Abstract
We have recently demonstrated that chronic infusion of exogenous ANG II, which induces blood pressure elevation, attenuates renal medullary endothelin B (ETB) receptor function in rats. Moreover, this was associated with a reduction of ETB receptor expression in the renal inner medulla. The aim of this present work was to investigate the effect of a physiological increase in endogenous ANG II (low-salt diet) on the renal ET system, including ETB receptor function. We hypothesized that endogenous ANG II reduces renal medullary ETB receptor function during low-salt intake. Rats were placed on a low-salt diet (0.01–0.02% NaCl) for 2 wk to allow an increase in endogenous ANG II. In rats on normal-salt chow, the stimulation of renal medullary ETB receptor by ETB receptor agonist sarafotoxin 6c (S6c) causes an increase in water (3.6 ± 0.4 from baseline vs. 10.5 ± 1.3 μl/min following S6c infusion; P < 0.05) and sodium excretion (0.38 ± 0.06 vs. 1.23 ± 0.17 μmol/min; P < 0.05). The low-salt diet reduced the ETB-dependent diuresis (4.5 ± 0.5 vs. 6.1 ± 0.9 μl/min) and natriuresis (0.40 ± 0.11 vs. 0.46 ± 0.12 μmol/min) in response to acute intramedullary infusion of S6c. Chronic treatment with candesartan restored renal medullary ETB receptor function; urine flow was 7.1 ± 0.9 vs. 15.9 ± 1.7 μl/min (P < 0.05), and sodium excretion was 0.4 ± 0.1 vs. 1.1 ± 0.1 μmol/min (P < 0.05) before and after intramedullary S6c infusion, respectively. Receptor binding assays determined that the sodium-depleted diet resulted in a similar level of ETB receptor binding in renal inner medulla compared with rats on a normal-salt diet. Candesartan reduced renal inner medullary ETB receptor binding (1,414 ± 95 vs. 862 ± 50 fmol/mg; P < 0.05). We conclude that endogenous ANG II attenuates renal medullary ETB receptor function to conserve sodium during salt deprivation independently of receptor expression.
Keywords: low-salt diet, ETB receptor, renal medulla, renin-angiotensin system, natriuresis
the endothelin (ET) and renin-angiotensin system (RAS) play an important role in sodium balance and blood pressure control. ET-1 is highly produced in the kidney, especially by the inner medullary collecting duct (23). ET-1 via ETB receptor activation in the renal medulla stimulates nitric oxide production to facilitate water and sodium excretion (27) via increased renal medullary blood flow (43) and inhibition of epithelial sodium channel activity (ENaC) (8, 9) as well as reabsorption in the medullary thick ascending limb (30). In contrast, ANG II prominently acts as an antinatriuretic hormone at least in part by increasing abundance of sodium transporters and channels along the nephron, mainly mediated by the AT1 receptor-dependent pathway (22).
Differences in salt intake affect both the ET system and RAS. A high-salt diet, which is a low-renin state, activates the renal ET system by increasing renal medullary ET-1 production as well as renal ETB receptor expression (31, 32, 34). A low-salt diet physiologically activates the RAS in the kidney and elsewhere (19). The increase in ANG II is mainly to conserve sodium. ANG II regulates other natriuretic factors, including prostaglandins and bradykinin, under low-salt diet conditions (17, 36). From in vitro studies, we know that ANG II increases ET-1 gene (edn1) activation in both endothelial and epithelial cells (38, 39). However, the interplay between the ET system and RAS during a low-salt feeding is still unknown.
Previously, we have shown that chronic infusion of a pressor dose of ANG II impairs the ETB-dependent natriuretic response, which is associated with a reduction of ETB receptor binding in the renal inner medulla (20). However, the pathological conditions induced by exogenous ANG II are also associated with a variety of changes, such as increased inflammation and oxidative stress that could produce changes in the ET system independently of a direct action of ANG II (5, 24). Such conditions mimic chronic kidney disease, which also affects ETB receptor expression in the kidney (35). However, the physiological regulation of the renal ET system by the RAS has not been investigated.
The present study was designed to examine the effect of the endogenous RAS on the renal medullary ET system. We hypothesized that renal medullary ETB receptor function, as well as renal ET-1 production, is reduced during salt deprivation through activation of the RAS. We tested this hypothesis by determining the effect of specific AT1 receptor blockade on the natriuretic and diuretic response to renal medullary ETB receptor activation.
