Abstract
Cigarette smoke (CS) is a well-established risk factor in the development of chronic obstructive pulmonary disease (COPD). In contrast, the extent to which CS exposure contributes to the development of the systemic manifestations of COPD, such as skeletal muscle dysfunction and wasting, remains largely unknown. Decreased skeletal muscle capillarization has been previously reported in early stages of COPD and might play an important role in the development of COPD-associated skeletal muscle abnormalities. To investigate the effects of chronic CS exposure on skeletal muscle capillarization and exercise tolerance, a mouse model of CS exposure was used. The 129/SvJ mice were exposed to CS for 6 mo, and the expression of putative elements of the hypoxia-angiogenic signaling cascade as well as muscle capillarization were studied. Additionally, functional tests assessing exercise tolerance/endurance were performed in mice. Compared with controls, skeletal muscles from CS-exposed mice exhibited significantly enhanced expression of von Hippel-Lindau tumor suppressor (VHL), ubiquitin-conjugating enzyme E2D1 (UBE2D1), and prolyl hydroxylase-2 (PHD2). In contrast, hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) expression was reduced. Furthermore, reduced muscle fiber cross-sectional area, decreased skeletal muscle capillarization, and reduced exercise tolerance were also observed in CS-exposed animals. Taken together, the current results provide evidence linking chronic CS exposure and induction of VHL expression in skeletal muscles leading toward impaired hypoxia-angiogenesis signal transduction, reduced muscle fiber cross-sectional area, and decreased exercise tolerance.
Keywords: capillaries, chronic obstructive pulmonary disease, hypoxia inducible factor-1α, pulmonary cachexia syndrome, vascular endothelial growth factor
exposure to cigarette smoke (CS) is a well-known risk factor for the development of irreversible airway limitation in chronic obstructive pulmonary disease (COPD) (2, 12). However, the contribution of CS exposure to the development of the extrapulmonary complications of COPD remains largely unknown. A significant number of patients with COPD develop generalized weight loss and peripheral muscle wasting, collectively termed as pulmonary cachexia syndrome (PCS) (1, 10, 39, 46, 47). Development of PCS is directly correlated with increased sense of fatigue, impaired exercise capacity, dyspnea, and increased rate of mortality in these patients (1, 25, 37, 40).
Skeletal muscles of patients with COPD are characterized by several structural, metabolic, and functional alterations associated with the loss of muscle strength and endurance. These include loss of muscle mass and cross-sectional area (9, 16), fiber type switch toward the more fatigue-susceptible type-II glycolytic fibers (14, 20, 50), decrease in muscle oxidative capacity (25), and significantly decreased capillarization (20). Interestingly, fiber type shift, reduction in oxidative enzyme activity, and decreased muscle fiber diameter have been also observed in skeletal muscles of healthy smokers before development of COPD (23, 30, 51). Moreover, smokers exhibit lower peripheral muscle fatigue-resistance than nonsmokers (51). The possibility of directly implicating chronic CS exposure to development of PCS in COPD is further supported by recent findings that CS induces direct oxidative damage of several important proteins involved in the regulation of muscle metabolism and bioenergetics and that these changes precede the respiratory changes (4, 30). In analogy, skeletal muscles from several murine models chronically exposed to CS exhibited patterns of morphological, metabolic, and functional changes similar to those observed in healthy smokers and patients with COPD (3, 13, 22, 29, 45).
The presence of an intact skeletal muscle vasculature is crucial for responding to the various metabolic changes and local tissue insults, e.g. hypoxia (15). An important element in this adaptive mechanism is the hypoxia inducible factor-1α (HIF-1α) transcription factor acting as a molecular sensor capable of detecting a fine fluctuation in intracellular oxygen concentrations (41). During normoxia, HIF-1α levels are kept under tight control through hydroxylation by a family of prolyl hydroxylases (PHD1–4) (11) and subsequent ubiquitination and proteosomal degradation mediated by the von Hippel-Lindau tumor suppressor (VHL) protein, a member of RING finger E3-ligase family (21, 33). The latter involves binding of VHL/HIF-1α complex to ubiquitin-conjugating enzyme E2D1 (UBE2D1), which carries out multiple transfers of activated ubiquitin molecules to specific lysil residues within the HIF-1α structure (18, 36). Intracellular hypoxia inactivates VHL, thus allowing HIF-1α stabilization and transcriptional activation of a large panel of genes involved in metabolism, erythropoiesis, and angiogenesis including vascular endothelial growth factor (VEGF) (41). Beside involvement in transcriptional control of VEGF expression via HIF-1α pathway, VHL regulates VEGF expression on the posttranscriptional and translation levels (52). Recently, we have reported first evidence demonstrating VHL overexpression in skeletal muscles from patients with COPD accompanied with decreased capillarization and muscle wasting (19). These changes were observed in patients with COPD with the mild, Stage-1 disease, where normal respiratory function has not been significantly compromised (19). This suggested that the observed abnormalities in skeletal muscle vascularity and hypoxia-angiogenesis signaling are an early, primary event, rather than a secondary occurrence to development of COPD and its complications.
The objectives of the present investigation are specifically focused on the assessment of the effects of the chronic CS exposure on the expression of putative elements of the hypoxia-angiogenic signaling cascade and muscle capillarization in an experimental animal model. For this purpose, we have used a mouse model exposed to CS for 6 mo. The 129/SvJ mice strain used in this study was previously demonstrated to exhibit higher intrinsic resistance to CS-induced lung emphysema compared with other susceptible strains such as C57BL/6J and DBA/2 (17, 38, 54).
MATERIALS AND METHODS
Animals and CS exposure.
The 129/SvJ mice (Jackson Laboratory, Bar Harbor, ME) were bred and maintained under specific pathogen-free conditions involving 12-h:12-h dark/light cycles inside adequate vivarium facilities at the University of Rochester. For CS exposure, 3R4F research cigarettes (University of Kentucky, Lexington, KY) were used to generate a mixture of sidestream smoke (89%) and mainstream smoke (11%) by a Teague smoking machine (Model TE-10; Teague Enterprises, Woodland, CA) at a concentration of ∼100 mg TPM/m3 to avoid the possible toxicity to mice at a high concentration of long-term CS exposure (N = 6) (53, 54). The level of carbon monoxide in the chamber was 350 ppm. The 129/SvJ mice (8–10 wk old, 22–25 g body wt) received 5-h exposures per day, 5 days/wk for 6 mo, and were killed 24 h after the last CS exposure (53). No mouse mortality was found due to chronic CS exposure, but body weight was significantly reduced in 6 mo of CS-exposed mice (31.6 ± 1.16 g) compared with air-exposed mice (36.9 ± 1.01 g, n = 3, P < 0.05). Control mice (N = 8) were exposed to filtered air in an identical chamber according to the same protocol described for CS exposure. All animal procedures described in this study were approved by the University Committee on Animal Research Committee of the University of Rochester.
Exercise endurance determination.
