Abstract
The rate of alveolar fluid clearance (AFC) is associated with mortality in clinical acute lung injury (ALI). Patients with ALI often develop circulatory shock, but how shock affects the rate of AFC is unknown. To determine the effect of circulatory shock on the rate of AFC in patients with ALI, the rate of net AFC was measured in 116 patients with ALI by serial sampling of pulmonary edema fluid. The primary outcome was the rate of AFC in patients with shock compared with those without shock. We also tested the effects of shock severity and bacteremia. Patients with ALI and shock (n = 86) had significantly slower rates of net AFC compared with those without shock (n = 30, P = 0.03), and AFC decreased significantly as the number of vasopressors increased. Patients with positive blood cultures (n = 21) had slower AFC compared with patients with negative blood cultures (n = 96, P = 0.023). In addition, the edema fluid-to-plasma protein ratio, an index of alveolar-capillary barrier permeability, was highest in patients requiring the most vasopressors (P < 0.05). Patients with ALI complicated by circulatory shock and bacteremia had slower rates of AFC compared with patients without shock or bacteremia. An impaired capacity to reabsorb alveolar edema fluid may contribute to high mortality among patients with sepsis-induced ALI. These findings also suggest that vasopressor use may be a marker of alveolar-capillary barrier permeability in ALI and provide justification for new therapies that enhance alveolar epithelial and endothelial barrier integrity in ALI, particularly in patients with shock.
Keywords: acute respiratory distress syndrome, alveolar epithelium, circulatory shock, pulmonary edema, vasopressors, sepsis
every year 190,000 patients in the U.S. are diagnosed with acute lung injury (ALI) or its more severe form acute respiratory distress syndrome (ARDS) (29). Despite advances in prevention and treatment, the mortality rate is still ∼40% in unselected cases (29). Many factors have been identified that are associated with poor outcomes from ALI/ARDS including advanced age (4), sepsis as the underlying cause of lung injury, chronic liver disease (7, 21, 30), and presence of nonpulmonary organ failures including circulatory shock (25, 27). The rate of alveolar fluid clearance (AFC) measured by serial sampling of pulmonary edema fluid has also been associated with outcomes in clinical ALI/ARDS. Alveolar fluid clearance is impaired in the majority of patients with clinical ALI/ARDS, and survival is highest in patients who have the fastest rates of AFC (34).
In the last 30 yr, experimental studies have led to a better understanding of the mechanisms that control lung fluid balance under normal and pathological conditions. Active sodium ion transport is the key mechanism that drives net alveolar fluid clearance, and the main site of alveolar fluid clearance is in the alveolar epithelium and distal airways (5, 8, 12, 17, 24, 32). However, the factors that regulate alveolar fluid clearance in clinical ALI/ARDS are not well understood. Because there is strong evidence that impaired alveolar fluid clearance is associated with increased mortality in patients diagnosed with ALI/ARDS (34), identifying and studying factors that impair alveolar epithelial fluid transport may be of major importance, potentially leading to a better understanding of the resolution phase of ALI/ARDS and better treatments for ALI/ARDS.
In a prior single center study of the rates of alveolar fluid clearance in ALI/ARDS, sepsis was strongly associated with impaired alveolar fluid clearance (34). Lower arterial pH and higher severity of illness as measured by the Simplified Acute Physiology II Score were also associated with impaired alveolar fluid clearance. Because sepsis is frequently complicated by shock, and shock is associated with metabolic acidosis and high severity of illness scores, in this study we hypothesized that circulatory shock might be a primary underlying mechanism of impaired alveolar fluid clearance in patients with ALI/ARDS. Furthermore, an association of shock with impaired alveolar fluid clearance rates might explain in part why patients with circulatory shock have the worst outcomes from ALI/ARDS (16). To test this hypothesis, we measured the rate of net alveolar fluid clearance in patients with clinical ALI/ARDS enrolled in a two-center, prospective observational cohort study and tested the association of vasopressor exposure as an index of shock severity with the rate of alveolar fluid clearance. Additionally, we tested the association of bacteremia with impaired alveolar fluid clearance because bacteremia may be a marker of injury to the alveolar epithelial barrier that could also contribute to impaired net alveolar fluid clearance (35).
MATERIALS AND METHODS
Patients.
Clinical data were obtained from a two-center prospective observational study that enrolled patients with acute pulmonary edema due to ALI/ARDS or cardiogenic causes at University of California at San Francisco and Vanderbilt University between 1981 and 2011. The study was approved by the University of California at San Francisco Committee on Human Research and the Vanderbilt Institutional Review Board. Patients were eligible if they had acute pulmonary edema and acute respiratory failure requiring mechanical ventilation with bilateral alveolar infiltrates on chest radiograph and hypoxemia and had serial sampling of pulmonary edema fluid to calculate the rate of net alveolar fluid clearance. Patients were identified as having ALI/ARDS if they had a predisposing condition known to cause ALI and met the American European consensus definition of 1) bilateral pulmonary infiltrates on chest radiograph, 2) hypoxemia with PaO2/FiO2 less than or equal to 300, and 3) absence of left heart failure (3). To be defined as having ALI/ARDS, patients were also required to have a pulmonary edema fluid-to-plasma protein ratio (EF/PL) of greater than or equal to 0.65. Patients with cardiogenic edema or with mixed cardiogenic edema and ALI/ARDS were excluded.