METHODS
Animal Preparation
Male Sprague-Dawley rats (8–10 wk old) from Harlan Laboratories (Indianapolis, IN) were housed under conditions of constant temperature and humidity and exposed to a 12:12-h light-dark cycle. All experiments and surgery procedures were approved and monitored by the Georgia Health Sciences University Institutional Animal Care and Use Committee in accordance with the American Physiological Society's Guide for the Care and Use of Laboratory Animals. Rats were placed on a normal-salt (0.4% NaCl, Harlan Teklad, Madison, WI) or low-salt diet (0.01–0.02% NaCl, Harlan Teklad) for 2 wk. Another set of rats received candesartan (5 mg·kg−1·day−1 in the drinking water) with a normal- or low-salt diet for 2 wk. Water and food intake were measured daily. The dose of candesartan was adjusted twice a week if necessary to ensure proper dosing.
Metabolic and Renal Function Measurement
Rats were placed in metabolic cages for 24-h acclimation followed by a 24-h urine collection during day 0 (baseline) or day 14 of a normal- or low-salt diet with or without candesartan treatment. After the baseline urine collection, a blood sample was collected from the tail vein (<50 μl). After 2 wk of treatment, rats were anesthetized with pentobarbital sodium (50 mg/kg ip). Plasma was collected from the descending aorta to measure ANG II concentration. The renal inner medulla was collected for receptor-binding analyses. Urinary and plasma creatinine were measured to determine creatinine clearance as previously described (2, 7). Urinary ET-1 excretion was measured by radioimmunoassay via the manufacturer's protocol (Peninsula Laboratories, San Carlos, CA).
Plasma ANG II Concentration
ANG II was measured in plasma samples from rats that received a normal- and low-salt diet. Plasma ANG II was extracted with methanol and measured by an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) as previously described (40).
Intramedullary Infusion of an ETB Receptor Agonist
Surgery and intramedullary infusion was performed under inactin anesthesia (100 mg/kg ip) as previously described (20). The jugular vein was catheterized for infusion of saline (0.9% NaCl) containing 4% BSA at a rate of 30 μl/min and changed to 15 μl/min after surgery to maintain euvolemia. A midline incision was made, and a catheter was inserted 5 mm in the left kidney to infuse saline or saline containing the ETB receptor agonist sarafotoxin 6c (S6c; American Peptide, Sunnyvale, CA) at the rate of 0.5 ml/h into the renal medulla as described below. Urine was collected from the ureter of each kidney. After surgery, saline was infused into the renal medulla of the left kidney. Following a 60-min equilibration period, a baseline urine collection was obtained (40 min) followed by an experimental period where saline or S6c (0.45 μg·kg−1·h−1) was infused into the renal medulla for an additional 80 min. Urine from the final 20 min of the baseline and experimental periods was used for analysis. Urinary sodium concentration was measured by atomic absorption. Mean arterial pressure was measured continuously via a femoral artery catheter.
Receptor-Binding Assay to ET-1 and ET-3
As previously described (20), inner medullary tissue was homogenized and plasma membrane fractions were prepared for binding studies. Plasma membrane and wheat germ agglutinin polyvinyltoluene beads (PerkinElmer, Boston, MA) were added into each well and incubated for 3 h. Then, [125I]-ET-1 (PerkinElmer) at a final concentration of 0.01–1.0 nM or [125I]-ET-3 (PerkinElmer) at a final concentration of 0.06–1.0 nM was added and incubated for 18 h. Total binding of [125I]-ET-1 or [125I]-ET-3 was determined using 0.3 or 1 μg plasma membrane protein, respectively. Nonspecific binding was determined in the presence of 10 μM unlabeled ET-1 or ET-3 ligand (American Peptide). All measurements were performed in duplicate.
Statistical Analysis
Urine flow rate, urinary sodium excretion, and mean arterial pressure in response to intramedullary infusion of saline or S6c were analyzed by two-way repeated-measures ANOVA followed by Bonferroni post hoc tests among rats receiving a normal- or low-salt diet with or without candesartan. Metabolic parameters, urinary ET-1 excretion, and receptor binding data were analyzed by two-way ANOVA followed by Bonferroni post hoc tests to compare the effects of candesartan on either a normal- or low-salt diet. Results are expressed as means ± SE, with P < 0.05 being considered statistically significant.