Exercise endurance in mouse was measured using a motorized rodent treadmill with an electric grid at the rear of the treadmill (Columbus Instruments, Columbus, OH) as described previously (28, 53). Run duration (min) and run distance (m, calculated from the run time and speed of the treadmill) were used as the parameters to reflect exercise tolerance. Mice were placed on the treadmill and allowed to adapt to the surroundings for 3–5 min before starting the exercise. The treadmill was started at a speed of 8.5 m/min with a 0° incline. After 9 min, the speed and incline were raised to 10 m/min and 5°, respectively. Speed was increased by 2.5 m/min every 3 min to a maximum of 40 m/min, and the incline increasing 5° every 9 min to a maximum of 15°. Exercise continued until mouse exhaustion, which is defined as an inability to maintain running despite repeated contact with the electric grid. At this stage, each mouse was immediately returned to its home cage.
Muscle biopsies.
Animal models were killed 24 h after the last CS exposure. Mice were anesthetized using 100 mg/kg pentobarbital sodium (Abbot Laboratories, Abbot Park, Illinois). Gastrocnemius muscle specimens (∼150 mg) in both legs were dissected, cleaned for fat and connective tissue, placed into sealed vials, snap frozen in liquid nitrogen, and stored at −80°C until analysis.
RNA isolation.
Muscle specimens were frozen in the liquid nitrogen, cut in pieces, and disrupted using Micro-Dismembrator II (B Braun, Melsungen, Germany). Total RNA was isolated from the obtained homogenized material with the RNEasy Fibrous Tissue Mini Kit (Qiagen, Valencia, CA) according to the instructions specified by the manufacturer. The total RNA was quantified by using the Nanovue Plus spectrophotometer (GE Healthcare, Buckinghamshire, UK). Isolated total RNA was stored on −80°C until further use.
Real-time PCR analysis.
Total RNA (0.75 μg) was reverse-transcribed to cDNA by using SuperScript II first-strand synthesis kit (Invitrogen, Carlsbad, CA) following detailed instructions described by the manufacturer. Synthesized complementary DNA was stored on −20°C until further use. Quantitative RT-PCR gene expression analysis was performed on ABI Prism Sequence Detection System 7900HT (PE Applied Biosystems, Foster City, CA). Genes targeted in the expression analysis including HIF-1α (catalogue no. Mm00468869_m1), VEGF (catalogue no. Mm00437304_m1), PHD2 (catalogue no. Mm00459770_m1), UBE2D1 (catalogue no. Mm01172638_m1), and VHL (catalogue no. Mm00494136_m1) were provided as Assay-on-Demand by Applied Biosystems. Gene expression analysis was normalized to the expression levels of β-microglobulin (catalogue no. Mm00432100_m1) gene expression. The probes were labeled using FAM as the reporter dye and TAMRA as the quencher dye.
Each sample was analyzed in duplicate under the following conditions: 2 min at 50°C, 10 min at 95°C, 15 s at 95°C, and 1 min at 60°C. PCR amplification was correlated against a standard curve. Reactions were performed in MicroAmp optical 96-well reaction plates (PE Applied Biosystems).
Western blot analysis.
The excised muscles were homogenized in the ice cold lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA) containing a protease inhibitor cocktail and centrifuged for 10 min on 10,000 g on 4°C. Supernatant was separated from the pellet and used in further steps. Concentration of total protein was determined using Bradford assay (Sigma Aldrich, Taufkirchen, Germany). Equal amounts of total protein (30 μg) were separated under reducing conditions using 7.5%, 10%, and 12% SDS page and transferred onto nitrocellulose membrane (Amersham) in a transblot electrophoretic transfer cell (Bio-Rad Laboratories, Hercules, CA). Membranes were probed overnight at 4°C using rabbit polyclonal anti-HIF-1α (Novus Biologicals, Cambridge, UK) in 1:1,000 dilution, rabbit polyclonal anti-VHL (Cell Signaling Technology, Beverly, MA) in 1:1,000 dilution, rabbit polyclonal anti-PHD2 in 1:1,000 dilution (Santa Cruz Biotechnology), rabbit polyclonal anti-VEGF in 1:1,000 dilution (Santa Cruz Biotechnology), rabbit polyclonal anti-UBE2D1 in 1:1,000 dilution (Abnova, Taipei, Taiwan), and rabbit polyclonal anti-α-tubulin in 1:10,000 (Abnova) used as loading control. Presence of nuclear protein yield was assessed and confirmed using rabbit polyclonal anti-TBP 1:1,000 (Abnova) used as nucleolar loading control (unpublished results). Membranes were developed using an enhanced chemiluminescence system (Amersham) and exposed to Hyperfilm enhanced chemiluminescence (Amersham). Densitometric analysis was performed using NIH software package Image J (ImageJ 1.32j; NIH, Bethesda, MD).
Determination of muscle fiber cross-sectional area.
Transverse sections (5 μm) were cut from the mid-belly of the gastrocnemius muscle on a cryostat at −22°C using Leica CM1850 cryostat attached to positively charged glass slides (Superfrost; MenzelGläser, Braunscweig, Germany). Hematoxylin and eosin (H & E) staining was performed on sections from six animals per group (N = 6) to assess general fiber morphology and measure fiber cross-sectional area. Digital photographs were taken from each H & E section at ×10 magnification using a Magnafire digital camera and software (Optronics, Galena, CA) with 30-ms exposure. Cross-sectional area for the muscles (CSA) was determined from the composite images of the entire muscle cross-section. More than 300 individual fibers per each cross-section have been included in the CSA evaluation. With the aid of an image morphometry program (ImageJ 1.32j), the outline of the individual fibers was traced and parameters such as fiber area expressed in μm2, smallest and biggest fiber diameter, and fiber cross-sectional roundness index were assessed for each individual fiber. The roundness index (recorded as a value between 0 and 1) is the ratio of the cell area relative to the area of a circle that fully enclosed that cell; circular cells have a value approaching 1, whereas noncircular cells have smaller values. Cut point roundness index for fiber inclusion in CSA evaluation was ≥0.8.
Immunohistochemistry and capillary measurements.
Serial transverse sections, 5 μm in thickness, were cut at −22°C using Leica CM1850 cryostat attached to positively charged glass slides (Superfrost). Before primary antibody incubation, the sections were incubated in 2.5% BSA for 5 min. Capillaries were identified by staining with monoclonal antibody detecting CD31 (MO823; Dako, Glostrup, Denmark) in 1:100 dilution and diaminobenzidine.
Capillary analysis was performed using light microscope (Olympus BX60) connected to a computerized image system (Cell Images). Capillary-to-fiber ratio parameter was determined by analyzing number of capillaries surrounding more than 200 individual fibers per section belonging to each animal (N = 5). Briefly, digital photographs of four to six randomly chosen cross-sectional areas were taken at ×40 magnification, area perimeter outlined using free-hand selection tool, and total number of fibers and surrounding capillaries determined. Quality of cross-sections was assessed by measuring roundness index of individual fibers with the aid of an image morphometry program (ImageJ 1.32j). Only fibers entirely included in outlined area and with roundness index ≥0.8 have been included in analysis.