The presence and severity of circulatory shock was based on requirement for vasopressors (intravenous infusion of norepinephrine, epinephrine, dopamine, or phenylephrine) and the number of total vasopressors administered on the day of edema fluid sampling. Bacteremia was defined as bacterial growth in blood cultures obtained on the day of or the day before pulmonary edema fluid sampling. Blood cultures that grew Staphylococcus epidermis were considered to be contaminated with skin flora and did not represent bacteremia.
Pulmonary edema fluid was collected at enrollment and then again within the first 6 h after enrollment as previously described (33, 34). Briefly, a suction catheter was advanced through the endotracheal tube until resistance was met; then gentle suction was applied, and undiluted edema fluid was collected and centrifuged at 3,000 g for 20 min to remove cells. The supernatant was stored at −80°C. Plasma samples were also obtained at same time to calculate the EF/PL ratio.
Calculation of the rate of AFC.
The total protein was measured in duplicate in plasma and edema fluid using the Biuret or BCA (Pierce Endogen) methods (18, 33). The rate of net alveolar clearance was calculated using this formula: (final protein concentration − initial protein concentration)/final protein concentration. The value was expressed as percentage of AFC per hour (%/h). For some analyses, the rate of AFC was classified as impaired (<3%/h), intact (≥3%/h), or maximal (>14%/h) as we did previously (33, 34).
Statistical analysis.
All statistical analysis was done using IBM SPSS for Macintosh version 19. Rates of net AFC were not normally distributed and were square root transformed for analysis. Student's t-test, ANOVA with post hoc Tukey's test, and Chi Square with linear-by-linear association test were used as appropriate. Multivariable logistic regression analysis was done to assess the independent effects of number of vasopressors, sex, age, sepsis, and severity of illness (Simplified Acute Physiology Score II, SAPS II score) on AFC.
RESULTS
Patient characteristics.
There were 116 patients with ALI/ARDS who met inclusion criteria for the study. Patient characteristics are summarized in Table 1. The majority of the patients were enrolled after 1990 (86%). The majority of patients (86, 74%) were receiving one or more vasopressors on the day of enrollment. The most common vasopressor was dopamine (n = 67), followed by phenylephrine (n = 35), norepinephrine (n = 31), and epinephrine (n = 11). Compared with patients with ALI who were not being treated with vasopressors, patients with ALI who were treated with vasopressors were more likely to be Caucasian, had a higher severity of illness as measured by the SAPS II score, and also had a higher mortality rate. However, there were no differences in severity of lung injury as measured by the Lung Injury Score (7) between the two groups.
Table 1.
Demographic and clinical characteristics
| ALI with Shock (N = 86) | ALI Without Shock (N = 30) | P Value | |
|---|---|---|---|
| Male sex | 62% | 47% | 0.20 |
| Age, yr | 45 (32–60) | 37 (28–52) | 0.17 |
| Caucasian race | 65% | 41% | 0.04 |
| SAPS II | 55 (42–69) | 34 (29–42) | <0.001 |
| LIS | 3.0 (2.5–3.7) | 3.0 (2.7–3.3) | 0.82 |
| Etiology of ALI | 0.16 | ||
| Nonpulmonary Sepsis | 35% | 17% | |
| Pneumonia | 29% | 30% | |
| Aspiration | 12% | 10% | |
| Other | 24% | 43% | |
| Tidal volume, ml/kg actual body weight | 10.4 (6.7–12.8) | 10.0 (6.4–12.0) | 0.68 |
| PEEP, cm H2O | 10 (7.4–15) | 10 (5.0–12) | 0.26 |
| Hospital mortality | 65% | 33% | 0.005 |
Data in first two columns are expressed as median (interquartile range) or percent of patients. Data in the third column are categorical variables compared using Fisher's Exact Test or Pearson's Chi Square as appropriate. Continuous variables compared using Student's t-test. SAPS II, Simplified Acute Physiology Score II; LIS, Lung Injury Score; ALI, acute lung injury.
AFC and vasopressors.
