Abstract
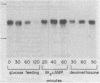
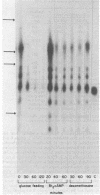
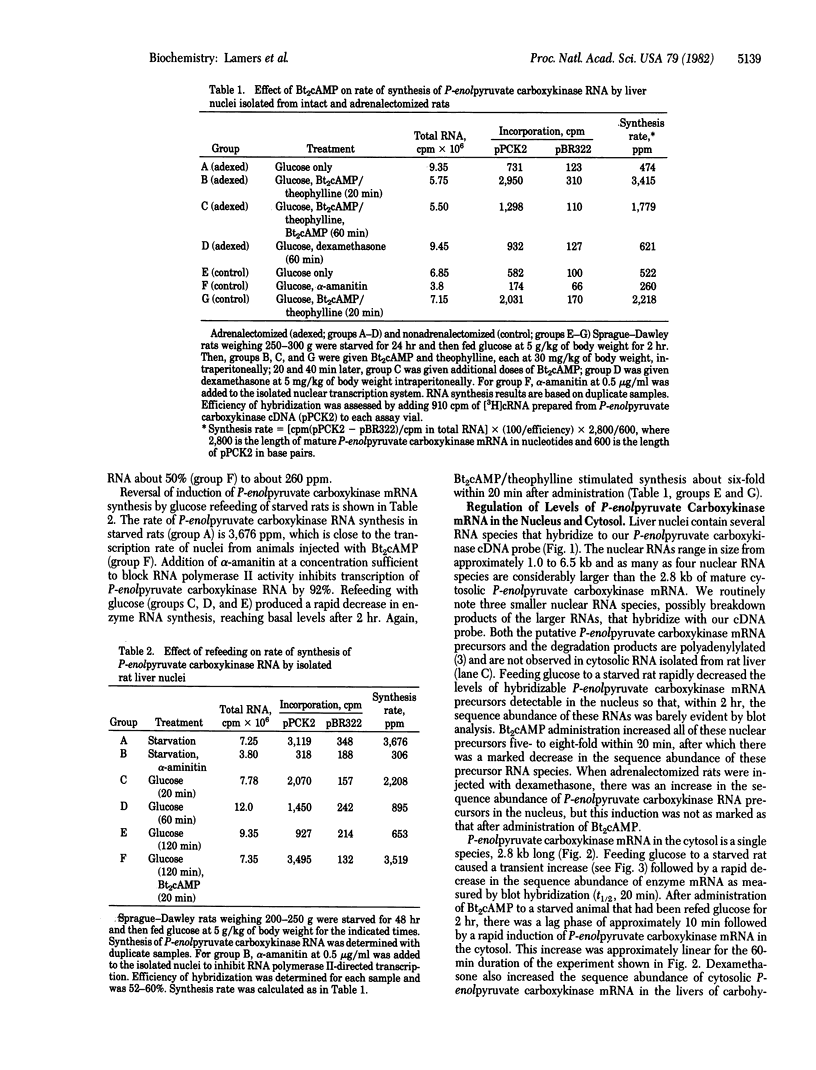
The effects of starvation, glucose refeeding, dibutyryl cAMP, and dexamethasone on expression of the gene for phosphoenolpyruvate carboxykinase (GTP) [GTP:oxaloacetate carboxy-lyase (transphosphorylating), EC 4.1.1.32] from rat liver cytosol was studied by using a cloned cDNA probe. The rate of transcription of the gene for phosphoenolpyruvate carboxykinase in hepatic nuclei isolated from starved rats decreased rapidly after refeeding with glucose. Administration of dibutyryl cAMP to glucose-refed animals increased the rate of phosphoenolpyruvate carboxykinase gene transcription seven-fold within 20 min. Phosphoenolpyruvate carboxykinase mRNA in the cytosol is 2.8 kilobases long whereas liver nuclei contain four precursor RNA species that are up to 6.5 kilobases long. Feeding glucose to starved rats rapidly decreased the sequence abundance of enzyme mRNA in both nuclei and cytosol. However, the decrease in cytosolic phosphoenolpyruvate carboxykinase mRNA was preceded by a transient increase in enzyme mRNA over the first 20 min after glucose refeeding. Administration of dibutyryl cAMP to glucose-refed starved animals increased the concentration of the nuclear RNA precursors of phosphoenolpyruvate carboxykinase five- to eight-fold within 30 min and induced the mRNA for the cytosolic enzyme over a period of 60 min. We conclude that cAMP induces phosphoenolpyruvate carboxykinase mRNA by increasing the rate of gene transcription.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale E. G., Hartley J. L., Granner D. K. N6,O2'-dibutyryl cycle AMP and glucose regulate the amount of messenger RNA coding for hepatic phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1982 Feb 25;257(4):2022–2028. [PubMed] [Google Scholar]
- Beale E. G., Katzen C. S., Granner D. K. Regulation of rat liver phosphoenolpyruvate carboxykinase (GTP) messenger ribonucleic acid activity by N6, O2'-dibutyryladenosine 3',5'-phosphate. Biochemistry. 1981 Aug 18;20(17):4878–4883. doi: 10.1021/bi00520a012. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe E., Chen B., Anderson W. B., Gottesman M. E., Perlman R. L., Pastan I. Role of cyclic adenosine 3',5'-monophosphate and the cyclic adenosine 3',5'-monophosphate receptor protein in the initiation of lac transcription. J Biol Chem. 1971 Dec 10;246(23):7343–7348. [PubMed] [Google Scholar]
- Harpold M. M., Dobner P. R., Evans R., Bancroft F. C., Darnell J. E., Jr The synthesis and processing of a nuclear RNA precursor to rat pregrowth hormone messenger RNA. Nucleic Acids Res. 1979 Jul 11;6(9):3133–3144. doi: 10.1093/nar/6.9.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iynedjian P. B., Hanson R. W. Increase in level of functional messenger RNA coding for phosphoenolpyruvate carboxykinase (GTP) during induction by cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1977 Jan 25;252(2):655–662. [PubMed] [Google Scholar]