Statistical analysis.
Obtained data for each experimental group were not normally distributed. The results were represented as median [interquartile range, (IQR)] as determined using Mann-Whitney U rank test for unpaired data. Differences were considered significant at P < 0.05. Statistical analysis was performed with SPSS v.16 Statistics Software.
RESULTS
CS exposure increases skeletal muscle levels of VHL, UBE2D1, and PHD2.
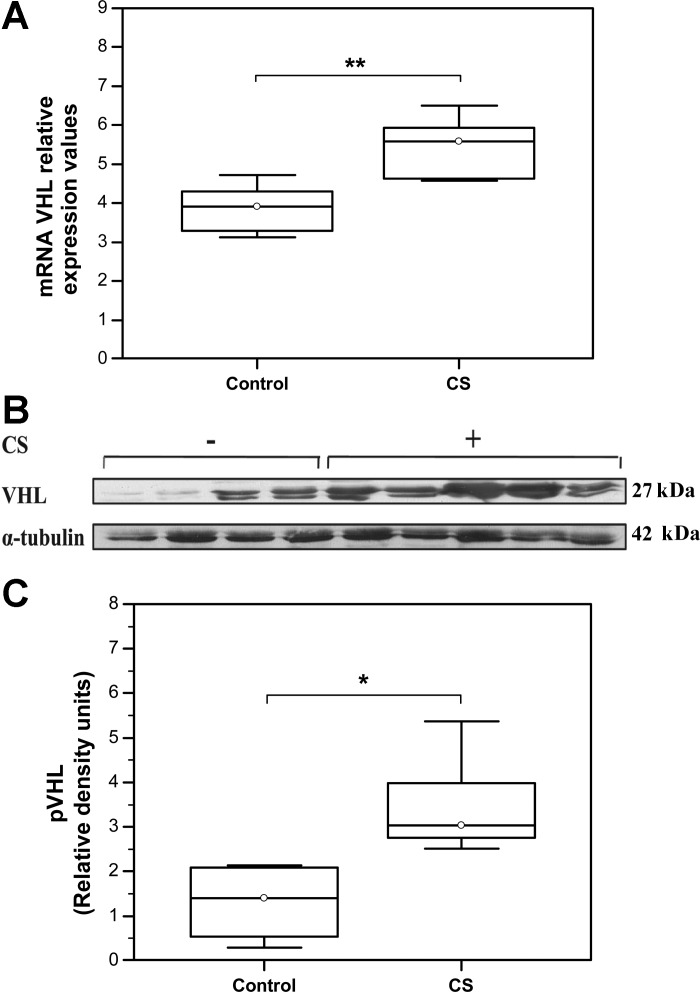
VHL mRNA expression levels were significantly increased in skeletal muscles from mice exposed to CS relative to the matching control group (Control: Median = 3.91, IQR = 3.31–4.3; N = 8 vs. CS: Median = 5.58, IQR = 4.6–5.9; N = 6; **P = 0.0055, Fig. 1A). Similarly, Western blot analysis demonstrated an increase in VHL protein levels in skeletal muscles from CS-exposed animals compared with the matching controls (Control: Median = 1.41, IQR = 0.53–2.1 vs. CS: Median = 3.04, IQR = 2.77–3.98; N = 6, *P = 0.016) (Fig. 1, B and C).
Fig. 1.
Increased expression levels of von Hippel-Lindau tumor suppressor (VHL) in skeletal muscles of cigarette smoke (CS)-exposed mice. A: qRT PCR analysis of VHL mRNA levels [Control: Median = 3.91, interquartile range (IQR) = 3.31–4.3; N = 8 vs. CS: Median = 5.58, IQR = 4.6–5.9; N = 6; **P = 0.0055]. B: representative Western blot showing VHL protein expression levels. C: quantification of VHL protein expression levels by densitometry. VHL density signals were normalized to α-tubulin signal values (Control: Median = 1.41, IQR = 0.53–2.1 vs. CS: Median = 3.04, IQR = 2.77–3.98; N = 6, *P = 0.016).
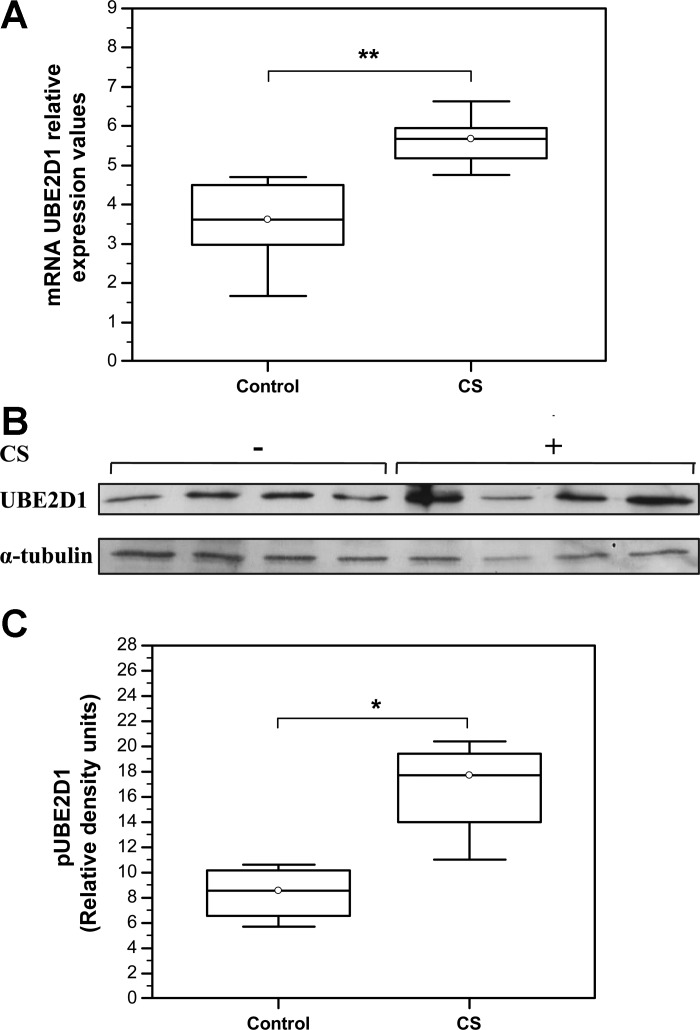
An important element of VHL-regulated ubiquitination machinery is UBE2D1 ubiquitin-conjugating enzyme, belonging to the E2 family of ubiquitin ligases. UBE2D1 is required for supply and transfer of the activated ubiquitin molecules to the VHL/target protein complex, therefore facilitating the process of ubiquitination and degradation of VHL target proteins (18, 36). The results of this study demonstrated statistically significant overexpression of UBE2D1 on both, mRNA (Control: Median = 3.62, IQR = 2.98–4.5, N = 7 compared with the CS: Median = 5.68, IQR = 5.17–5.95; N = 5; **P = 0.0057) (Fig. 2A) and protein level (Control: Median = 0.86, IQR = 0.66–1.02 compared with the CS: Median = 1.77, IQR = 1.4–1.94; N = 6; *P = 0.03) (Fig. 2, B and C).