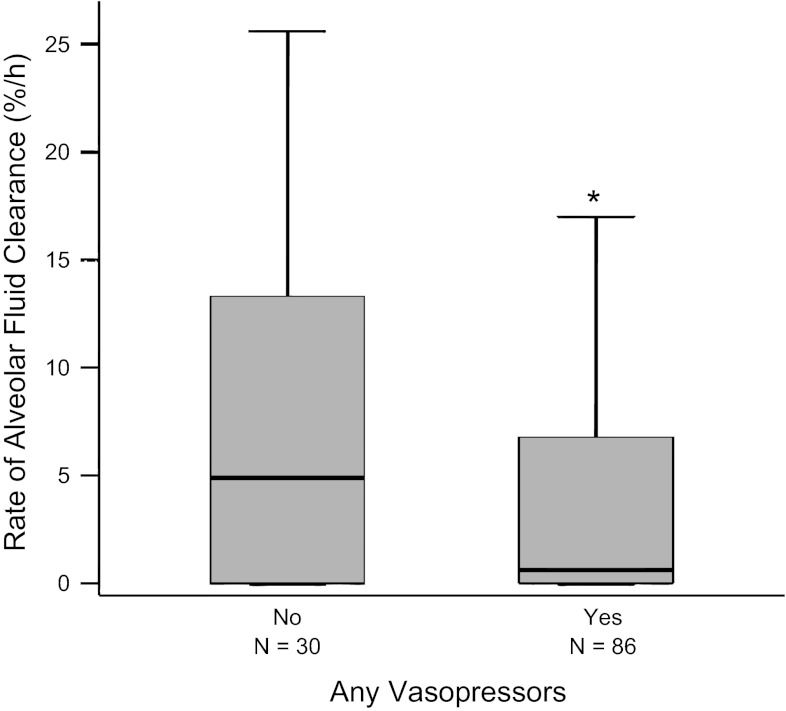
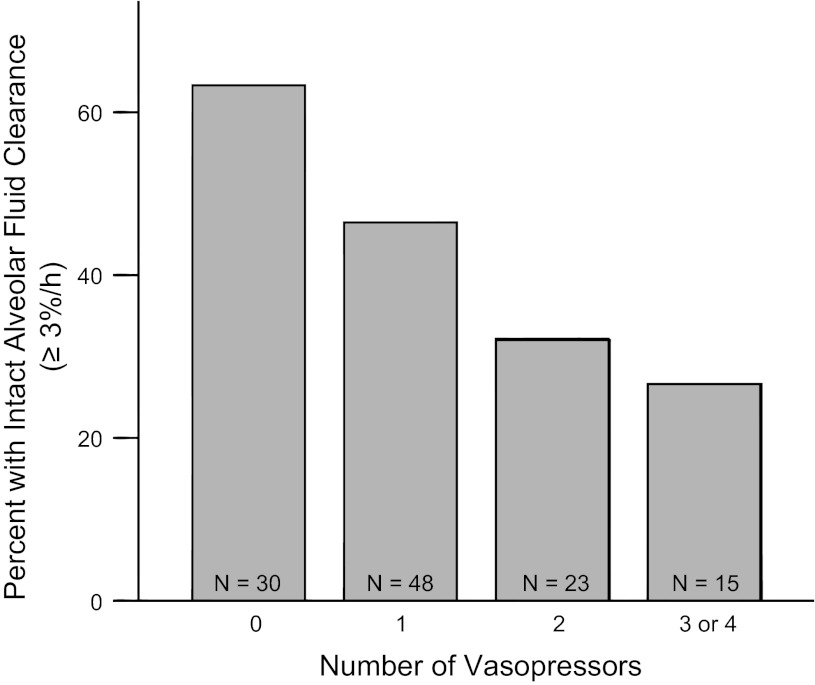
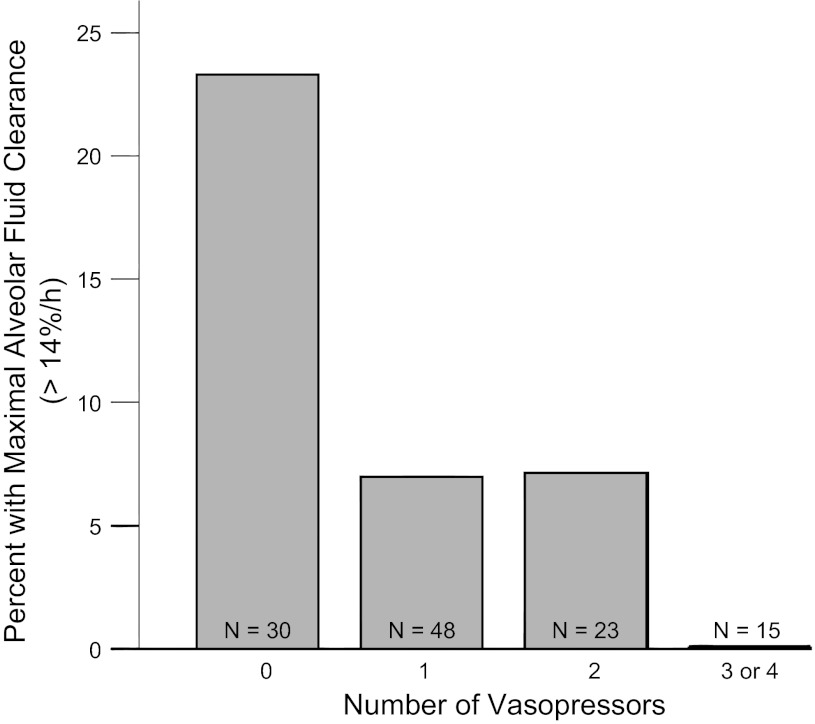
Patients who were receiving any vasopressors had significantly slower rates of AFC compared with those not receiving vasopressors (Fig. 1). The percentage of patients with intact AFC (AFC ≥3%/h) progressively decreased as the number of vasopressors increased (Fig. 2), as did the percentage of patients with maximal rates of AFC (AFC >14%/h) (Fig. 3). The same associations were observed for individual vasopressors, indicating that the association of vasopressor use with lower rates of AFC was most likely related to presence of shock and shock severity rather than indicative of a specific pharmacological effect of any vasopressor. For example, patients receiving dopamine (n = 67) had lower rates of AFC than those who were not (P = 0.045); patients receiving neosynephrine (n = 35) had lower rates of AFC than those who were not (P = 0.058); patients receiving norepinephrine had lower rates of AFC than those who were not (P = 0.021). There were too few patients receiving epinephrine to support an analysis of this pressor alone. The association of increasing vasopressor use with a lower likelihood of intact AFC persisted even when patients enrolled before 1990 were excluded (P = 0.02). To determine whether any demographic or clinical variables such as severity of illness influenced the association between vasopressor requirement and impaired AFC, we used a multivariable logistic regression model for impaired AFC. In the unadjusted analysis, for each additional vasopressor required, there was an odds ratio of 1.8 (95% CI, 1.2–2.6, P = 0.006) for the presence of impaired AFC. In an analysis adjusted for age, sex, sepsis, and SAPS II (14), the relationship between vasopressor requirement and impaired AFC was unchanged (OR 1.9, 95% CI 1.2–3.2, P = 0.008). Excluding patients enrolled before 1990 did not change the association (OR 1.7, 95% CI 1.03–2.97, P = 0.04).
