isolated perfused beating mdx hearts should allow delineation of both the locus (regional and intramural) and the cycle dependence (diastole vs. systole) of the principle cardiac defects in dystrophin-deficiency and potentially shed light on whether these defects might be reversible (i.e., functional) consequences of calcium mishandling or be nonreversible consequences of fibrosis (i.e., remodeling).
MATERIALS AND METHODS
Animals
Adult male mdx (C57BL/10ScSn-Dmdmdx/J, N = 14) and age-matched male wild-type (WT: C57BL/10SnJ, N = 10; and C57BL/6, N = 3) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and the National Institute on Aging (18) and housed under an American Association for Accreditation of Laboratory Animal Care-approved facility to 16 mo of age. All mice were housed in the same animal facility and cared for under approval by Washington University's Animal Study Committee. All mice were separated into two groups for DTI and optical mapping studies.
DTI
Isolated heart preparation for DTI.
Ten mdx mice and 10 WT mice (C57BL/10SnJ) were used for DTI measurement. Mice were heparinized (1,000 U/kg) and anesthetized with 0.05 isoflurane. Hearts were excised and cannulated for retrograde perfusion using a procedure modified from Chen et al. (14). Briefly, hearts were first perfused with Krebs buffer at 60 mmHg constant pressure for ∼5 min to wash out the residue blood. Five mdx hearts and five WT hearts were perfusion arrested in diastole (perfusion pressure = 60 mmHg) with the regular St. Thomas' cardioplegic solution containing normal [Ca2+] of 1.2 mmol/l (denoted as mdx-NC and WT-NC, respectively) under room temperature. DTI of diastolic arrested hearts were performed for 1.5 h. After that, hearts were reperfused with 37°C Krebs buffer to resume beating. Upon stabilization of cardiac work (∼6 min, up to 300 beats/min), 2.5 mmol/l Ba2+ were introduced to induce systolic cardiac contracture while Ca2+ was lowered to 0.078 mmol/l (14, 49, 67). The systolic arrest of mdx-NC, WT-NC, mdx-LC, and WT-LC (defined below) are all achieved with the same modified Tyrode solution containing 2.5 mmol/l Ba2+ and 0.078 mmol/l Ca2+ (13). All solutions were equilibrated with 95% O2-5% CO2 (pH 7.4) and contained adenosine (45 μmol/l) to maximally dilate the coronary vessels (39). Bovine serum albumin (61 μmol/l) was added to the perfusate to minimize interstitial edema (20, 74). DTI of systolic hearts was imaged for ∼1.5 h. Upon the completion of MRI, all hearts were perfused with 10% formalin to fix in systole for histological study.
To evaluate the effect of [Ca2+] on the diastolic cardiomyocyte architecture in mdx mice, another five mdx hearts and five WT hearts were perfusion arrested in diastole with a modified cardioplegic solution containing low [Ca2+] of 0.078 mmol/l (denoted as mdx-LC and WT-LC, respectively). The 0.078 mmol/l [Ca2+] was chosen over the calcium-free condition to minimize the risk of complete depletion of intracellular calcium, which can cause calcium overload upon reperfusion with solutions containing normal [Ca2+], thereby exerting unfavorable effects on certain ion channels (49, 55).
A MR-compatible, viable mouse heart perfusion system.
A dedicated MRI-compatible, retrograde mouse heart perfusion system was used to acquire high signal-to-noise diffusion tensor MR images. This system provides a quantitative and nondestructive assessment of diastolic and systolic myocardial architecture from the same heart. The system includes 1) a custom-built 15-mm-diameter solenoid coil, which provides high signal to noise (65 for nondiffusion-weighted images, and 40 for diffusion-weighted images) and 2) a Langendorff constant-pressure perfusion apparatus that allows arrest of a viable heart sequentially in diastole and then in systole.
MRI procedure.
All MRI experiments were performed on a Varian 11.7-T small animal MR scanner (Varian Associates; Palo Alto, CA), and a custom-built 10-mm-diameter solenoid coil was used to acquire images. Long-axis scout images were acquired as previously described (14). A multislice spin-echo sequence with diffusion-sensitizing bipolar gradient was used to acquire short-axis, diffusion-weighted images. Diffusion-sensitizing gradients were applied in six noncolinear directions. Imaging parameters are as follows: echo time, 46 ms; time interval between diffusion-sensitizing gradients, 30 ms; b value, 0 and 800 s/mm2; in-plane resolution, 125 × 125 μm2; repetition time, 3 s; and image-acquisition time, 1 h. For diastolic-arrested hearts, eight contiguous slices (thickness = 0.7 mm) extending from the base to apex were acquired. For systolic-arrested hearts, eight contiguous slices were acquired with slice thickness adjusted to 0.55–0.65 mm based on the fraction of longitudinal shortening after barium-induced contracture. Attention was paid to keep the slice selection and the heart orientation the same between diastole and systole imaging.
DTI data analysis.
All MR images were processed using custom-developed software written in Matlab (MathWorks) as previously described (14). Fiber helix angle (α) and sheet angle (β) are calculated from the tensor according to the literature (14) (see the DTI paragraph in Introduction for background). Briefly, the fiber helix angle (α) was calculated as the angle between the circumferential cardiac axis and the projection of the first eigenvector of the diffusion tensor onto the circumferential-longitudinal plane; the right-handed helix was set as positive (60). Sheet angle (β) was determined as the angle between the secondary eigenvector and the radial axis; the sheet inclined toward the base from endocardium to epicardium was set as positive (Fig. 1) (60, 68). Because regional wall thickening is accompanied by a reduced magnitude of the sheet angle (|β|) despite the sign of sheet angle (14, 42), the |β| was used to analyze regional diastolic and systolic sheet function. Left ventricular (LV) wall thickness was calculated as the mean distance between epicardial and endocardial borders. The through-wall difference of α, defined as the difference in endocardial and epicardial helix angles, i.e., Δα = αendocardium − αepicardium, was used to quantify transmural changes of fiber orientation.