- Kioussis D., Reshef L., Cohen H., Tilghman S. M., Iynedjian P. B., Ballard F. J., Hanson R. W. Alterations in translatable messenger RNA coding for phosphoenolpyruvate carboxykinase (GTP) in rat liver cytosol during deinduction. J Biol Chem. 1978 Jun 25;253(12):4327–4332. [PubMed] [Google Scholar]
- Lis J. T., Neckameyer W., Dubensky R., Costlow N. Cloning and characterization of nine heat-shock-induced mRNAs of Drosophila melanogaster. Gene. 1981 Oct;15(1):67–80. doi: 10.1016/0378-1119(81)90105-0. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- Mory Y., Gefter M. RNA synthesis in isolated nuclei: the use of mercurated nucleotides. Nucleic Acids Res. 1978 Oct;5(10):3899–3912. doi: 10.1093/nar/5.10.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Diesterhaft M., Granner D. Evidence for a dual effect of dibutyryl cyclic AMP on the synthesis of tyrosine aminotransferase in rat liver. J Biol Chem. 1982 Mar 10;257(5):2386–2390. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Reshef L., Faliks D., Bentor-Getter V., Glaser G. Effect of glucose feeding to fasted rats on the translational efficiency of liver cytosol phosphoenolpyruvate carboxykinase mRNA. FEBS Lett. 1979 Jan 1;97(1):96–100. doi: 10.1016/0014-5793(79)80060-5. [DOI] [PubMed] [Google Scholar]
- Roper M. D., Wicks W. D. Evidence for acceleration of the rate of elongation of tyrosine aminotransferase nascent chains by dibutyryl cyclic AMP. Proc Natl Acad Sci U S A. 1978 Jan;75(1):140–144. doi: 10.1073/pnas.75.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek G. T., van de Poll K. W., Voorma H. O., van Wijk R. Studies on the posttranscriptional site of cAMP action in the regulation of the synthesis of tyrosine aminotransferase. Eur J Biochem. 1981;114(1):27–31. doi: 10.1111/j.1432-1033.1981.tb06166.x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. Rapid loss of translatable messenger RNA of phosphoenolpyruvate carboxykinase during glucose repression in liver. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1304–1308. doi: 10.1073/pnas.71.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks W. D., McKibbin J. B. Evidence for translational regulation of specific enzyme synthesis by N 6 , O 2' -dibutyryl cyclic AMP in hepatoma cell cultures. Biochem Biophys Res Commun. 1972 Jul 11;48(1):205–211. doi: 10.1016/0006-291x(72)90364-6. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Tsang A. S., Mahbubani H. A change in the rate of transcription of a eukaryotic gene in response to cyclic AMP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7171–7175. doi: 10.1073/pnas.77.12.7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung D., Oliver I. T. Induction of phosphopyruvate carboxylase in neonatal rat liver by adenosine 3',5'-cyclic monophosphate. Biochemistry. 1968 Sep;7(9):3231–3239. doi: 10.1021/bi00849a028. [DOI] [PubMed] [Google Scholar]
- Yoo-Warren H., Cimbala M. A., Felz K., Monahan J. E., Leis J. P., Hanson R. W. Identification of a DNA clone to phosphoenolpyruvate carboxykinase (GTP) from rat cytosol. Alterations in phosphoenolpyruvate carboxykinase RNA levels detectable by hybridization. J Biol Chem. 1981 Oct 25;256(20):10224–10227. [PubMed] [Google Scholar]