Fig. 1.
Net alveolar fluid clearance (AFC) rates are slower in patients with acute lung injury (ALI) with shock. Boxplot summary of rates of AFC comparing patients with ALI who were not receiving vasopressors (n = 30) and patients with ALI who were receiving vasopressors (n = 86). Patients on any vasopressor had slower rate of AFC compared with those not on any vasopressors. *P = 0.03 by Student's t-test on square root transformed data.
Fig. 2.
Severity of shock is associated with impaired AFC. Percentage of patients with intact AFC vs. number of vasopressors required on the day of edema fluid sampling. Intact AFC (≥3%/h) was less frequent in patients on higher numbers of vasopressors. P = 0.006 for trend across groups by linear-by-linear association test.
Fig. 3.
Patients with the most severe shock (3 or 4 vasopressors) do not have maximal rates of AFC. Percentage of patients with maximal rates of AFC vs. number of vasopressors required on the day of edema fluid sampling. Maximal AFC (>14%/h) was less frequent in patients on higher numbers of vasopressors. P = 0.014 for trend across groups by linear-by-linear association test.
AFC and bacteremia.
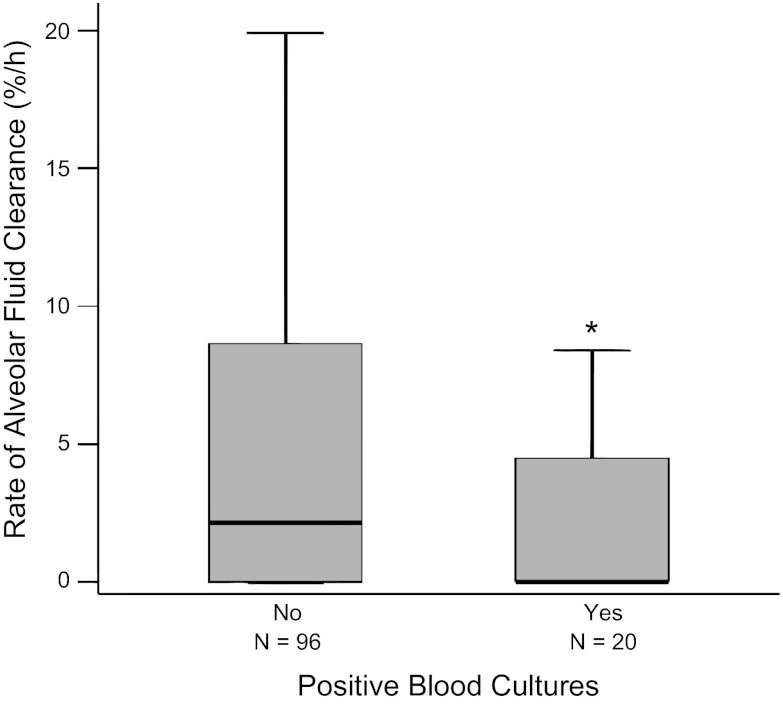
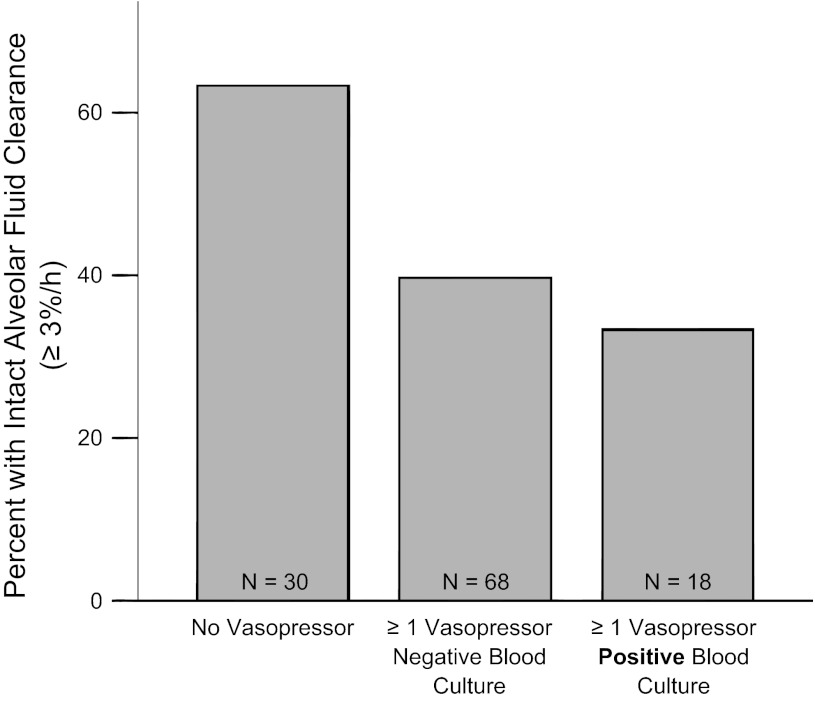
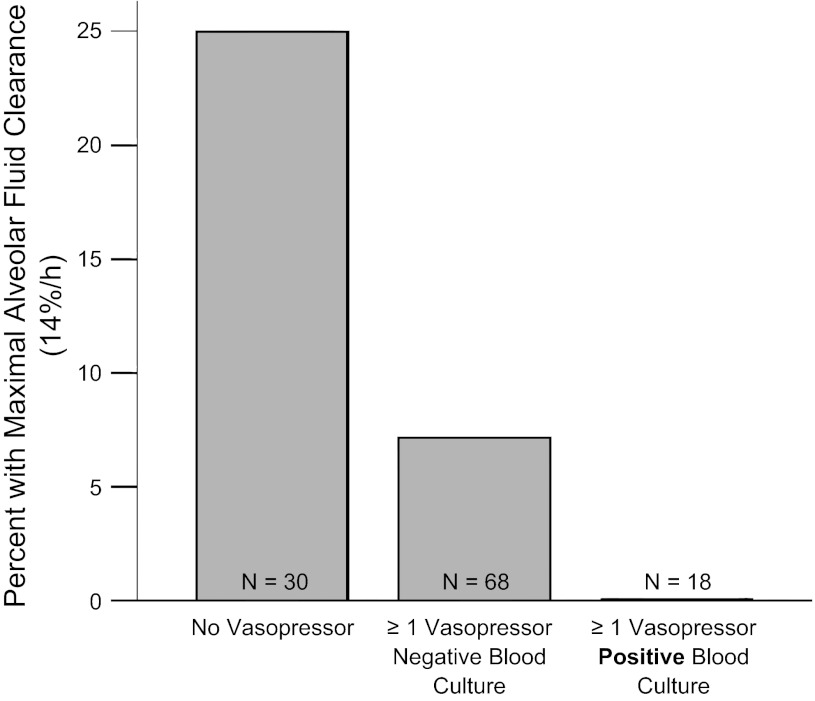
Patient characteristics are compared between patients with and without bacteremia in Table 2. Overall, 17% of patients had bacteremia. Among these, 35% of isolates were Gram negative, and 65% of isolates were Gram positive. Patients who had bacteremia as determined by positive blood cultures had significantly slower rates of AFC than patients with negative blood cultures or no blood cultures obtained (Fig. 4). Patients with Gram-negative isolates were less