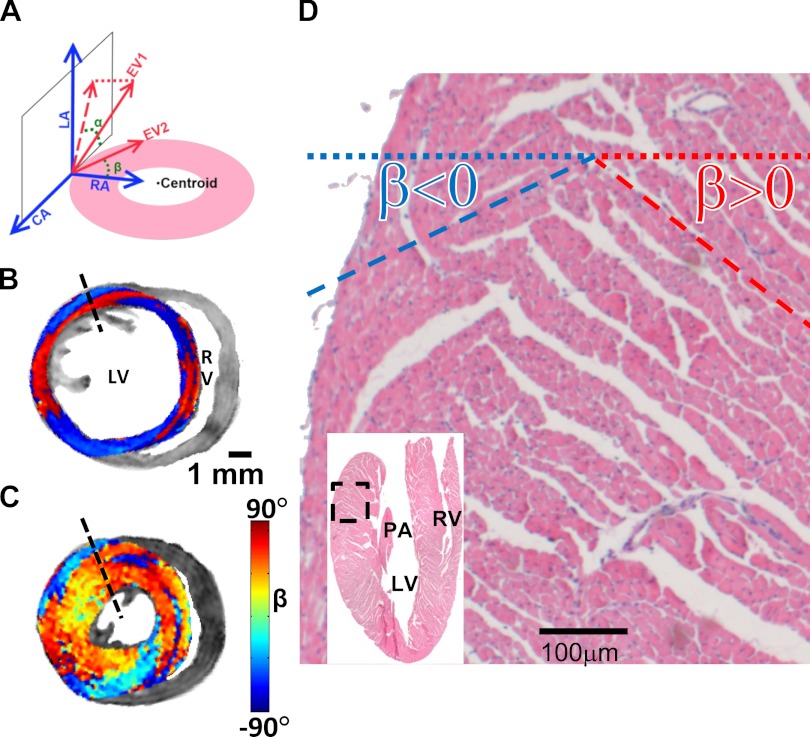
Fig. 1.
Representative diffusion tensor MRI (DTI) and histology images of sheet architecture in wild-type (WT) mouse hearts. A: a local cylindrical coordinate system was used to determine left ventricular (LV) myocardial structures. The primary eigenvector of the diffusion tensor is characterized by helix angle (α), which is the angle between the circumferential axis (CA) and the projection of the first eigenvector (EV1) onto the circumferential-longitudinal plane; the α shown in A is set as negative. The secondary eigenvector (EV2) is characterized by sheet angle (β), which is the angle between the secondary eigenvector and the radial axis (RA); the β shown in A, toward base and toward endocardium, is set as negative. B and C: short-axis views of DTI determined sheet angle (β) in a basal slice of WT heart that was sequentially arrested in diastole (B) and systole (C). The posterior-lateral wall of LV manifested two populations of β, exhibiting positive (red) and negative (blue) values. D: histology of sheet organization in a WT heart. Images were acquired from the basal-lateral wall, which is highlighted on the hematoxylin and eosin-stained image (inset). Two populations of β were observed. RV, right ventricle; PA, papillary muscle; P, posterior; L, left; R, right; A, anterior.
Histology.
After DTI acquisition, five mdx and five WT hearts were rapidly fixed in 10% formalin. Afterward, fixed hearts were sliced into four blocks with 2 mm thickness from base to apex. Each slice was embedded in paraffin and sectioned at 4 μm thickness. The tissue sections were stained with picrosirius red for the identification of fibrosis (71). Quantitative light microscopic analysis of histological samples was performed by video microscopy. The extent of myocardial fibrosis was quantified from digitized histological images using a thresholding algorithm implemented in MatLab. Regions that demonstrate stronger red-to-green ratio in picrosirius red staining were considered to represent interstitial fibrosis. The percentage of tissue fibrosis was calculated as the ratio of pixels representing fibrotic tissue versus. pixels of the entire short-axis slice, and the average value in two adjacent slices were used in analysis.
Optical Mapping
Isolated heart preparation and calcium dye loading.
Seven Langendorff-perfused hearts from 16-mo-old mice were separated into two groups [mdx, N = 4; and WT (C57BL/6), N = 3]. The isolated heart preparation was according to a Langendorff perfusion protocol modified for murine hearts (40). In brief, after anesthesia and heart excision, a short section of aorta was attached to a 21-gauge cannula. After cannulation, hearts were superfused and retrogradely perfused with oxygenated (95% O2-5% CO2) constant temperature (37 ± 1°C), modified Tyrode solution consisting of (in mmol/l) 128.2 NaCl, 4.7 KCl, 1.19 NaH2PO4, 1.05 MgCl2, 1.3 CaCl2, 20.0 NaHCO3, and 11.1 glucose (pH 7.3) that was passed through a 5-μm filter (Millipore, Billerica, MA). Perfusion was performed using a peristaltic pump (Peri-Star, WPI, Sarasota, FL) under a constant aortic pressure of 60–80 mmHg.
The isolated heart was pinned at the edge of ventricular apex to the Sylgard bottom of the chamber to prevent stream-induced movement. A small silicon tube was inserted into the left ventricle through the pulmonary vein, left atria, and tricuspid valve to prevent solution congestion and subsequent ischemia. A small black tape was used to cover the atria to avoid fluorescence scattering pollution from the atria. Excitation-contraction uncoupler blebbistatin (10 μM, Tocris Bioscience) was used to prevent the effect of motion artifact on the action potential duration estimation. The heart was then stained with a calcium indicator (30 μl of 1 mg/ml dimethyl sulfoxide, 1:1 mixed with Fluronic F127, Invitrogen, Carlsbad, CA, in Tyrode solution; Rhod-2 AM, Invitrogen) for 5–7 min.
Optical imaging system.
The excitation light was generated by a halogen lamp (Newport Oriel Instruments, Stratford, CT; SciMedia, Costa Mesa, CA) and passed through a heat filter, shutter, and excitation band-pass filter (520 ± 45 nm). A flexible light guide directs the band-pass filtered light onto the preparation. The optical mapping apparatus comprised an MiCAM Ultima-L CMOS camera (SciMedia) with high-spatial (100 × 100 pixels, 230 ± 20 μm per pixel) and temporal (1,000–3,000 frames/s) resolution. A band-pass filter (590 ± 15 nm, Thorlabs, Newton, NJ) was fixed in front of the calcium-imaging camera.
Optical mapping protocols.
After isolation and cannulation, motion suppression, and dye staining, prepared hearts were equilibrated for 5–10 min before imaging. The optical mapping signals were recorded sequentially under unloaded and stretched conditions. The unloaded condition was achieved by a pressure releasing silicon tube inserted into the LV chamber of the Langendorff heart preparation. The stretched condition was achieved by inflating the LV chamber with Tyrode solution to 80 mmHg pressure through the silicon tube inserted into the left ventricle. The perfusion pressure was increased from 60 to 100 mmHg to maintain the direction of the retrograde perfusion and the viability of the perfused heart. A steady-state restitution (S1-S1) (40) pacing protocol was applied under both conditions.
Optical mapping data processing.
A custom-designed MatLab program was used to analyze the optical mapping signals. The signals were filtered with a low-pass Butterworth filter at 256 Hz. Regional calcium dynamics were quantified in WT and mdx mouse ventricles (base vs. apex) under both unloaded and stretched conditions. Hand-drawn regions of interest were used to define basal (upper 1/3 area of left ventricle) versus apical regions (lower 1/3 area of left ventricle). Because the calcium relaxation process is not monoexponential, only the latter half of the calcium signal decay could be fitted exponentially to derive the decay constant. Typically, both 50% relaxation decay time (T50) and the decay constant are used to characterize calcium relaxation kinetics (12, 29, 47, 48, 57, 58, 64, 72, 75, 80). Specifically, the Ca2+ relaxation time from the calcium transient peak value to 50% relaxation (T50) was calculated to define the calcium reuptake phase, and Ca2+ decay constant was calculated using an exponential curve fitting program from the 50% calcium peak value to full relaxation. Taken together, the T50 (for the early relaxation phase) and the decay constant (for the late relaxation phase) represent the whole process of Ca2+ reuptake-dependent relaxation.