Fig. 2.
Chronic CS exposure increases expression levels of ubiquitin-conjugating enzyme E2D1 (UBE2D1) in mice skeletal muscles. A: qRT PCR analysis of UBE2D1 mRNA levels. **P < 0.001 (Control: Median = 3.62, IQR = 2.98–4.5, N = 7 compared with the CS: Median = 5.68, IQR = 5.17–5.95; N = 5; **P = 0.0057). B: representative Western blot showing UBE2D1 protein expression levels. C: quantification of UBE2D1 protein expression levels by densitometry. UBE2D1 density signals were normalized to α-tubulin signal values (Control: Median = 0.86, IQR = 0.66–1.02 compared with the CS: Median = 1.77, IQR = 1.4–1.94; N = 6; *P = 0.03).
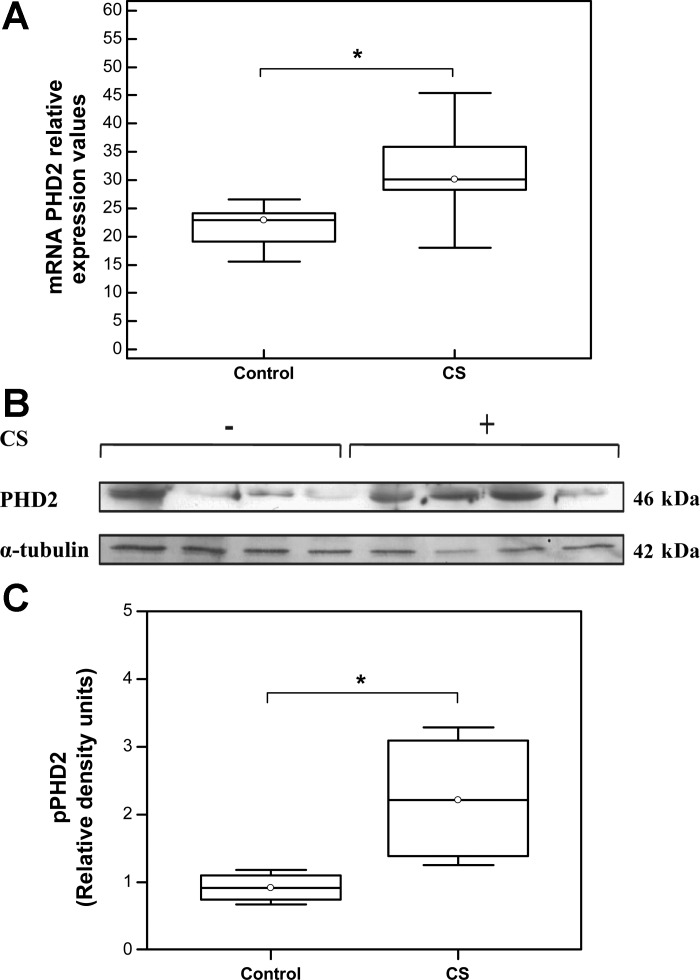
VHL-mediated polyubiquitination and subsequent proteosomal degradation of intracellular HIF-1α is facilitated via its O2-dependent hydroxylation by a family of PHDs (PHD1–4) (6). PHD2, a key member of this family, is responsible for maintaining basal levels of HIF-1α in normoxia and was selected for analysis (11). Our results demonstrated a significant increase in PHD2 mRNA levels (Fig. 3A) in muscles from CS-exposed mice compared with controls (Control: Median = 22.87, IQR = 19.13–24.06, N = 7 vs. CS: Median = 30.13, IQR = 28.27–35.9, N = 6; *P = 0.038). In this regard, 6 mo of CS exposure significantly elevated PHD2 protein levels in gastrocnemius muscle of exposed animals (Control: Median = 0.92, IQR = 0.75–1.1 vs. CS: Median = 2.22, IQR = 1.39–3.1, N = 6; *P = 0.021) (Fig. 3, B and C).
Fig. 3.
Increased expression levels of prolyl hydroxylase-2 (PHD2) in skeletal muscle of CS-exposed mice. qRT PCR analysis of PHD2 mRNA levels. *P < 0.05 (Control: Median = 22.87, IQR = 19.13–24.06, N = 7 vs. CS: Median = 30.13, IQR = 28.27–35.9, N = 6; *P = 0.038). B: representative Western blot showing VHL protein expression levels. C: quantification of PHD2 protein expression levels (Control: Median = 0.92, IQR = 0.75–1.1 vs. CS: Median = 2.22, IQR = 1.39–3.1, N = 6; *P = 0.021).
CS exposure decreases stability of HIF-1α protein.
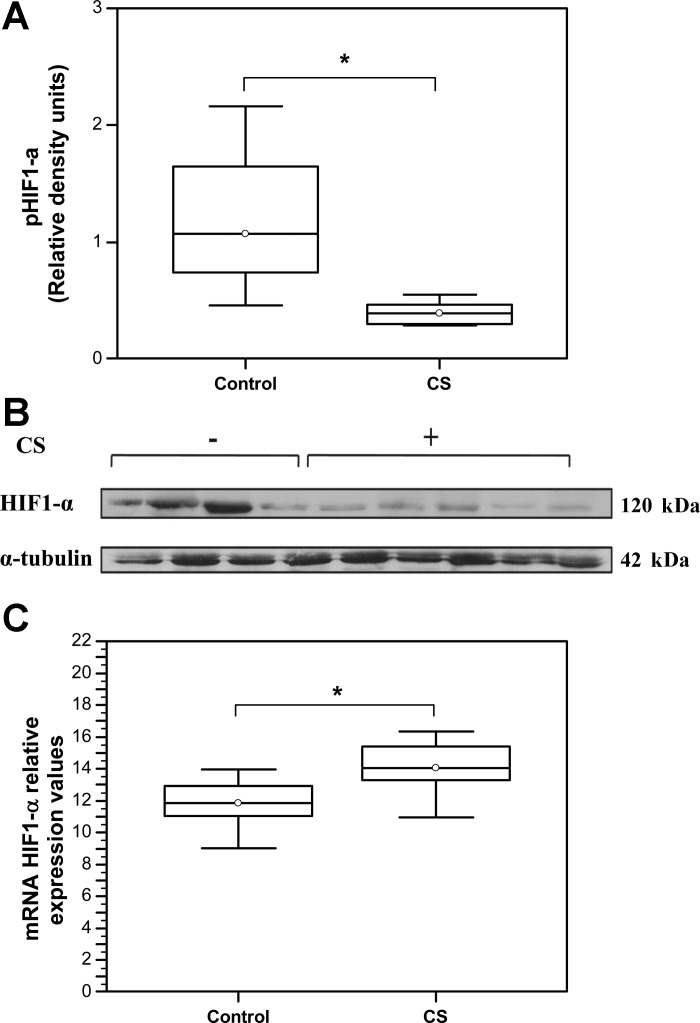
Enhanced VHL and PHD2 activity strongly suggested the possibility of increased HIF1-protein degradation and reduced availability in skeletal muscles of CS-exposed animals. Indeed, a significant decrease in HIF-1α protein levels was observed in CS-exposed animals relative to controls (Control: Median = 1.07, IQR = 0.74–1.64 vs. CS: Median = 0.39, IQR = 0.3–0.47, N = 6; *P = 0.027) (Fig. 4, A and B). By contrast, qRT-PCR analysis of HIF-1α mRNA expression level revealed mild but statistically significant increase in expression (*P = 0.045) in muscles of CS-exposed animals (Median = 11.83, IQR = 11.04–12.91, N = 6) relative to the control (Median = 14.06, IQR = 13.27–15.39, N = 8).