RESULTS
Plasma ANG II Concentration
To confirm that a sodium-depleted diet activates the RAS, we measured plasma ANG II in rats fed a normal- or low-salt diet for 2 wk. We found that rats on a low-salt diet had significantly elevated plasma ANG II levels compared with rats on a normal-salt diet (611 ± 78 vs. 279 ± 68 pg/ml; P < 0.05).
Intramedullary ETB Receptor Function in Rats on a Low-Salt Diet
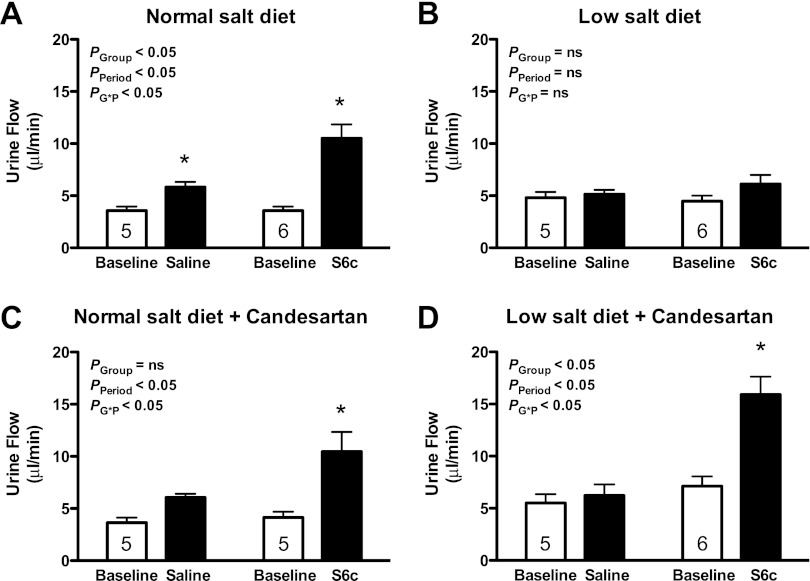
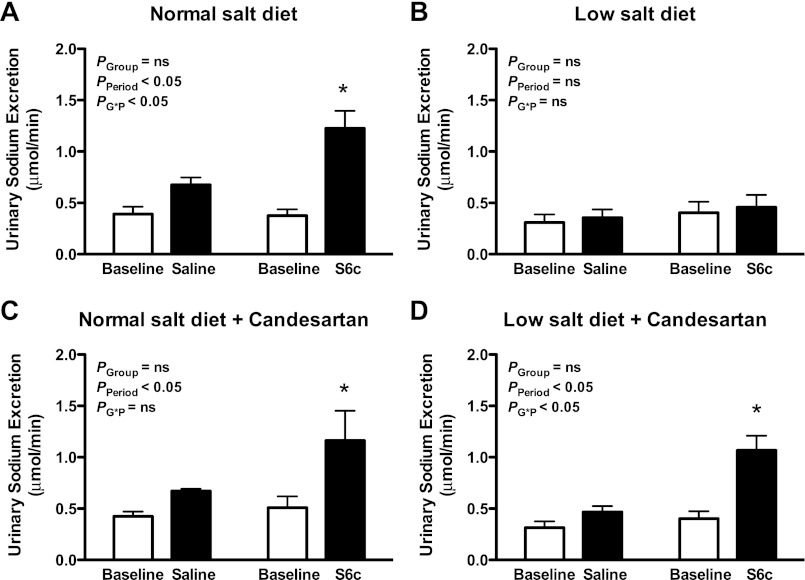
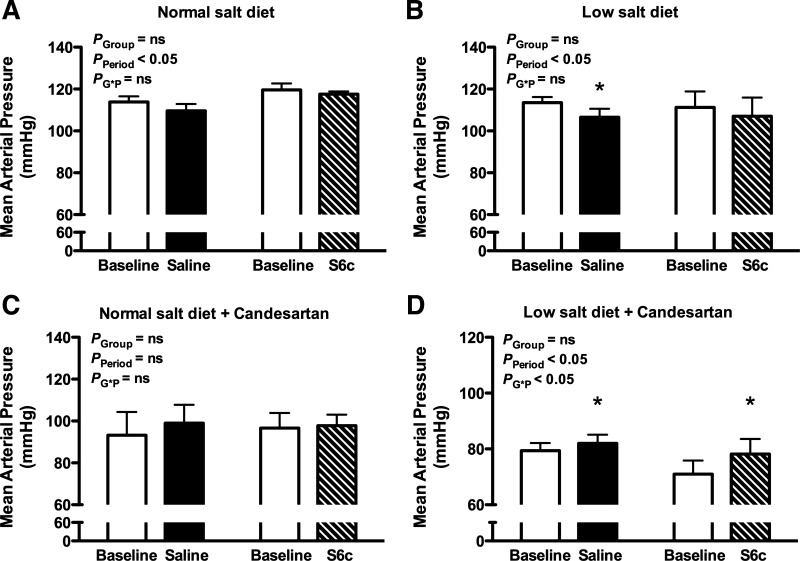
As previously reported, intramedullary infusion of the ETB receptor agonist S6c produced a significant increase in urine flow rate (P < 0.05; Figure 1A) and urinary sodium excretion (P < 0.05; Fig. 2A) in rats on a normal-salt diet. Moreover, the increases in water and sodium excretion during S6c infusion were significantly greater than those observed during saline infusion at the same time period (P < 0.05). In contrast, intramedullary infusion of S6c did not increase the urine flow rate (Fig. 1B) or urinary sodium excretion (Fig. 2B) in rats on a salt-depleted diet. Mean arterial pressure remained unchanged during intramedullary infusion of saline or S6c for rats on either diet (Fig. 3, A and B).