Statistics.
Statistical differences were assessed with the use of analysis of variance (ANOVA) or multivariate analysis of variance (MANOVA) as appropriate. Bonferroni correction was applied to post hoc comparisons. A value of P < 0.05 was considered significant. Data were expressed as means ± SE. All statistics were conducted with the IBM SPSS statistics program.
RESULTS
mdx Hearts Manifest Abnormal but Recoverable Regional Diastolic Sheet Function
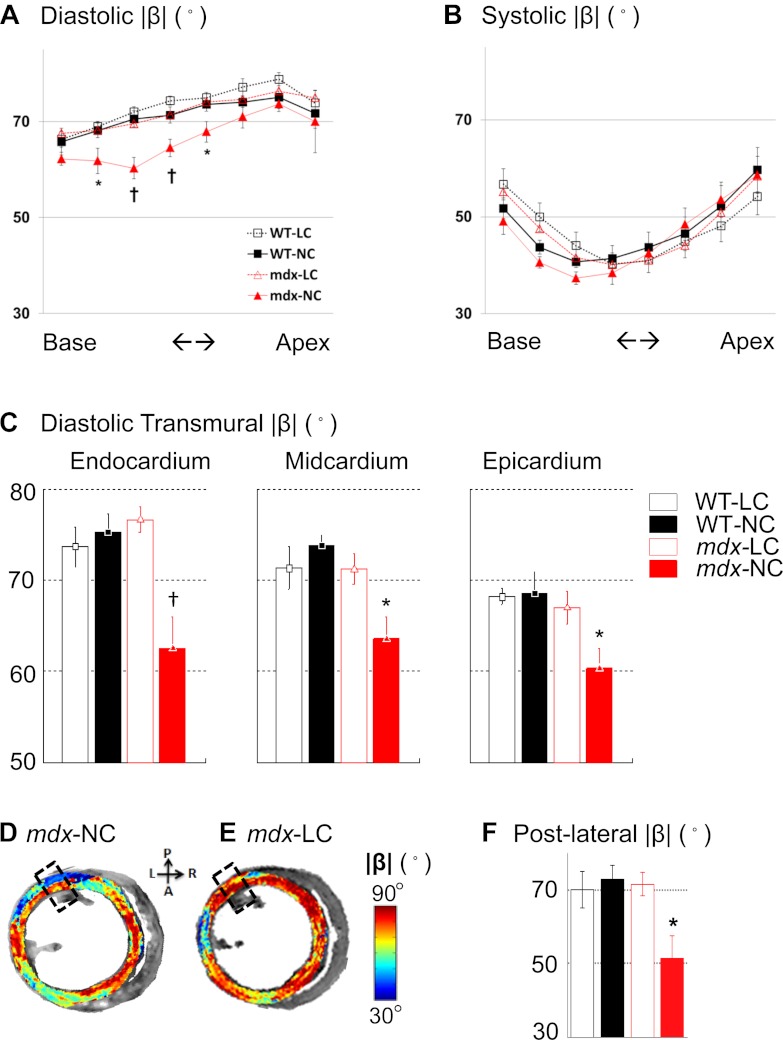
DTI defined two populations of β with opposite signs in the LV wall of WT and mdx mice, as confirmed by several previous reports (14, 28, 33, 41, 52, 68). The |β| value is decreased in systole (Figs. 1, B and C, and 2, A and B), reflecting the reorientation of myocardial sheets, which contributes ∼50% of systolic focal wall thickening (14, 16, 41, 42). Under conditions of barium-induced systolic arrest, the systolic |β| was comparable among all groups of hearts [P = not significant (NS)]. However, the diastolic |β| of mdx hearts (mdx-NC) was ∼10° lower than that of WT hearts (WT-NC) at the base when perfused with the regular cardioplegic solution (P < 0.05, Fig. 2). The |β| at the apex of mdx-NC hearts appeared normal (P = NS compared with WT-NC at each corresponding short-axis imaging slice), demonstrating that sheet diastolic dysfunction in mdx hearts occurred focally at the base.
Fig. 2.
DTI-determined diastolic and systolic magnitude of sheet angle (|β|) in short-axis slices. A: muscular dystrophy (mdx) hearts exhibited abnormally lower diastolic |β| that was recovered to normal value after reducing [Ca2+] in the perfusate. For hearts perfusion arrested in diastole with normal calcium solution, mdx hearts (mdx-NC) exhibited lower |β| than did WT (WT-NC) hearts with the maximal ∼10° difference occurred at the basal region. In contrast, mdx hearts perfused with the low calcium solution (mdx-LC) exhibited normal diastolic |β|. Varying [Ca2+] had no effect on diastolic |β| in WT hearts (WT-LC). B: systolic |β| exhibited no difference among 4 groups of hearts. C: diastolic |β| in the epicardium, midcardium, and endocardium at the base of WT and mdx hearts. In all regions, diastolic |β| of mdx-LC hearts were comparable with that of WT-LC and WT-NC hearts, but all were higher than that of mdx-NC hearts. The largest difference in diastolic |β| between mdx-LC and mdx-NC was observed in the endocardium. D and E: representative DTI-determined diastolic |β| maps on short-axis slices at the base of mdx-NC and mdx-LC hearts. The lower |β| in the mdx-NC heart, indicated by fewer regions with red color, was visually appreciable. F: in the posterior-lateral region (the areas enclosed by dashed line in D and E), the diastolic |β| of mdx-NC hearts was approximated 20° lower than that of mdx-LC hearts and of all WT hearts. Data represent means ± SE; N = 5 for each group. *P < 0.05 and †P < 0.005 for the indicated short-axis location.
By the reduction of the [Ca2+] of the cardioplegic solution to 7% of its regular concentration, a complete and rapid restoration of diastolic |β| in mdx hearts (mdx-LC) to normal values was observed. No effect of calcium manipulation on WT diastolic |β| was observed (WT-LC, P = NS compared with WT-NC at each corresponding short-axis imaging slice), indicating that the diastolic sheet dysfunction in mdx hearts was substantially calcium dependent. These data suggest that even at this advanced age, a principal mechanical abnormality in mdx mice likely is associated with incomplete sarcomere relaxation that is regionally dependent.