likely to have intact AFC than patients with Gram-positive isolates (14% vs. 46% with intact AFC, P = 0.15), but this difference did not reach statistical significance in this small group of 20 patients with bacteremia. Patients who had both positive blood cultures and received one or more vasopressors had slower rates of AFC as measured by the percentage of patients with intact AFC (Fig. 5) and the percentage of patients with maximal AFC (Fig. 6).
Table 2.
Comparison of patients with and without positive blood cultures
| ALI with Positive Blood Cultures (N = 20) | ALI with Negative Blood Cultures (N = 96) | P Value | |
|---|---|---|---|
| Male sex | 75% | 54% | 0.13 |
| Age, yr | 41 (29–59) | 44 (31–59) | 0.77 |
| Caucasian race | 67% | 58% | 0.61 |
| SAPS II | 61 (51–78) | 44 (33–61) | 0.019 |
| LIS | 2.9 (2.3–3.7) | 3.0 (2.7–3.6) | 0.29 |
| Etiology of ALI | |||
| Nonpulmonary Sepsis | 25% | 31% | 0.043 |
| Pneumonia | 55% | 24% | |
| Aspiration | 5% | 13% | |
| Other | 15% | 32% | |
| Hospital mortality | 70% | 54% | 0.22 |
Data in the first two columns are expressed as median (interquartile range) or percent of patients. Data in the third column are categorical variables compared using Fisher's Exact Test or Pearson's Chi Square as appropriate. Continuous variables compared using Student's t-test.
Fig. 4.
Net AFC rates are slower in patients with ALI with bacteremia. Boxplot summary of rates of AFC in patients with ALI who had bacteremia (positive blood cultures, n = 20) compared with patients without bacteremia (negative blood cultures, n = 96). Patients with bacteremia had significantly slower rates of AFC compared with those without bacteremia. *P = 0.023 by Student's t-test on square root transformed data.
Fig. 5.