Fig. 1.
Urine flow rate before (baseline) and during an intramedullary infusion of saline or the ETB receptor agonist sarafotoxin 6c (S6c) for 80 min in rats on a normal- or low-salt diet. Separate groups of rats were pretreated with the AT1 receptor antagonist candesartan for 2 wk before the study. ns, Not significant. *P < 0.05 vs. baseline period in the same group; n = 5–6 rats/group.
Fig. 2.
Sodium excretion before (baseline) and during an intramedullary infusion of saline or the ETB receptor agonist S6c for 80 min in rats on a normal- or low-salt diet. Separate groups of rats were pretreated with the AT1 receptor antagonist candesartan for 2 wk before the study. *P < 0.05 vs. baseline period in the same group; n = 5–6 rats/group.
Fig. 3.
Mean arterial pressure before (baseline) and during an intramedullary infusion of saline or the ETB receptor agonist sarafotoxin 6c (S6c) for 80 min in rats on a normal or low salt diet. Separate groups of rats were pretreated with the AT1 receptor antagonist, candesartan, for 2 wk prior to study. *P < 0.05 vs. baseline period in the same group; n = 5–6 rats/group.
For the time control group, intramedullary infusion of saline produced a modest, but significant increase in the urine flow rate over time (P < 0.05; Fig. 1A); however, urinary sodium excretion remained stable during saline infusion compared with the baseline period in rats on a normal-salt diet (Fig. 2A). In contrast, saline infusion did not increase the urine flow rate (Fig. 1B) or urinary sodium excretion (Fig. 2B) in a low-salt diet group.
AT1 Receptor Blockade and ETB Function in Rats on a Low-Salt Diet
To test the hypothesis that AT1 receptor activity regulates renal medullary ETB receptor function during low-salt feeding, the AT1 receptor antagonist candesartan was given to rats on a normal- or low-salt diet for 2 wk. Intramedullary infusion of S6c significantly increased urine flow (Fig. 1C; P < 0.05) and urinary sodium excretion (Fig. 2C; P < 0.05) in rats on a normal-salt diet treated with candesartan, indicating that AT1 receptors do not affect ETB receptor function with a normal-salt diet. Interestingly, candesartan restored the S6c-induced urine flow rate (Fig. 1D; P < 0.05) and urinary sodium excretion (Fig. 2D; P < 0.05) in rats on a low salt diet. Mean arterial pressure was slightly increased over the course of the acute period of both saline and S6c infusion in rats on a low-salt diet (Fig. 3D; P < 0.05).
Effects of AT1 Receptor Blockade on 24-h Intake and Excretion During Salt Deprivation
As depicted in Table 1, the baseline intake and excretion variables before any diet manipulations were similar among groups. After 14 days of treatment, a low-salt diet significantly reduced food consumption and urinary sodium excretion compared with rats on a normal-salt diet (P < 0.05). Urine volume was comparable between salt diets. There was no difference in creatinine clearance between normal- and low-salt-fed rats.
Table 1.