Fig. 4.
CS exposure destabilizes hypoxia-inducible factor-1α (HIF-1α) protein. A: representative Western blot showing HIF-1α protein expression levels. B: quantification of HIF-1α protein expression levels by densitometry. HIF-1α density signals were normalized to α-tubulin signal values. (Control: Median = 1.07, IQR = 0.74–1.64 vs. CS: Median = 0.39, IQR = 0.3–0.47, N = 6; P = 0.027). C: qRT PCR analysis of HIF-1α mRNA values (Control: Median = 11.83, IQR = 11.04–12.91, N = 6 vs. CS: Median = 14.06, IQR = 13.27–15.39, N = 8, *P = 0.045).
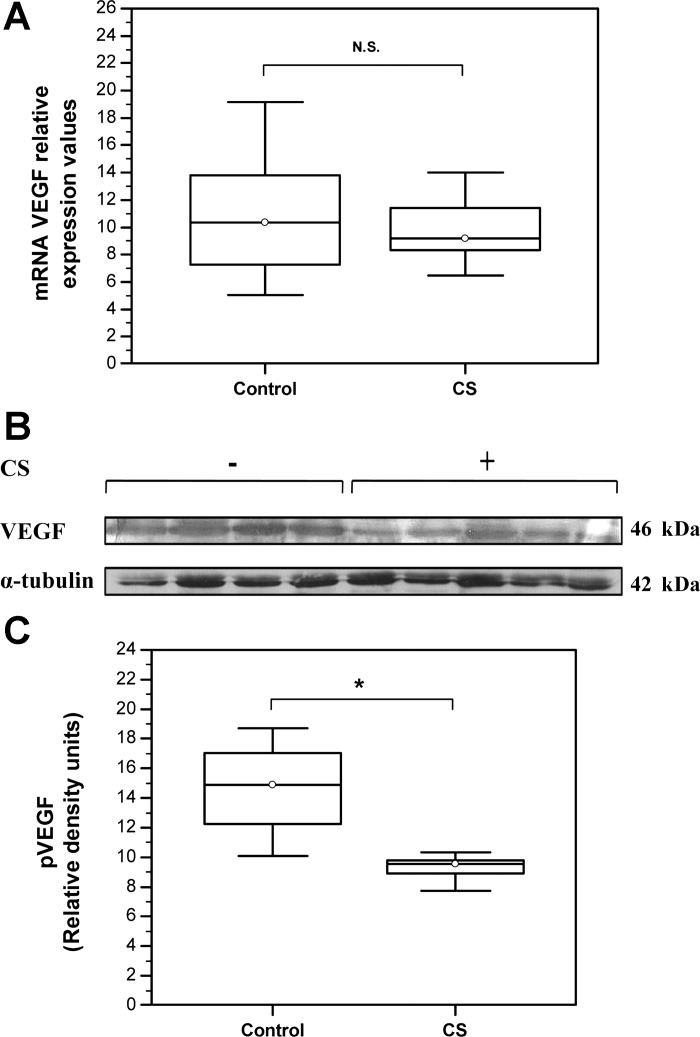
Decreased VEGF expression is observed in CS-exposed mice.
Decreased HIF-1α protein expression is expected to exert a negative impact on downstream gene expression including VEGF, which is central in neocapillary formation and maintenance of adult tissue vascularization. Indeed, VEGF mRNA levels demonstrated a tendency toward decreased expression in CS-exposed mice (Control: Median = 10.37, IQR = 7.28–13.82, N = 8 vs. CS: Median = 9.16, IQR = 8.31–11.42, N = 6; P = 0.75) (Fig. 5A). Likewise, immunoblot analysis detected a statistically significant decrease in VEGF protein levels in skeletal muscles from CS-exposed mice relative to the control (Control: Median = 14.9, IQR = 12.27–17.05 vs. CS: Median = 9.55, IQR = 8.92–9.82, N = 6; *P = 0.037) (Fig. 5, B and C).
Fig. 5.
Decreased vascular endothelial growth factor (VEGF) abundance in skeletal muscles of CS-exposed mice. A: qRT PCR analysis of VEGF mRNA levels (Control: Median = 10.37, IQR = 7.28–13.82, N = 8 vs. CS: Median = 9.16, IQR = 8.31–11.42, N = 6; P = 0.75). B: representative Western blot showing VEGF protein expression levels. C: quantification of VEGF protein expression levels by densitometry. VEGF density signals were normalized to α-tubulin signal values (Control: Median = 14.9, IQR = 12.27–17.05 vs. CS: Median = 9.55, IQR = 8.92–9.82, N = 6; *P = 0.037).
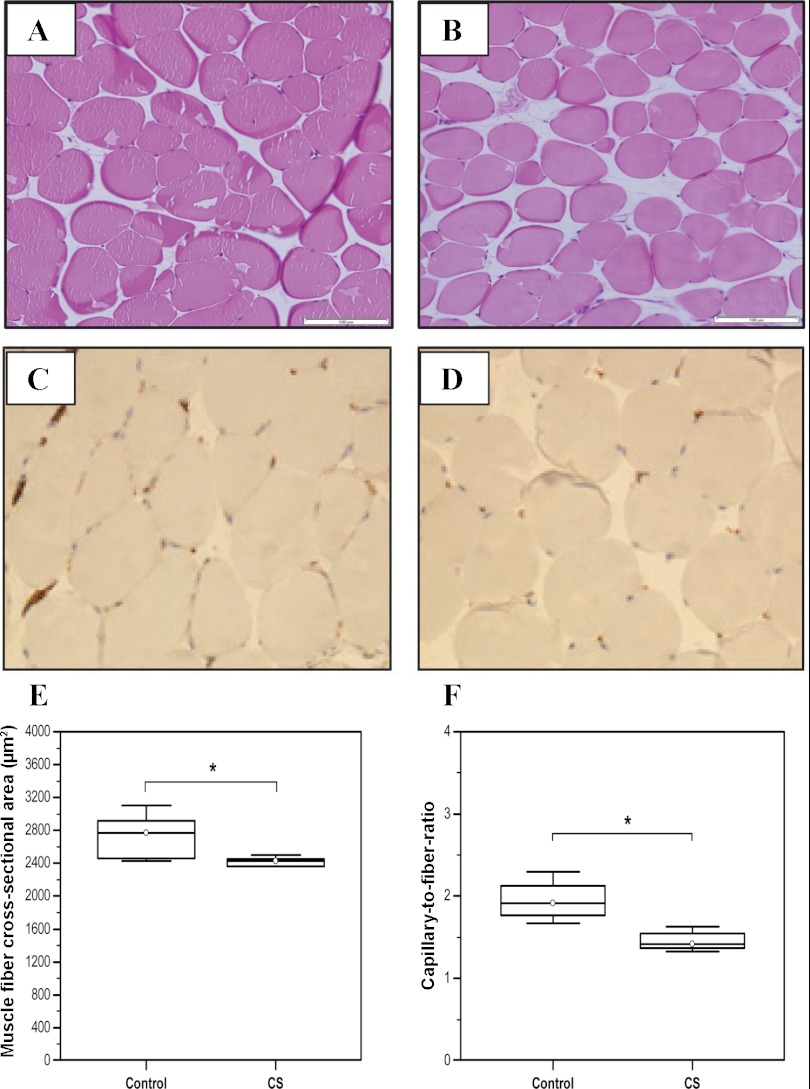
CS exposure reduces muscle fiber cross-sectional area.