At the base, further regional analysis indicated that the maximal decrease of diastolic |β| in mdx-NC hearts occurred in the endocardium and in the posterior-lateral wall. When compared with WT-NC, mdx-NC exhibited 13° lower |β| (75 ± 2 vs. 62 ± 3°, P < 0.005) in the endocardium (inner 1/3 wall depth in transmural direction), 10° lower |β| (74 ± 1 vs. 64 ± 2°, P < 0.05) in the midcardium (middle 1/3 wall depth in transmural direction), and 8° lower |β| (68 ± 2 vs. 60 ± 2°, P < 0.05) in the epicardium (outer 1/3 wall depth in transmural direction) (Fig. 2C). In the posterior-lateral wall, the diastolic |β| was 51 ± 6° in mdx-NC (Fig. 2F). This value was ∼20° lower than that of mdx-LC (70 ± 4°), as well as that of WT-NC (72 ± 4°) or WT-LC (72 ± 6°) groups (P < 0.05 for all comparisons). Together, these findings indicate a clear regional and transmural dependence of incomplete relaxation despite the ubiquitous dystrophin mutation.
mdx and WT hearts exhibit similar transmural fiber organization and function but lower FA.
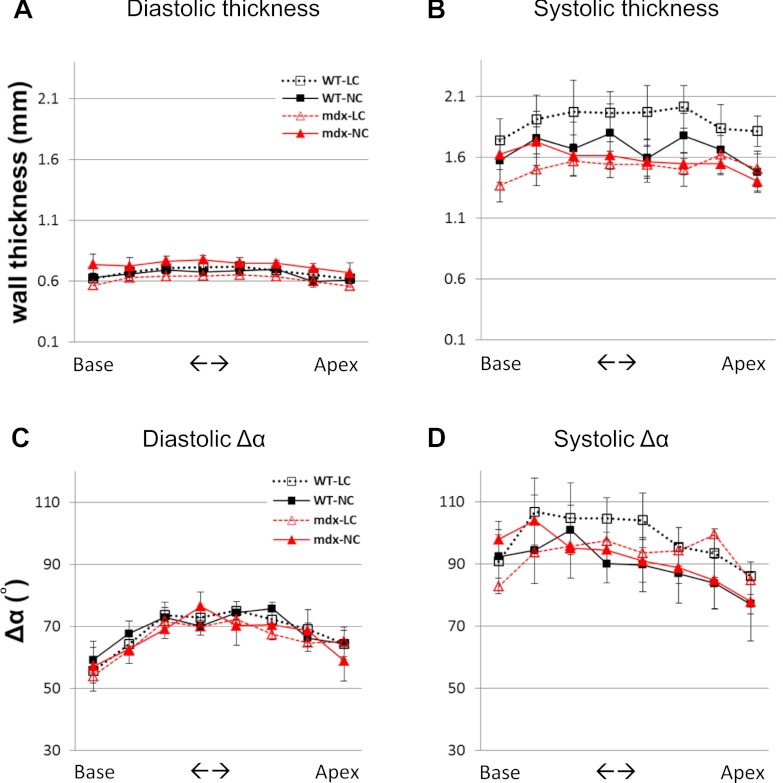
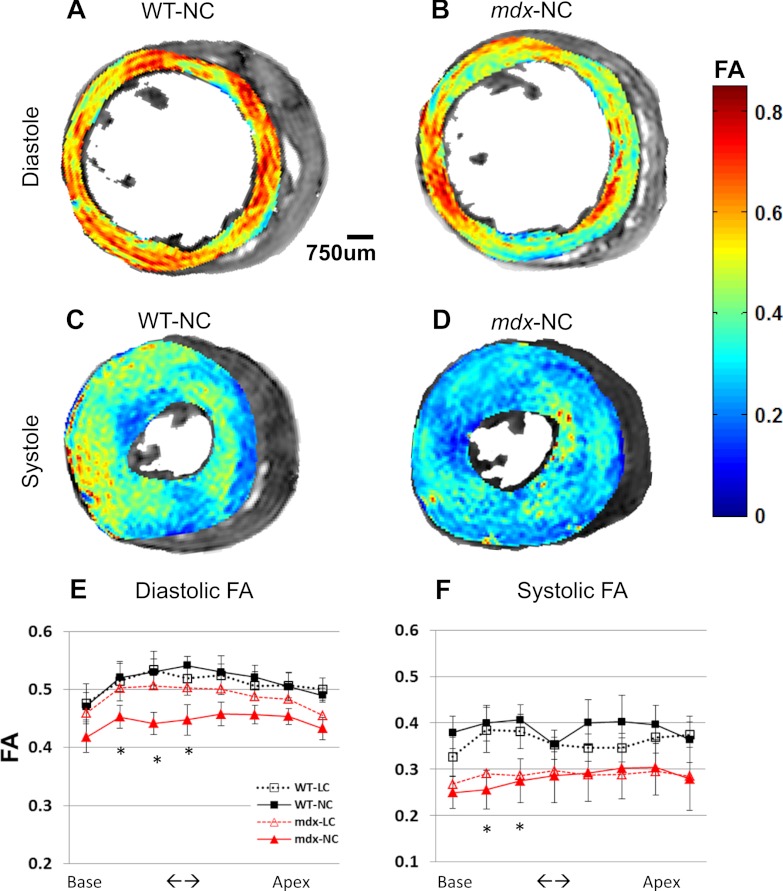
Wall thickness and through-wall differences of myofiber helix angle (Δα) were similar between mdx and WT mice (Fig. 3), suggesting that the major mechanical abnormality was confined to sheets rather than fibers. The normal wall thickness agrees with data of former publications (44, 63). In all hearts, systolic FA was ∼40% lower than diastolic FA (P < 0.05 for all comparisons, Fig. 4), indicating that a more isotropic diffusion environment was elicited by cardiac contraction (19, 26), as expected from fibers shortening in the long axis and expanding in the short axis (i.e., becoming rounder or less oblong).
Fig. 3.
Averaged wall thicknesses in each short-axis slice are similar in all WT and mdx groups, both in diastole (A) and in systole (B). Averaged through-wall myofiber helix angle differences (Δα) in each short-axis slice are similar in all WT and mdx groups, both in diastole (C) and in systole (D). Overall, Δα is increased in systole, agreeing with a prior publication (14). No significance was detected in all comparisons in each panel.
Fig. 4.
A–D: representative LV fractional anisotropy (FA) maps at base of a WT and a mdx heart sequentially arrested in diastole and then in systole. When compared with FA in diastole, lower FA in systole is visually appreciable. E and F: when compared with WT-NC hearts, mdx-NC hearts exhibit ∼15% lower FA in diastole (E) and ∼30% lower FA in systole (F). Reducing [Ca2+] in cardioplegic solution recovered diastolic FA in mdx-LC hearts to normal value. *P < 0.05. In barium-arrested systole, both mdx-LC and mdx-NC hearts exhibit lowered FA than do WT-LC and WT-NC, indicating a more isotropic tissue organization/cell shape in systole despite similar sheet angles. Please see materials and methods for the systolic arrest condition of all 4 groups.