Metabolic characteristics of rats on a normal-salt diet, a low-salt diet, a normal-salt diet with candesartan, and a low-salt diet with candesartan on day 0 (baseline before any diet manipulations) and day 14 of treatment
| Normal Salt | Normal Salt+Candesarten | Low Salt | Low Salt+Candesarten | |
|---|---|---|---|---|
| Baseline | ||||
| Food intake, g/day | 21.6 ± 0.5 | 21.9 ± 1.5 | 24.2 ± 0.4 | 22.0 ± 1.2 |
| Water intake, g/day | 33.0 ± 0.7 | 31.5 ± 0.7 | 28.3 ± 2.7 | 29.4 ± 2.5 |
| UV, ml/day | 15.8 ± 1.0 | 17.2 ± 1.1 | 15.2 ± 1.2 | 16.1 ± 0.6 |
| UNaV, mmol/day | 1.73 ± 0.04 | 1.88 ± 0.13 | 1.81 ± 0.12 | 1.84 ± 0.10 |
| Ccreat, ml/min | 1.02 ± 0.05 | 1.01 ± 0.09 | 1.03 ± 0.10 | 1.15 ± 0.12 |
| Day 14 | ||||
| Food intake, g/day | 20.8 ± 0.9 | 21.7 ± 0.6 | 16.4 ± 1.3* | 14.5 ± 0.7* |
| Water intake, g/day | 30.8 ± 2.2 | 32.0 ± 2.1 | 22.9 ± 1.7 | 31.7 ± 3.8 |
| UV, ml/day | 14.9 ± 1.8 | 15.3 ± 2.1 | 11.8 ± 1.7 | 17.5 ± 2.2 |
| UNaV, mmol/day | 1.67 ± 0.07 | 1.86 ± 0.25 | 0.005 ± 0.001* | 0.028 ± 0.008* |
| Ccreat, ml/min | 0.88 ± 0.06 | 1.17 ± 0.10† | 0.71 ± 0.07 | 0.74 ± 0.05* |
Values are means ± SE. UV, urine flow rate; UNaV, urinary sodium excretion; Ccreat, creatinine clearance.
P < 0.05 vs. a normal-salt diet.
P < 0.05 vs. the same salt diet alone; n = 5–6 rats/group.
Candesartan did not change food intake, urine flow, or urinary sodium excretion during either normal- or low-salt feeding. Although candesartan increased creatinine clearance when rats received a normal-salt diet (P < 0.05), candesartan did not influence creatinine clearance in rats on a low-salt diet.
Urinary ET-1 Excretion in Rats on a Low-Salt Diet
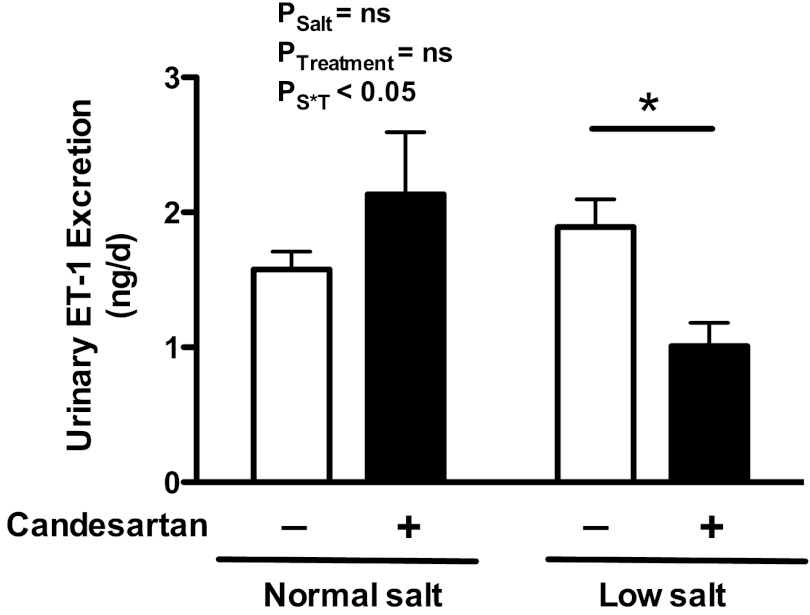
After 14 days of treatment, low-salt feeding had no effect on urinary ET-1 excretion compared with a normal-salt diet. Candesartan did not affect urinary ET-1 excretion in rats on a normal-salt diet. However, candesartan significantly reduced urinary ET-1 excretion in rats on a low-salt diet (Fig. 4; P < 0.05).