Statistically significant reduction in muscle fiber cross-sectional area was observed in gastrocnemius muscles of 129 SvJ mice after 6 mo of chronic CS exposure (Control: Median = 2,771.16, IQR = 2,462.83–2,914.93 vs. CS-exposed animals: Median = 2,429.3, IQR = 2,360.46–2,454.91; N = 6, *P = 0.025) (Fig. 6, A–C).
Fig. 6.
Mean skeletal muscle cross-sectional area (CSA) and capillary-to-fiber ratio in skeletal muscles of CS- and air-exposed mice. Representative hematoxylin and eosin staining of gastrocnemius cross-section from 129 SvJ mice Control group (A), CS-exposed group (B), Mean fiber CSA in control animals (C) relative to the CS-exposed animal group (Control: Median = 2,771.16, IQR = 2,462.83–2,914.93 vs. CS-exposed animals: Median = 2,429.3, IQR = 2,360.46–2,454.91; N = 6, *P = 0.025). Representative CD31 staining for control group (D) and mice exposed to cigarette smoke (CS) (E). F: capillary-to-fiber ratio (Control: Median = 1.91, IQR = 1.77–2.13 vs. CS: Median = 1.42, IQR = 1.37–1.55, N = 5; *P = 0.014). Arrows point to endothelial cells positive for CD31 staining.
Muscle capillarization is impaired in CS-exposed mice.
To determine the impact of decreased HIF-1α and VEGF expression on muscle capillarization, capillary density was analyzed by anti-CD31 immunostaining, and capillary-to-fiber ratio was assessed in CS-exposed animals and matching controls. As expected, capillary-to-fiber ratio was decreased by 33% in skeletal muscles from CS-exposed mice relative to controls (Control: Median = 1.91, IQR = 1.77–2.13, vs. CS: Median = 1.42, IQR = 1.37–1.55, N = 5; *P = 0.014) (Fig. 6, D–F).
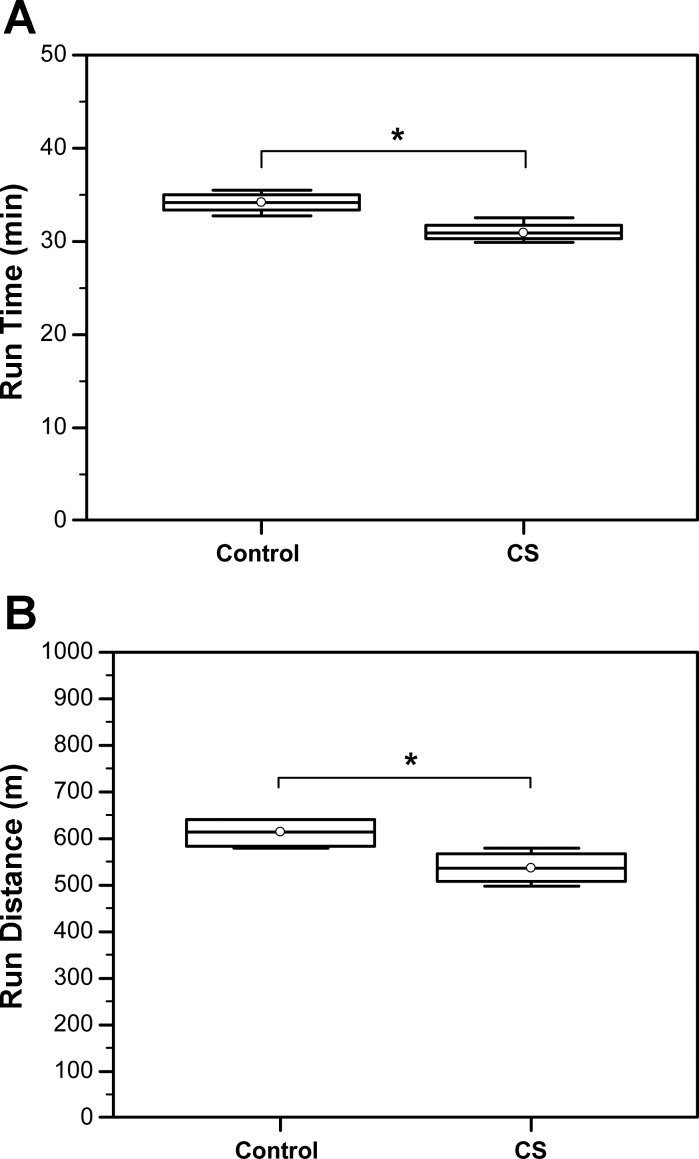
Chronic CS exposure decreases exercise tolerance in mice.
Exercise capacity is an important parameter to evaluate and determine limitations of skeletal muscle function, which is known to be impaired in patients with COPD (31). Exercise capacity was, thus, evaluated in CS-exposed mice vs. controls. We found that run time was significantly decreased in CS-exposed mice compared with controls (Control: Median = 34.2, IQR = 33.35–35 vs. CS: Median = 30.9, IQR = 30.35–31.75, N = 4; *P = 0.029). Moreover, the mouse run distance was also reduced in CS-exposed vs. control mice (Control: Median = 614, IQR = 584–640 vs. CS: Median = 536, IQR = 507.567, N = 4; *P = 0.03) (Fig. 7).
Fig. 7.
Chronic CS exposure decreases exercise tolerance in mice. A: comparison of run time (min) (Control: Median = 34.2, IQR = 33.35–35.0 vs. CS: Median = 30.9, IQR = 30.35–31.75, N = 4; *P = 0.029). B: run distance (m) before exhauster (Control: Median = 614, IQR = 584–640 vs. CS: Median = 536, IQR = 507–567, N = 4; *P = 0.03).