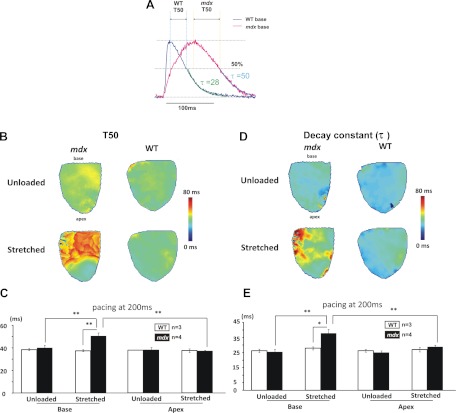
Optical imaging reveals longer calcium decay time (T50) with regional dependencies on stretch.
Figures 5 illustrates the calcium T50, as defined by optical mapping, which represents the duration of [Ca2+]i decay from 100 to 50% of its peak value. Under unloaded conditions, T50 was comparable between mdx and WT hearts. However, ventricular stretch elicited an ∼25% increase in T50 (from 40 ± 2 to 51 ± 3 ms, P < 0.05) and ∼50% increase of decay constant (from 25 ± 1 to 37 ± 1 ms, P < 0.05) at the base of mdx hearts, confirming abnormal calcium uptake in this region. Ventricular stretch, however, did not affect the T50 or decay constant at the apex of mdx hearts, nor in the entire left ventricle of WT hearts, indicating a heterogeneous regional dependency on the development of abnormal calcium handling in mdx.
Fig. 5.
Calcium relaxation for mdx and WT. A: representative calcium transients at the base of a WT (blue line) and a mdx (red line) heart. Signals were averaged from adjacent 25 charge-coupled device elements. Definitions of T50 and decay constant τ are illustrated. B and C: representative T50 and decay constant maps in a mdx and a WT heart under unloaded vs. stretched condition. D and E: under unloaded conditions, both T50 and decay constant were comparable between mdx and WT hearts. Ventricular stretch caused regional increases of T50 and decay constant at the base of mdx hearts. However, WT hearts exhibit similar T50 and decay constant. *P < 0.05; **P < 0.01.
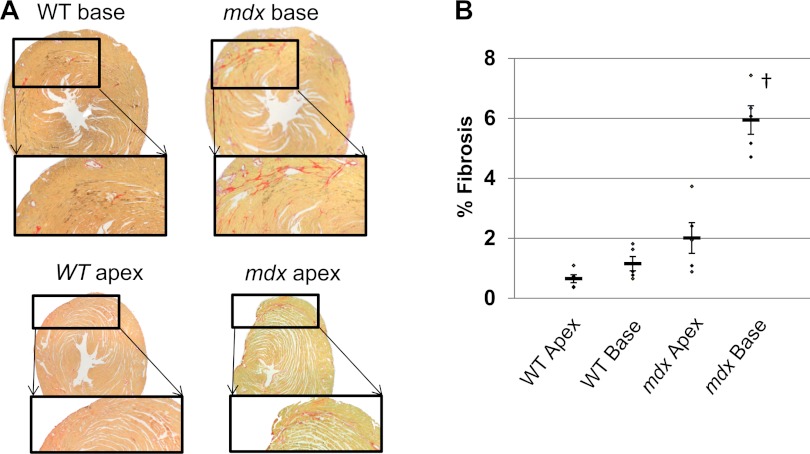
Mdx hearts exhibit higher fibrosis content at base compared with apex.
Figure 6 illustrates representative histopathology in picrosirius red stained mdx hearts. Scattered interstitial fibrosis staining was observed throughout the entire myocardium. Quantitative analysis demonstrated that WT mice exhibited similar collagen content at the base versus the apex. However, mdx mice exhibited approximately three times more collagen at the base compared with the apex, agreeing with prior patient studies (76, 77) and mdx mice studies (44, 63).
Fig. 6.
Regional variations of interstitial fibrosis in mdx hearts. A: representative picrosirius red stained slices at the base and apex of WT and mdx hearts. B: the percentage of fibrotic area at the base and the apex of mdx (N = 5) and WT (N = 5) hearts. Original data points are marked as dots. Thick bar, mean value; thin bar, means ± SE. †P < 0.005 for all comparisons to the base segment of mdx hearts.
DISCUSSION
This study shows for the first time that mdx hearts exhibit an abnormal myocardial sheet angle magnitude |β| in relaxation that is calcium dependent and rapidly reversible. Furthermore, regional and intramural gradients of diastolic dysfunction are evident, primarily in the more basal segments where abnormal calcium reuptake has been demonstrated. These colocalized abnormalities in calcium reuptake and diastolic sheet function strongly suggest that features other than the simple absence of dystrophin are critical to the evolution of heart failure in DMD. Indeed, it is well recognized that the mechanical defect in the dystrophin-encoding gene does not independently elicit DMD cardiac functional defects such as reduced circumferential wall strain (3), which emerge in a regionally heterogeneous fashion over time and not at all loci where dystrophin is missing.
Given that cardiac contraction induces sheet sliding, extension, and reorientation leading to the reduced |β|, the observed lower diastolic |β| may reflect the state that mdx cardiomyocytes are not fully relaxed in diastole. The low-diastolic FA of mdx hearts, indicating more round shaped fiber/sheets organization (19, 26), also implies this diastolic mechanical defect of mdx hearts. Systolic FA of mdx is lower than that of WT, indicating while the mechanical sheet orientation is not affected by the absence of dystrophin in systole, the microstructural shape of muscular architecture is affected, which requires further investigation. We also showed that this regional diastolic sheet dysfunction in mdx hearts is a likely consequence of persistently elevated cytosolic calcium, because it is quickly reversible upon perfusion with a lower level of calcium (Fig. 2).
The prior recognition of stretch-induced calcium mishandling in isolated mdx cardiomyocytes informs the present work (23, 75). Specifically, mechanical stretch increases the opening chance of stretch-activated channels (75) and develops transient membrane microruptures in mdx cardiomyocytes (65, 66). The resultant leakage of extracellular calcium into the cytosol then compromises calcium clearance during relaxation and renders mdx cardiomyocytes vulnerable to stretch-induced injury (59). In this study of intact perfused hearts, we employed a stretch protocol to mimic cardiomyocyte activity under moderate strain regimes by inflating LV pressure to 80 mmHg, which is similar to that used for investigating fibrillation by several groups (10, 21, 37, 50, 78). Under the stretched condition, mdx hearts exhibited regional deficiency in calcium uptake, as evidenced by the prolonged T50 and decay time constant, at the base where the sheet functional defect was observed. Thus our results illustrate a regional correspondence between abnormal diastolic sheet mechanics and disturbed calcium kinetics.
Calcium kinetic signals from the optical mapping dyes can be recorded from up to 300-μm depth of the tissue preparation, among which about half of the total signals come from first 100 μm (30). Although optical mapping data may not reliably represent the tissue beyond 300-μm depth, the surface signal provides a reliable and well-accepted surrogate measure of regional tissue calcium kinetics. Regional calcium mishandling, together with the abnormal β and FA, are observed together in all mdx hearts but not in any WT heart, which offers a suitable negative control.