Patients with one or more vasopressors and bacteremia are less likely to have intact AFC. Shock with bacteremia is associated with the lowest rates of AFC. Percentage of patients with intact AFC in patients with ALI by vasopressor and bacteremia status. Patients with both a vasopressor requirement and bacteremia had the lowest percentage of patients with intact (≥3%/h) AFC. P = 0.026 for trend across groups by linear-by-linear association test.
Fig. 6.
Patients with one or more vasopressors and bacteremia are less likely to have maximal AFC. Percentage of patients with maximal AFC in patients with ALI by vasopressor and bacteremia status. Patients with both a vasopressor requirement and bacteremia had the lowest percentage of patients with maximal (>14%/h) AFC. P = 0.006 for trend across groups by linear-by-linear association test.
EF/PL ratio and vasopressor use.
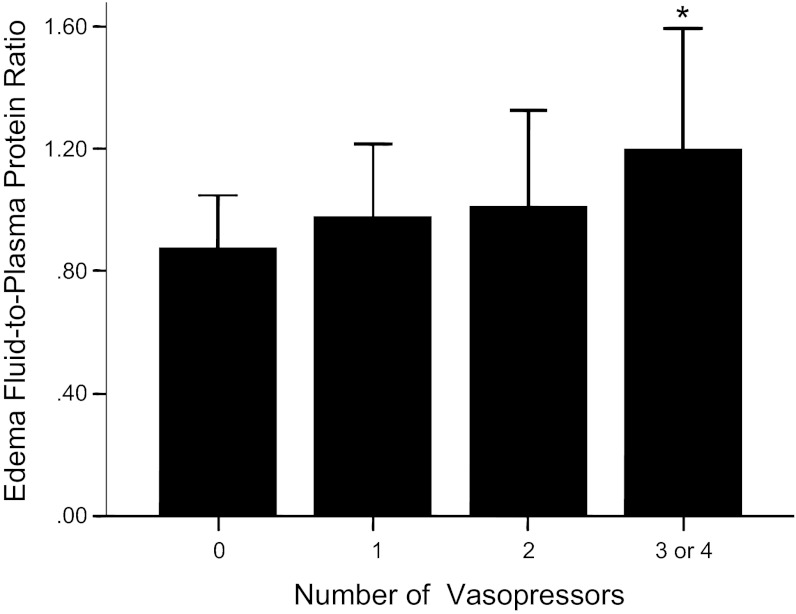
There was a strong association between the number of vasopressors and the initial EF/PL ratio, an index of alveolar-capillary barrier permeability with patients who required the most vasopressors having the highest EF/PL ratios (Fig. 7).
Fig. 7.
Need for vasopressors is associated with increased alveolar-capillary barrier permeability as measured by the edema fluid-to-plasma protein ratio at study enrollment. Patients with the most severe shock (3 or 4 vasopressors) had the highest edema fluid-to-plasma protein ratio. *P < 0.05 vs. all other groups.
DISCUSSION
In 116 patients with ALI/ARDS, the rate of net AFC was significantly slower in those patients who required vasopressors compared with those who did not, an effect that was dose dependent and independent of age, sex, diagnosis of sepsis, or severity of illness. Bacteremia was also significantly associated with slower rates of net AFC, and patients with both vasopressor use and bacteremia had the slowest rates of AFC. These findings have potential clinical significance for explaining the association between shock and poor clinical outcomes in patients with ALI (16). In addition, these findings could help to explain the failure of β2-adrenergic agonists to improve clinical outcomes in two large phase-III clinical trials in patients with ALI (11, 16).
Rates of AFC are decreased in the setting of sepsis in both animals (2) and humans (34), but whether these effects are related to the severity of shock has not been studied. There are several mechanisms by which shock, vasopressor use, and bacteremia might impair AFC. Vasopressor use may be an indicator of the severity of sepsis and the release of proinflammatory mediators, as well as reactive oxygen and nitrogen species, which can increase lung endothelial permeability and depress alveolar fluid clearance. Higher levels of reactive nitrogen species have previously been associated with lower rates of AFC in patients with ALI/ARDS (37). The proinflammatory intra-alveolar environment in ALI/ARDS can also impair AFC mechanisms. Exposure of primary human alveolar epithelial type II cells to pulmonary edema fluid from patients with ALI/ARDS resulted in a marked decrease in gene and protein expression for several ion transport proteins (ENaC, NaK ATPase, and CFTR) and decreased vectorial epithelial fluid transport (15). This effect was driven in part by high levels of cytokines, such as IL-1β. Direct instillation of IL-1β into the lung leads to increased alveolar capillary permeability and increased permeability pulmonary edema (10). IL-1β also decreases expression of the α-subunit of the lung epithelial sodium channel (ENaC) (28) that is responsible for apical epithelial sodium reabsorption. In mouse models, the IL-8 homolog KC decreased AFC in an respiratory syncytial virus model through impaired β-adrenergic receptor signaling. (6).