Fig. 4.
Urinary endothelin (ET)-1 excretion (24 h) in rats on a normal- or low-salt diet with (+) or without (−) candesartan treatment for 14 days. *P < 0.05 vs. a low-salt diet alone; n = 5–6 rats/group.
ET-1 and ET-3 Binding in the Inner Medulla of Rats on a Low-Salt Diet
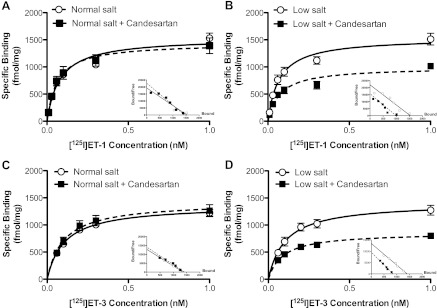
ET-1 has a similar affinity to both ETA and ETB receptors, while ET-3 has weak affinity for ETA receptors and binds only ETB receptors at low concentrations. Thus the saturation curve of [125I]-ET-1 was performed to determine ETA and ETB receptor binding, and the saturation curve of [125I]-ET-3 was used to determine ETB receptor binding. Both [125I]-ET-1 and [125I]-ET-3 binding curves reached saturation at 0.3 nM. [125I]-ET-1-specific binding sites in the renal inner medullary preparations were no different between normal- and low-salt diet groups (Fig. 5, A and B). Administration of candesartan to rats on a normal-salt diet did not affect saturation binding for [125I]-ET-1 (Fig. 5A). In rats on a low-salt diet, candesartan reduced [125I]-ET-1 saturation binding (Fig. 5B). Similar to [125I]-ET-1 binding, renal inner medullary preparations from rats on a normal- and low-salt diet had comparable [125I]-ET-3-specific binding curves (Fig. 5, C and D). Candesartan did not affect saturation binding for [125I]-ET-3 in rats on a normal-salt diet (Fig. 5C). [125I]-ET-3 binding was significantly reduced in candesartan-treated rats on a low-salt diet compared with a low-salt diet alone (Fig. 5D).
Fig. 5.
Saturation binding curves for [125I]-ET-1 (A and B) or [125I]-ET-3 (C and D) in plasma membrane preparations of renal inner medullary tissue from rats on a normal (A and C)- or low (B and D)-salt diet with or without candesartan. Insets: Scatchard analysis of [125I]-ET-1 and [125I]-ET-3; n = 3–4 rats/group.
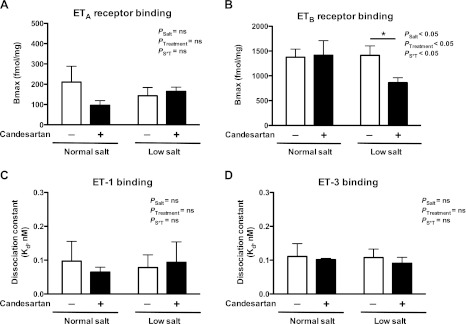
A Scatchard analysis was used to determine maximum binding (Bmax) values of [125I]-ET-1 binding sites that represent total ETA and ETB receptor binding, and Bmax values of [125I]-ET-3 binding sites represent specific ETB receptor binding as previously described (20). The difference between Bmax values of [125I]-ET-1 and [125I]-ET-3 binding was used to determine ETA receptor binding (Fig. 6A). Bmax values for ETB receptor binding in renal inner medullary tissue were comparable between rats on a normal- and low-salt diet (Fig. 6B). Administration of candesartan did not change Bmax values for ETB receptor binding in rats on a normal-salt diet. In contrast, Bmax values for ETB receptor binding were significantly reduced in inner medullary tissue from rats on a low-salt diet treated with candesartan. Candesartan treatment did not affect Bmax values for ETA receptor binding in tissues from either normal- or low-salt-fed rats (Fig. 6A). Dissociation constants (Kd) for [125I]-ET-1 or [125I]-ET-3 binding were comparable between inner medullary preparations from rats on a normal- or low-salt diet (Fig. 6, C and D, respectively). Candesartan did not change Kd of [125I]-ET-1 or [125I]-ET-3 binding in either group.
Fig. 6.
Maximum binding (Bmax) of ETA (A) and ETB (B) receptors and dissociation constants (Kd) derived from [125I]-ET-1 (C) and [125I]-ET-3 (D) in renal inner medullary tissue from rats on a normal- or low-salt diet with (+) or without (−) candesartan treatment for 2 wk; n = 3–4 rats/group.