The prolonged T50 and decay constant observed in mdx mouse hearts may reflect calcium leakage through the destabilized sarcolemma as a direct effect of the lack of infrastructural integrity that otherwise would be provided by the dystrophin-associated glycoprotein complex. Accordingly, a therapeutic strategy to inhibit calcium leakage during the resting state (i.e., unrelated to the operation of voltage-gated L-type channels for cell excitation), could be effective in restoring cardiac function in myopathic mdx heart. The promising results with several novel compounds such as P188 that is claimed to repair sarcolemma microruptures (54), and S107 that stabilizes ryanodine receptor 2 (8, 9), together with the ineffectiveness of L-type calcium channel blockers as demonstrated in earlier clinical trials (51), attests to the potential clinical utility of the approach.
Several potential limitations merit discussion. The ∼10° difference of diastolic |β| may not contribute to MR detectable wall thickness change because of the limited image resolution relative the wall thickness of mouse hearts and the partial volume effect on edge detection common to MRI exams. Because the measurement of |β| in ventricular wall is not sensitive to these limitations, our results suggest that |β| is more sensitive than is MR-measured wall thickness to detect subtle changes in myocardial organization. Quantitative histological validation of DTI-measured sheet angle was not conducted because it has been reported and validated in numerous earlier studies (33, 36, 60, 68). Because mdx mice can only be purchased from commercial vendors at <2 mo of age, the considerable time, high cost, and susceptibility to sudden death during even light anesthesia militated against acquiring in vivo cardiac functional readout before DTI experiments. Finally, although the current study did not investigate regional cardiac mechanics, the previous work of our group and others have shown such abnormal regional wall strain is detectable in both patients with DMD (3) and in mdx mice before ventricular function is abnormal (44).
Although the perfused viable whole heart preparation may not entirely replicate the in vivo condition, the present observations have elucidated striking functional differences between mdx and WT hearts and provided useful insights into the regional macroscopic ventricular mechanical dysfunction and related cellular calcium kinetics that cannot be defined by isolated cell preparations. Because sheet function is a key determinant of ventricular wall strain (16, 69) and calcium mishandling in mdx cardiomyocytes is associated with cellular injury (45, 53, 75), the observed stretch-induced regional calcium mishandling in mdx hearts may be responsible in part for the local progression of diastolic sheet dysfunction. In combination with the expected heterogeneous distribution of wall strain and stress, increased diastolic stiffness may facilitate a vicious cycle of continuing damage to fragile cell membranes that ultimately results in global heart failure.(17, 31, 43, 44, 79).
GRANTS
This study was supported by American Heart Association Grant 12PRE9310044 (to Y.-J. Cheng) and National Institutes of Health Grants R01-HL-085369 (to I. R. Efimov), R21-HL-108617 (to I. R. Efimov), and R01-AR-056223 (to S. A. Wickline).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.-J.C., D.L., and J.C. conception and design of research; Y.-J.C. and D.L. performed experiments; Y.-J.C. and D.L. analyzed data; Y.-J.C., D.L., S.D.C., I.R.E., J.C., and S.A.W. interpreted results of experiments; Y.-J.C. and D.L. prepared figures; Y.-J.C., D.L., and J.C. drafted manuscript; Y.-J.C., D.L., S.D.C., I.R.E., J.C., and S.A.W. edited and revised manuscript; Y.-J.C., D.L., I.R.E., J.C., and S.A.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lei Zhang and Lingzhi Hu for suggestions regarding MRI; Noriko Yanaba and Huiying Zhang for assistance with histology; Dr. Lung-Chang Chien for assistance with statistical analysis; Bor-Yiing Su for helpful suggestions on the imaging segmentation algorithm; and Matthew Goette, Kirk Hou, and Jan Kubanek for helpful discussions and manuscript review.
REFERENCES
- 1. Ahuja R, Kalra V, Saxena A, Dua T. Prevalence and patterns of cardiac involvement in duchenne muscular dystrophy. Indian Pediatr 37: 1246–1251, 2000 [PubMed] [Google Scholar]
- 2. Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol 88: 83–91, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Ashford MW, Jr, Liu W, Lin SJ, Abraszewski P, Caruthers SD, Connolly AM, Yu X, Wickline SA. Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation 112: 2462–2467, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Balzer P, Furber A, Delepine S, Rouleau F, Lethimonnier F, Morel O, Tadei A, Jallet P, Geslin P, le Jeune JJ. Regional assessment of wall curvature and wall stress in left ventricle with magnetic resonance imaging. Am J Physiol Heart Circ Physiol 277: H901–H910, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Banks GB, Chamberlain JS. The value of mammalian models for duchenne muscular dystrophy in developing therapeutic strategies. Curr Top Dev Biol 84: 431–453, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8: 333–344, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J 66: 259–267, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15: 325–330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, Marks AR. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA 105: 2198–2202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bode F, Katchman A, Woosley RL, Franz MR. Gadolinium decreases stretch-induced vulnerability to atrial fibrillation. Circulation 101: 2200–2205, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81: 1189–1192, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chase A, Orchard CH. Ca efflux via the sarcolemmal Ca ATPase occurs only in the t-tubules of rat ventricular myocytes. J Mol Cell Cardiol 50: 187–193, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Chen F, Suzuki Y, Nagai N, Peeters R, Marchal G, Ni Y. Dynamic susceptibility contrast-enhanced perfusion MR imaging at 1.5 T predicts final infarct size in a rat stroke model. J Neurosci Methods 141: 55–60, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Liu W, Zhang H, Lacy L, Yang X, Song SK, Wickline SA, Yu X. Regional ventricular wall thickening reflects changes in cardiac fiber and sheet structure during contraction: quantification with diffusion tensor MRI. Am J Physiol Heart Circ Physiol 289: H1898–H1907, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Costa KD, Takayama Y, McCulloch AD, Covell JW. Laminar fiber architecture and three-dimensional systolic mechanics in canine ventricular myocardium. Am J Physiol Heart Circ Physiol 276: H595–H607, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Costa KD, Takayama Y, McCulloch AD, Covell JW. Laminar fiber architecture and three-dimensional systolic mechanics in canine ventricular myocardium. Am J Physiol Heart Circ Physiol 276: H595–H607, 1999 [DOI] [PubMed] [Google Scholar]