DISCUSSION
The results of the present study demonstrated overexpression of VHL as well as other elements in the ubiquitination cascade including PHD2 and UBE2D1 in skeletal muscles of mice exposed to CS for 6 mo. This provides first evidence that chronic exposure of normal mice to CS directly enhances VHL expression in skeletal muscles. The present results are in agreement with and further strengthen our recent findings in skeletal muscles of patients with COPD (19). In the latter study, VHL overexpression was observed already at the early (Stage-1) of the disease, before development of significantly impaired pulmonary function (19). It is unclear whether an early VHL overexpression also occurs in lungs from mice exposed to CS, this needs to be addressed in future studies specifically addressing this point. Hence, our findings in humans (19) together with the results of the present study indicate that enhanced VHL expression in skeletal muscles might be an early, primary event induced by chronic CS exposure rather than a secondary occurrence to development of COPD.
VHL, a member of the E3 ubiquitin ligase family, tightly regulates intracellular levels of HIF-1α through the mechanism that includes transfer of activated ubiquitin molecules from UBE2D1-conjugating enzyme to lysil residues of HIF-1α protein marking it for rapid proteosomal degradation (18, 21, 33, 36). HIF-1α availability within the cell is further regulated by the family of PHD1–4, which catalyze HIF-1α hydroxylation in positions 402 and 564 required for VHL-HIF-1α recognition and interaction during the ubiquitination process (6, 11). Overexpression of VHL, UBE2D1, and PHD2 observed in the present study was accompanied by significantly decreased HIF-1α protein expression in skeletal muscles of CS-exposed animals. This can account for the impaired transduction of the HIF-1α signal toward VEGF expression, resulting in decreased skeletal muscle capillarization. Of interest, our results demonstrate a small but statistically significant increase in HIF-1α mRNA expression in CS-exposed animals, whereas the protein expression levels were significantly decreased. The mechanisms behind this finding are unclear. However, increased HIF-1α mRNA expression in skeletal muscles has been previously reported after short intermittent hypoxic training and in ischemic muscle tissue (8, 48) and represents a compensatory response to inadequate fiber perfusion and O2 availability in the muscle tissue. The findings of the present study provides further evidence to the ongoing debate (7) that increased ubiquitination might be an important mechanism mediating development of systemic complications in COPD.
VEGF plays a central role in the maintenance of adult skeletal muscle capillarity (34). Decreased VEGF expression in skeletal muscles of CS-exposed animals in the present study was accompanied with an ∼33% decrease in muscle capillarization. This is in an agreement with a previous report demonstrating significant capillary loss in the gastrocnemius-specific VEGF-deficient mice (34, 35, 42). Moreover, decreased VEGF expression and inhibition of VEGF-dependent signaling have been associated with a substantial endothelial cell apoptosis (42, 49, 54), which might represent potential mechanism underlying capillary loss observed in CS-exposed animals in the present study. Previously, significantly decreased capillarization was reported only in the oxidative soleus muscle but not the glycolytic extensor digitorum longus in the CS-exposed C57BI/6J mice (44). A likely explanation to the apparent discrepancy between the two studies may be the three-times longer CS exposure period in our study (24 vs. 8 wk). In this study, VEGF mRNA expression was not significantly decreased in variance with the significantly decreased protein levels. The mechanisms behind the discrepant levels of mRNA and protein expression in this study cannot be readily explained. However, as observed in our human study (19), decreased VEGF protein levels, which represent the active signaling component, can be explained by the concurrently decreased HIF-1 and increased VHL protein levels. In this regard, VEGF mRNA steady-state levels do not necessarily mirror transcriptional rate, especially in hypoxia (24, 43). This suggestion is also supported with the recent findings by Xin et al. (52), which provided evidence that VHL regulates posttranscriptional levels of VEGF.
Parallel to the disturbed hypoxia/angiogenic signaling in CS exposed mice, a significant decrease in skeletal muscle exercise tolerance was also observed. Previously, reduced exercise tolerance was demonstrated in mice with specific loss of HIF-1α in the skeletal muscle (25). Moreover, skeletal muscle capillary adaptation to exercise training was shown to be highly dependent on myocyte-expressed VEGF (33). However, additional mechanisms, such as CS-induced oxidative damage of proteins important for muscle bioenergetics and metabolism or inflammatory processes, might also play a role in development of skeletal muscle dysfunction and reduced exercise tolerance in the present study animals (3, 4, 22, 44).
Taken together, the results of the present study provide first evidence that chronic CS exposure significantly enhances elements of the ubiquitination cascade including VHL, UBE2D1, and PHD2. This has adversely affected transduction of the HIF-1α signal toward VEGF expression and capillary formation. Impaired hypoxic signaling in the CS-exposed mice was simultaneously accompanied by morphological skeletal muscle changes and decreased exercise capacity. The findings of this study are in agreement with those reported in skeletal muscles from patients with COPD and PCS (19) and further identify activation of the ubiquitin-proteolysis cascade as an important mechanism mediating decreased skeletal muscle capillarization due to CS exposure.
GRANTS
This study was supported by the Olle Engkvist Byggmästare Fund, Sweden and NIH 1R01HL085613 (to I. Rahman), 1R01HL097751 (to I. Rahman), 1R01HL092842 (to I. Rahman), and NIEHS Environmental Health Science Center grant P30-ES01247.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.T.B., I.R., A.S., and S.M.A.-H. conception and design of research; V.T.B., E.T., A.A.E., and H.Y. performed experiments; V.T.B., E.T., A.A.E., H.Y., and I.R. analyzed data; V.T.B., A.A.E., H.Y., I.R., A.S., and S.M.A.-H. interpreted results of experiments; V.T.B., E.T., and H.Y. prepared figures; V.T.B., I.R., A.S., and S.M.A.-H. drafted manuscript; V.T.B., A.A.E., I.R., A.S., and S.M.A.-H. edited and revised manuscript; I.R., A.S., and S.M.A.-H. approved final version of manuscript.