Circulatory shock itself may have deleterious effects on alveolar epithelial fluid transport mechanisms. In a rat model of prolonged hemorrhagic shock, there was no upregulation of AFC after fluid resuscitation despite a significant increase in plasma epinephrine levels (20). This effect was mediated by neutrophil-dependent oxidant mechanisms. The association of vasopressor use with slower rates of AFC could also reflect an adverse effect of exogenous catecholamines on AFC. Multiple studies have shown that exogenous β-adrenergic agonists and endogenous catecholamines can increase the rate of epithelial sodium transport and AFC in the acute setting (1). Xu et al. (36) showed that endogenous catecholamines increased in a rat pancreatitis model and pharmacological inhibition of these catecholamines negatively impacted the rate of AFC (36). Administration of exogenous catecholamines (epinephrine) to the distal airspaces also augments AFC in isolated, perfused human lungs (31). However, prolonged exposure to catecholamines can impair AFC (23) likely through downregulation of the β-adrenergic receptor on alveolar epithelial type II cells (22).
It is also possible that any stimulatory effect of exogenous catecholamines (vasopressors) on alveolar epithelial sodium transport was counterbalanced by higher rates of alveolar fluid formation due to increased microvascular permeability in those with the most severe shock and bacteremia. The net rate of AFC is dependent, not only on the rate of fluid cleared from the airspace, but also on the rate of alveolar edema fluid accumulation. Thus it is possible that the observed decrease in net AFC in patients requiring vasopressors reflects more severe injury to the alveolar capillary barrier and a faster rate of alveolar fluid formation or alveolar flooding. Bacteremia has also been associated with increased lung epithelial injury and permeability in rabbit models of Pseudomonas pneumonia and decreased AFC in septic rats (2, 13, 26). The strong association between the number of vasopressors and the initial EF/PL ratio, an index of alveolar-capillary barrier permeability (Fig. 7), supports the hypothesis that vasopressor requirement may be an indirect marker of the extent of both systemic endothelial and alveolar-capillary barrier injury.
In the setting of experimental lung injury, higher tidal volumes decrease net AFC in rats (9). However, in this clinical study, there was no difference in the tidal volume or positive end-expiratory pressure between patients with or without shock (Table 1). Thus differences in the applied tidal volumes do not explain the differences in the rate of AFC in the patients with and without shock. The causes of lung injury differed between patients with and without shock with more patients in the shock group having nonpulmonary sepsis and more patients in the no-shock group having other causes of lung injury. However, vasopressor use was common in both septic and nonseptic patients, suggesting that sepsis itself is not the primary determinant of AFC rates in this study.
There are some limitations to this study. Although the patients were enrolled prospectively, the effect of vasopressor use on AFC was not the original focus of the study. The indications for vasopressor use were not captured, nor was the use of vasopressors protocolized. In addition, the doses of vasopressors used were not available. A second limitation is that we measured the net rate of AFC and thus are not able to distinguish between an increase in edema fluid formation vs. a decrease in edema fluid clearance. This is an important distinction and may influence potential therapeutic options for improving net AFC in this patient population. A third limitation is that patients were enrolled over a long time period, and clinical management of patients may have changed over this time period, influencing the findings. However, the major findings persisted even when patients enrolled before 1990 were excluded. In addition, neither vasopressor use nor rates of AFC were different in our subjects when compared over time by decade of enrollment (data not shown). Finally, in this clinical study, we are unable to determine causation. It may be that the number of vasopressors, bacteremia, and lower rates of net AFC are all markers of severity of lung injury and that bacteremia and vasopressor use do not directly affect net AFC. However, whatever mechanisms account for the inverse relationship of alveolar fluid transport and the presence of shock and bacteremia in patients with ALI, AFC measures a fundamental property of the lung, namely the capacity to reabsorb edema fluid across the alveolar epithelium, the first step in the resolution of ALI (19).
In summary, in patients with ALI/ARDS, the rate of net AFC was significantly slower in those who required vasopressors and those with bacteremia, effects that were dose dependent and independent of other severity factors. These findings may be important for several reasons. First they may explain in part the high mortality in sepsis-induced ALI/ARDS despite aggressive treatment. The rate of net AFC influences clinical outcomes in patients with ARDS with the patients having the fastest rates of AFC also having the highest chance of survival (34). Second, the results identify two key factors that may have direct effects on net AFC and the resolution phase of ALI. Finally, these findings suggest that vasopressor use may be a marker of alveolar-capillary barrier permeability and provide additional rationale for novel therapies that could enhance the resolution of alveolar edema by accelerating repair of the injured lung endothelium and the alveolar epithelium.
GRANTS
This study was supported in part by NIH grants HL090785 (J. Bastarache), HL51856 (M. Matthay), HL103836 (L. Ware) and an American Heart Association Established Investigator Award (L. Ware).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.F.Z., M.A.M., and L.B.W. conception and design of research; Y.F.Z., M.A.M., and L.B.W. analyzed data; Y.F.Z., J.A.B., M.A.M., and L.B.W. interpreted results of experiments; Y.F.Z. drafted manuscript; Y.F.Z., J.A.B., M.A.M., and L.B.W. edited and revised manuscript; Y.F.Z., J.A.B., M.A.M., and L.B.W. approved final version of manuscript; L.B.W. performed experiments; L.B.W. prepared figures.
ACKNOWLEDGMENTS
The authors thank Nancy Wickersham for invaluable help processing samples and assisting with protein measurements.