- 17. DeAnda A, Jr, Komeda M, Moon MR, Green GR, Bolger AF, Nikolic SD, Daughters GT, 2nd, Miller DC. Estimation of regional left ventricular wall stresses in intact canine hearts. Am J Physiol Heart Circ Physiol 275: H1879–H1885, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Dellorusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil 22: 467–475, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Deux JF, Malzy P, Paragios N, Bassez G, Luciani A, Zerbib P, Roudot-Thoraval F, Vignaud A, Kobeiter H, Rahmouni A. Assessment of calf muscle contraction by diffusion tensor imaging. Eur Radiol 18: 2303–2310, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Dunphy G, Richter HW, Azodi M, Weigand J, Sadri F, Sellke F, Ely D. The effects of mannitol, albumin, and cardioplegia enhancers on 24-h rat heart preservation. Am J Physiol Heart Circ Physiol 276: H1591–H1598, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Eijsbouts SC, Majidi M, van Zandvoort M, Allessie MA. Effects of acute atrial dilation on heterogeneity in conduction in the isolated rabbit heart. J Cardiovasc Electrophysiol 14: 269–278, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Ennis DB, Kindlmann G. Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Reson Med 55: 136–146, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Fanchaouy M, Polakova E, Jung C, Ogrodnik J, Shirokova N, Niggli E. Pathways of abnormal stress-induced Ca2+ influx into dystrophic mdx cardiomyocytes. Cell Calcium 46: 114–121, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finsterer J, Stollberger C. Cardiac involvement determines the prognosis of Duchenne muscular dystrophy. Indian J Pediatr 74: 209; author reply 210–212, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Gailly P. New aspects of calcium signaling in skeletal muscle cells: implications in Duchenne muscular dystrophy. Biochim Biophys Acta 1600: 38–44, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Galban CJ, Maderwald S, Uffmann K, de Greiff A, Ladd ME. Diffusive sensitivity to muscle architecture: a magnetic resonance diffusion tensor imaging study of the human calf. Eur J Appl Physiol 93: 253–262, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Geerts L, Bovendeerd P, Nicolay K, Arts T. Characterization of the normal cardiac myofiber field in goat measured with MR-diffusion tensor imaging. Am J Physiol Heart Circ Physiol 283: H139–H145, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Gilbert SH, Benson AP, Li P, Holden AV. Regional localisation of left ventricular sheet structure: integration with current models of cardiac fibre, sheet and band structure. Eur J Cardiothorac Surg 32: 231–249, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Giordano FJ, He H, McDonough P, Meyer M, Sayen MR, Dillmann WH. Adenovirus-mediated gene transfer reconstitutes depressed sarcoplasmic reticulum Ca2+-ATPase levels and shortens prolonged cardiac myocyte Ca2+ transients. Circulation 96: 400–403, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Girouard SD, Laurita KR, Rosenbaum DS. Unique properties of cardiac action potentials recorded with voltage-sensitive dyes. J Cardiovasc Electrophysiol 7: 1024–1038, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Guccione JM, Costa KD, McCulloch AD. Finite element stress analysis of left ventricular mechanics in the beating dog heart. J Biomech 28: 1167–1177, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Gulati S, Saxena A, Kumar V, Kalra V. Duchenne muscular dystrophy: prevalence and patterns of cardiac involvement. Indian J Pediatr 72: 389–393, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Helm P, Beg MF, Miller MI, Winslow RL. Measuring and mapping cardiac fiber and laminar architecture using diffusion tensor MR imaging. Ann NY Acad Sci 1047: 296–307, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Hermans MC, Pinto YM, Merkies IS, de Die-Smulders CE, Crijns HJ, Faber CG. Hereditary muscular dystrophies and the heart. Neuromuscul Disord 20: 479–492, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Holmes AA, Scollan DF, Winslow RL. Direct histological validation of diffusion tensor MRI in formaldehyde-fixed myocardium. Magn Reson Med 44: 157–161, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Hsu EW, Muzikant AL, Matulevicius SA, Penland RC, Henriquez CS. Magnetic resonance myocardial fiber-orientation mapping with direct histological correlation. Am J Physiol Heart Circ Physiol 274: H1627–H1634, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Huang JL, Tai CT, Chen JT, Ting CT, Chen YT, Chang MS, Chen SA. Effect of atrial dilatation on electrophysiologic properties and inducibility of atrial fibrillation. Basic Res Cardiol 98: 16–24, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Ishikawa Y, Bach JR, Minami R. Cardioprotection for Duchenne's muscular dystrophy. Am Heart J 137: 895–902, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Judd RM, Rottman GA, Forder JR, Yin FC, Blackband SJ. Feasibility of 19F imaging of perfluorochemical emulsions to measure myocardial vascular volume. Magn Reson Med 28: 129–136, 1992 [DOI] [PubMed] [Google Scholar]
- 40. Lang D, Glukhov AV, Efimova T, Efimov IR. Role of Pyk2 in cardiac arrhythmogenesis. Am J Physiol Heart Circ Physiol 301: H975–H983, 2011 [DOI] [PubMed] [Google Scholar]
- 41. LeGrice IJ, Smaill BH, Chai LZ, Edgar SG, Gavin JB, Hunter PJ. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol Heart Circ Physiol 269: H571–H582, 1995 [DOI] [PubMed] [Google Scholar]
- 42. LeGrice IJ, Takayama Y, Covell JW. Transverse shear along myocardial cleavage planes provides a mechanism for normal systolic wall thickening. Circ Res 77: 182–193, 1995 [DOI] [PubMed] [Google Scholar]
- 43. LeWinter MM, Kent RS, Kroener JM, Carew TE, Covell JW. Regional differences in myocardial performance in the left ventricle of the dog. Circ Res 37: 191–199, 1975 [DOI] [PubMed] [Google Scholar]
- 44. Li W, Liu W, Zhong J, Yu X. Early manifestation of alteration in cardiac function in dystrophin deficient mdx mouse using 3D CMR tagging. J Cardiovasc Magn Reson 11: 40, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lynch GS. Role of contraction-induced injury in the mechanisms of muscle damage in muscular dystrophy. Clin Exp Pharmacol Physiol 31: 557–561, 2004 [DOI] [PubMed] [Google Scholar]
- 46. McNally EM. Duchenne muscular dystrophy: how bad is the heart? Heart 94: 976–977, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Mellor KM, Wendt IR, Ritchie RH, Delbridge LM. Fructose diet treatment in mice induces fundamental disturbance of cardiomyocyte Ca2+ handling and myofilament responsiveness. Am J Physiol Heart Circ Physiol 302: H964–H972, 2012 [DOI] [PubMed] [Google Scholar]