REFERENCES
- 1. Baarends EM, Schols AM, Mostert R, Wouters EF. Peak exercise response in relation to tissue depletion in patients with chronic obstructive pulmonary disease. Eur Respir J 10: 2807– 2813, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Barnes PJ. New concepts in chronic obstructive pulmonary disease. Annu Rev Med 54: 113– 129, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Barreiro E, Del Puerto-Nevado L, Puig-Vilanova E, Pérez-Rial S, Sánchez F, Martínez-Galán L, Rivera S, Gea J, González-Mangado N, Peces-Barba G. Cigarette smoke-induced oxidative stress in skeletal muscles of mice. Respir Physiol Neurobiol 182: 9– 17, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Barreiro E, Peinado VI, Galdiz JB, Ferrer E, Marin-Corral J, Sánchez F, Gea J, Barberà JA. ENIGMA in COPD Project. Cigarette smoke-induced oxidative stress: A role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am J Respir Crit Care Med 182: 477– 488, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Barreiro E, Schols A, Polkey MI, Galdiz JB, Gosker HR, Swallow EB, Cornell C, Gea J. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax 63: 100– 107, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22: 4082– 4090, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Debigaré R, Côté CH, Maltais F. Ubiquitination and proteolysis in limb and respiratory muscles of patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc 7: 84– 90, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Dehne N, Kerkweg U, Otto T, Fandrey J. The HIF-1 response to simulated ischemia in mouse skeletal muscle cells neither enhances glycolysis nor prevents myotube cell death. Am J Physiol Regul Integr Comp Physiol 293: R1693– R1701, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Engelen MP, Schols AM, Does JD, Wouters EF. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 71: 733– 738, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Engelen MP, Schols AM, Baken WC, Wesseling GJ, Wouters EF. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J 7: 1793– 1797, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Epstein ACR, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakola P, Barstead R, Hodgkin J, Maxvell PH, Pugh CW, Schofield CJ, Ratcliffe PJC. Elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43– 54, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Fabbri L, Pauwels RA, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: gold executive summary updated 2003. COPD 1: 105– 141, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Gosker HR, Langen RCJ, Bracke KR, Joos GF, Brusselle GG, Steele C, Ward KA, Wouters EF, Schols AM. Extrapulmonary manifestations of chronic obstructive pulmonary disease in a mouse model of chronic cigarette smoke exposure. Am J Respir Cell Mol Biol 40: 710– 716, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Gosker HR, Zeegers MP, Wouters EF, Schols AM. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax 62: 944– 949, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831– 838, 1984 [DOI] [PubMed] [Google Scholar]
- 16. Hopkinson NS, Tennant RC, Dayer MJ, Swallow EB, Hansel TT, Moxham J, Polkey MI. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res 13: 8– 25, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313– 322, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Iwai K, Yamanaka K, Kamura T, Minato N, Conaway RC, Conaway JW, Klausner RD, Pause A. Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci USA 96: 12436– 12441, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jatta K, Eliason G, Portela-Gomes GM, Grimelius L, Caro O, Nilholm L, Sirsjö A, Piehl-Aulin K, Abdel-Salim M. Overexpression of von Hippel-Lindau protein in skeletal muscles of patients with chronic obstructive pulmonary disease. J Clin Pathol 62: 70– 76, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Jobin J, Maltais F, Doyon JF, LeBlanc P, Simard PM, Simard AA, Simars C. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardio Pulm Rehab 18: 432– 437, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Kallio PJ, Wilson WJ, O'Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1α by the ubiquitin-proteasome pathway. J Biol Chem 274: 6519– 6525, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Langen RC, Haegens A, Vernooy JH, Wouters EF, de Winther MP, Carlsen H, Steele C, Shoelson SE, Schols AM. NF-kappa B activation is required for the transition of pulmonary inflammation to muscle atrophy. Am J Respir Cell Mol Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larsson L, Orlander J. Skeletal muscle morphology, metabolism and function in smokers and non-smokers. A study on smoking discordant monozygous twins. Acta Physiol Scand 120: 343– 352, 1984 [DOI] [PubMed] [Google Scholar]
- 24. Levy AP, Levy NS, Goldberg MA. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem 271: 2746– 2753, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Maltais F, Simard AA, Simard C, Jobin J, Desgagnes P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med 153: 288– 293, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Marquis K, Debigare R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166: 809– 813, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Mason SD, Howlett RA, Kim MJ, Olfert IM, Hogan MC, McNulty W, Hickey RP, Wagner PD, Kahn CR, Giordano FJ, Johnson RS. Loss of skeletal muscle HIF-1alpha results in altered exercise endurance. PLoS Biol 2: e288, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Massett MP, Berk BC. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol Regul Integr Comp Physiol 288: R1006– R1013, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Michaud SE, Menard C, Guy LG, Gennaro G, Rivard A. Inhibition of hypoxia-induced angiogenesis by cigarette smoke exposure: impairment of the HIF-1-α/VEGF pathway. FASEB J 17: 1150– 1152, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Montes de Oca M, Loeb E, Torres SH, De Sanctis J, Hernandez N, Talamo C. Peripheral muscle alterations in non-COPD smokers. Chest 133: 13– 18, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Nagal K, Betsuyaku T, Ito Y, Nasuhara Y, Nishimura M. Decrease of vascular endothelial growth factor in macrophages from long-term smokers. Eur Respir J 25: 626– 633, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Mishima M. Exercise capacity deterioration in patients with COPD: longitudinal evaluation over 5 years. Chest 128: 62– 69, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Ohh M, Park CW, Ivan N, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chao V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol 2: 423– 427, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol 587: 1755– 1767, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olfert IM, Howlett RA, Wagner PD, Breen EC. Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am J Physiol Regul Integr Comp Physiol 299: R1059– R1067, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paltoglou S, Roberts BJ. HIF-1alpha and EPAS ubiquitination mediated by the VHL tumour suppressor involves flexibility in the ubiquitination mechanism, similar to other RING E3 ligases. Oncogene 26: 604– 609, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J 20: 539– 544, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248– 1259, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rutten EP, Spruit MA, Wouters EF. Critical view on diagnosing muscle wasting by single frequency bioelectrical impedance in COPD. Respir Med 104: 91– 98, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157: 1791– 1797, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 24: 97– 106, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics 18: 63– 69, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Tang K, Breen EC, Wagner PD. Hu protein R-mediated posttranscriptional regulation of VEGF expression in rat gastrocnemius muscle. Am J Physiol Heart Circ Physiol 283: H1497– H1504, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Tang K, Wagner PD, Breen EC. TNF-alpha-mediated reduction in PGC-1 alpha may impair skeletal muscle function after cigarette smoke exposure. J Cell Physiol 222: 320– 327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thaikoottathil JV, Martin RJ, Zdunek J, Weinberger A, Rino JG, Chu HW. Cigarette smoke extract reduces VEGF in primary human airway epithelial cells. Eur Respir J 33: 835– 843, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Vermeeren MA, Wouters EF, Geraerts-Keeris AJ, Schols AM. Nutritional support in patients with chronic obstructive pulmonary disease during hospitalization for an acute exacerbation; a randomized controlled feasibility trial. Clin Nutr 23: 1184– 1192, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sørensen TI, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med 173: 79– 83, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Vogt M, Puntschart A, Geiser J, Zuleger C, Billeter R, Hoppeler H. Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J Appl Physiol 91: 173– 182, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 290: L209– L221, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, Belleau R, Maltais F. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc 30: 1467– 1474, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Wust RCI, Morse CI, de Haan A, Rittweger J, Jones DA, Degens H. Skeletal muscle properties and fatigue resistance in relation to smoking history. Eur J Appl Physiol 104: 103– 110, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xin H, Brown JA, Gong C, Fan H, Brewer G, Gnarra JR. Association of the von Hippel-Lindau protein with AUF1 and post-transcriptional regulation of vascular endothelial growth factor A mRNA. Mol Cancer Res 10: 108– 120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Rönty M, Kinnula VL, Rahman I. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 122: 2032– 2045, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yao H, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174– L1186, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Yamato H, Sun JP, Churg A, Wright JL. Cigarette smoke-induced emphysema in guinea pigs is associated with diffusely decreased capillary density and capillary narrowing. Lab Invest 75: 211– 219, 1996 [PubMed] [Google Scholar]