REFERENCES
- 1. Azzam ZS, Adir Y, Crespo A, Comellas A, Lecuona E, Dada LA, Krivoy N, Rutschman DH, Sznajder JI, Ridge KM. Norepinephrine increases alveolar fluid reabsorption and Na,K-ATPase activity. Am J Respir Crit Care Med 170: 730–736, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Berger G, Guetta J, Klorin G, Badarneh R, Braun E, Brod V, Saleh NA, Katz A, Bitterman H, Azzam ZS. Sepsis impairs alveolar epithelial function by downregulating Na-K-ATPase pump. Am J Physiol Lung Cell Mol Physiol 301: L23–L30, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Cooke CR, Shah CV, Gallop R, Bellamy S, Ancukiewicz M, Eisner MD, Lanken PN, Localio AR, Christie JD. A simple clinical predictive index for objective estimates of mortality in acute lung injury. Crit Care Med 37: 1913–1920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis IC, Matalon S. Epithelial sodium channels in the adult lung—important modulators of pulmonary health and disease. Adv Exp Med Biol 618: 127–140, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis IC, Xu A, Gao Z, Hickman-Davis JM, Factor P, Sullender WM, Matalon S. Respiratory syncytial virus induces insensitivity to β-adrenergic agonists in mouse lung epithelium in vivo. Am J Physiol Lung Cell Mol Physiol 293: L281–L289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med 152: 1818–1824, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol 35: 10–19, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med 165: 242–249, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G, Sheppard D, Violette SM, Weinreb PH, Horan GS, Matthay MA, Pittet JF. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ Res 102: 804–812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, Khan Z, Lamb SE. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 379: 229–235, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ingbar DH, Bhargava M, O'Grady SM. Mechanisms of alveolar epithelial chloride absorption. Am J Physiol Lung Cell Mol Physiol 297: L813–L815, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest 104: 743–750, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270: 2957–2963, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Lee JW, Fang X, Dolganov G, Fremont RD, Bastarache JA, Ware LB, Matthay MA. Acute lung injury edema fluid decreases net fluid transport across human alveolar epithelial type II cells. J Biol Chem 282: 24109–24119, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT. Randomized, placebo-controlled clinical trial of an aerosolized beta-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 184: 561–568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142: 1250–1257, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 33: 319–327, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Modelska K, Matthay MA, Brown LA, Deutch E, Lu LN, Pittet JF. Inhibition of β-adrenergic-dependent alveolar epithelial clearance by oxidant mechanisms after hemorrhagic shock. Am J Physiol Lung Cell Mol Physiol 276: L844–L857, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Monchi M, Bellenfant F, Cariou A, Joly LM, Thebert D, Laurent I, Dhainaut JF, Brunet F. Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med 158: 1076–1081, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Morgan EE, Hodnichak CM, Stader SM, Maender KC, Boja JW, Folkesson HG, Maron MB. Prolonged isoproterenol infusion impairs the ability of β(2)-agonists to increase alveolar liquid clearance. Am J Physiol Lung Cell Mol Physiol 282: L666–L674, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Morgan EE, Stader SM, Hodnichak CM, Mavrich KE, Folkesson HG, Maron MB. Postreceptor defects in alveolar epithelial β-adrenergic signaling after prolonged isoproterenol infusion. Am J Physiol Lung Cell Mol Physiol 285: L578–L583, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol 289: L685–L695, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Page B, Vieillard-Baron A, Beauchet A, Aegerter P, Prin S, Jardin F. Low stretch ventilation strategy in acute respiratory distress syndrome: eight years of clinical experience in a single center. Crit Care Med 31: 765–769, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Pittet JF, Kudoh I, Wiener-Kronish JP. Endothelial exposure to Pseudomonas aeruginosa proteases increases the vulnerability of the alveolar epithelium to a second injury. Am J Respir Cell Mol Biol 18: 129–135, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Roupie E, Lepage E, Wysocki M, Fagon JY, Chastre J, Dreyfuss D, Mentec H, Carlet J, Brun-Buisson C, Lemaire F, Brochard L. Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. SRLF Collaborative Group on Mechanical Ventilation Societe de Reanimation de Langue Francaise. Intensive Care Med 25: 920–929, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM, Pittet JF. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem 280: 18579–18589, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Rubin DB, Wiener-Kronish JP, Murray JF, Green DR, Turner J, Luce JM, Montgomery AB, Marks JD, Matthay MA. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest 86: 474–480, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakuma T, Gu X, Wang Z, Maeda S, Sugita M, Sagawa M, Osanai K, Toga H, Ware LB, Folkesson G, Matthay MA. Stimulation of alveolar epithelial fluid clearance in human lungs by exogenous epinephrine. Crit Care Med 34: 676–681, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vadasz I, Raviv S, Sznajder JI. Alveolar epithelium and Na,K-ATPase in acute lung injury. Intensive Care Med 33: 1243–1251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verghese GM, Ware LB, Matthay BA, Matthay MA. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J Appl Physiol 87: 1301–1312, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest 88: 864–875, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu J, Wang Z, Ma G, Sagawa M, Shimazaki M, Ueda Y, Sakuma T. Endogenous catecholamine stimulates alveolar fluid clearance in rats with acute pancreatitis. Respirology 14: 195–202, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Zhu S, Ware LB, Geiser T, Matthay MA, Matalon S. Increased levels of nitrate and surfactant protein a nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med 163: 166–172, 2001 [DOI] [PubMed] [Google Scholar]