- 48. Mukherjee R, Apple KA, Squires CE, Kaplan BS, McLean JE, Saunders SM, Stroud RE, Spinale FG. Protein kinase C isoform activation and endothelin-1 mediated defects in myocyte contractility after cardioplegic arrest and reperfusion. Circulation 114: I308–I313, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Munch DF, Comer HT, Downey JM. Barium contracture: a model for systole. Am J Physiol Heart Circ Physiol 239: H438–H442, 1980 [DOI] [PubMed] [Google Scholar]
- 50. Nazir SA, Lab MJ. Mechanoelectric feedback in the atrium of the isolated guinea-pig heart. Cardiovasc Res 32: 112–119, 1996 [PubMed] [Google Scholar]
- 51. Phillips MF, Quinlivan R. Calcium antagonists for Duchenne muscular dystrophy. Cochrane Database Syst Rev CD004571, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pope AJ, Sands GB, Smaill BH, LeGrice IJ. Three-dimensional transmural organization of perimysial collagen in the heart. Am J Physiol Heart Circ Physiol 295: H1243–H1252, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prosser BL, Ward CW, Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333: 1440–1445, 2011 [DOI] [PubMed] [Google Scholar]
- 54. Quinlan JG, Wong BL, Niemeier RT, McCullough AS, Levin L, Emanuele M. Poloxamer 188 failed to prevent exercise-induced membrane breakdown in mdx skeletal muscle fibers. Neuromuscul Disord 16: 855–864, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Robinson LA, Harwood DL. Lowering the calcium concentration in St. Thomas' Hospital cardioplegic solution improves protection during hypothermic ischemia. J Thorac Cardiovasc Surg 101: 314–325, 1991 [PubMed] [Google Scholar]
- 56. Romfh A, McNally EM. Cardiac assessment in duchenne and becker muscular dystrophies. Curr Heart Fail Rep 7: 212–218, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Sahoo SK, Kim do H. Calumenin interacts with SERCA2 in rat cardiac sarcoplasmic reticulum. Mol Cells 26: 265–269, 2008 [PubMed] [Google Scholar]
- 58. Sahoo SK, Kim T, Kang GB, Lee JG, Eom SH, Kim do H. Characterization of calumenin-SERCA2 interaction in mouse cardiac sarcoplasmic reticulum. J Biol Chem 284: 31109–31121, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sapp JL, Bobet J, Howlett SE. Contractile properties of myocardium are altered in dystrophin-deficient mdx mice. J Neurol Sci 142: 17–24, 1996 [DOI] [PubMed] [Google Scholar]
- 60. Scollan DF, Holmes A, Winslow R, Forder J. Histological validation of myocardial microstructure obtained from diffusion tensor magnetic resonance imaging. Am J Physiol Heart Circ Physiol 275: H2308–H2318, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Sosnovik DE, Wang R, Dai G, Reese TG, Wedeen VJ. Diffusion MR tractography of the heart. J Cardiovasc Magn Reson 11: 47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Streeter DD, Jr, Hanna WT. Engineering mechanics for successive states in canine left ventricular myocardium II Fiber angle and sarcomere length. Circ Res 33: 656–664, 1973 [DOI] [PubMed] [Google Scholar]
- 63. Stuckey DJ, Carr CA, Camelliti P, Tyler DJ, Davies KE, Clarke K. In vivo MRI characterization of progressive cardiac dysfunction in the mdx mouse model of muscular dystrophy. PLoS One 7: e28569, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taylor AJ, Al-Saadi N, Abdel-Aty H, Schulz-Menger J, Messroghli DR, Gross M, Dietz R, Friedrich MG. Elective percutaneous coronary intervention immediately impairs resting microvascular perfusion assessed by cardiac magnetic resonance imaging. Am Heart J 151: e891–e897, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther 15: 1086–1092, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Townsend D, Yasuda S, Metzger J. Cardiomyopathy of Duchenne muscular dystrophy: pathogenesis and prospect of membrane sealants as a new therapeutic approach. Expert Rev Cardiovasc Ther 5: 99–109, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Toyota E, Fujimoto K, Ogasawara Y, Kajita T, Shigeto F, Matsumoto T, Goto M, Kajiya F. Dynamic changes in three-dimensional architecture and vascular volume of transmural coronary microvasculature between diastolic- and systolic-arrested rat hearts. Circulation 105: 621–626, 2002 [DOI] [PubMed] [Google Scholar]
- 68. Tseng WY, Wedeen VJ, Reese TG, Smith RN, Halpern EF. Diffusion tensor MRI of myocardial fibers and sheets: correspondence with visible cut-face texture. J Magn Reson Imaging 17: 31–42, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Usyk TP, Mazhari R, McCulloch AD. Effect of laminar orthotropic myofiber architecture on regional stress and strain in the canine left ventricle. J Elasticity 61: 143–164, 2000 [Google Scholar]
- 70. van Deutekom JC, Bremmer-Bout M, Janson AA, Ginjaar IB, Baas F, den Dunnen JT, van Ommen GJ. Antisense-induced exon skipping restores dystrophin expression in DMD patient derived muscle cells. Hum Mol Genet 10: 1547–1554, 2001 [DOI] [PubMed] [Google Scholar]
- 71. Van Erp C, Irwin NG, Hoey AJ. Long-term administration of pirfenidone improves cardiac function in mdx mice. Muscle Nerve 34: 327–334, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Vassalle M, Lin CI. Calcium overload and cardiac function. J Biomed Sci 11: 542–565, 2004 [DOI] [PubMed] [Google Scholar]
- 73. Wagner KR, Lechtzin N, Judge DP. Current treatment of adult Duchenne muscular dystrophy. Biochim Biophys Acta 1772: 229–237, 2007 [DOI] [PubMed] [Google Scholar]
- 74. Watts JA, Maiorano PC. Trace amounts of albumin protect against ischemia and reperfusion injury in isolated rat hearts. J Mol Cell Cardiol 31: 1653–1662, 1999 [DOI] [PubMed] [Google Scholar]
- 75. Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol 292: H846–H855, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Yilmaz A, Gdynia HJ, Baccouche H, Mahrholdt H, Meinhardt G, Basso C, Thiene G, Sperfeld AD, Ludolph AC, Sechtem U. Cardiac involvement in patients with Becker muscular dystrophy: new diagnostic and pathophysiological insights by a CMR approach. J Cardiovasc Magn Reson 10: 50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yilmaz A, Gdynia HJ, Ludolph AC, Klingel K, Kandolf R, Sechtem U. Images in cardiovascular medicine. Cardiomyopathy in a Duchenne muscular dystrophy carrier and her diseased son: similar pattern revealed by cardiovascular MRI. Circulation 121: e237–e239, 2010 [DOI] [PubMed] [Google Scholar]
- 78. Zarse M, Stellbrink C, Athanatou E, Robert J, Schotten U, Hanrath P. Verapamil prevents stretch-induced shortening of atrial effective refractory period in langendorff-perfused rabbit heart. J Cardiovasc Electrophysiol 12: 85–92, 2001 [DOI] [PubMed] [Google Scholar]
- 79. Zhong L, Su Y, Yeo SY, Tan RS, Ghista DN, Kassab G. Left ventricular regional wall curvedness and wall stress in patients with ischemic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 296: H573–H584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhu X, Altschafl BA, Hajjar RJ, Valdivia HH, Schmidt U. Altered Ca2+ sparks and gating properties of ryanodine receptors in aging cardiomyocytes. Cell Calcium 37: 583–591, 2005 [DOI] [PubMed] [Google Scholar]