DISCUSSION
Physiologically, a low-sodium diet increases activity of the RAS to maintain blood pressure (19). The elevation of ANG II helps to conserve sodium. Our laboratory and others have provided convincing evidence to demonstrate that ETB receptors in the renal medulla, especially in collecting ducts, promote water and sodium excretion as a means of blood pressure regulation (14, 27). The present study provides new evidence that the interaction between the RAS and ET-1 system underlies physiological regulation of sodium retention during salt deprivation. The major findings of the current study are 1) renal medullary ETB receptor function is reduced in rats on a sodium-depleted diet; 2) attenuated ETB-dependent water and sodium excretion during salt deprivation is mediated by ANG II; and 3) renal ET-1 production and renal medullary ETB receptor expression are regulated by ANG II through AT1 receptors during conditions of low-salt intake. Thus this study provides evidence for a contribution of the renal medullary ET system in the ANG II-induced sodium conservation associated with low sodium intake.
We previously reported that chronic infusion of exogenous ANG II at a prohypertensive dose attenuated ETB-dependent natriuresis (20). In the present study, physiological elevation of ANG II activity induced by a shift in dietary sodium intake to a low-salt diet also reduced renal medullary ETB receptor function. Blockade of AT1 receptor activity restored renal medullary ETB receptor function during salt deprivation. These data clearly indicate that the antinatriuretic effects of a low-salt diet on ETB-dependent function are mediated by ANG II and the AT1 receptor. These data are consistent with the hypothesis that renal medullary ETB receptor function is shut down to prevent water and sodium loss.
Several studies have shown that ANG II reduces ETB receptor expression both in vitro and in vivo. Wong et al. (44) demonstrated that ANG II, via the AT1 receptor pathway, downregulated ET receptor binding in cultured inner medullary collecting duct cells. Blockade of ANG II formation by an angiotensin-converting enzyme inhibitor increases ETB receptor density in the kidney of cardiomyopathic hamsters with moderate heart failure (45). Moreover, our own laboratory demonstrated that chronic infusion of ANG II at a pressor dose decreases ETB receptor binding in the renal inner medulla (20). In the current study, however, a low-salt diet, which activates the endogenous RAS, had comparable ETB receptor binding in the renal inner medulla to a normal-salt diet. The reason physiological and pathological increases in ANG II levels differently regulate ETB receptor expression is unknown but could be due to different downstream signaling pathways that are activated under these circumstances. For example, Banes-Berceli et al. (3) showed that hypertension produced by exogenous infusion of ANG II was dependent upon Janus kinase 2 (JAK2) activation. However, physiological stimulation of endogenous ANG II by a low-salt diet did not activate the JAK2 pathway (3). How such pathways could influence ETB receptor expression and/or signaling has yet to be determined.
Urinary ET-1 excretion reflects ET-1 production within the kidneys (1), and the collecting duct is a major source of ET-1 synthesis (41). In vitro studies suggest that flow or sodium stimulates ET-1 mRNA expression in tubular cells, including in the collecting duct or thick ascending limb (16, 26, 29). Furthermore, animal studies show that increased tubular flow rate (26), dietary salt intake (16, 31), and renal interstitial osmolality (6, 16) elevate renal ET-1 production. These data indicate that renal tubular cells may play a role in renal ET-1 production in response to a high sodium intake. In our experiments, urine flow rate was comparable, while urinary sodium excretion was dramatically reduced with low-salt feeding compared with a normal-salt diet. We expected that urinary ET-1 excretion would be reduced in a low-salt diet. To our surprise, a low-salt diet had comparable urinary ET-1 excretion to a normal-salt diet. We know that a high-salt diet and increases in renal tubular flow rate increase ET-1 production within the collecting duct system, so we presumed that a low-salt diet would decrease urinary ET-1 excretion. However, ANG II activates ET-1 gene expression and production in endothelial (11, 13) and tubular cells (15). Therefore, these effects appear to negate one another such that urinary ET-1 excretion is no different between normal- and low-salt-treated animals. Pharmacological blockade of ANG II activity with candesartan had no effect on ET-1 excretion in rats on a normal-salt diet but reduced urinary ET-1 excretion during low-salt feeding. These data suggest that ANG II contributes to the maintenance of renal ET-1 production during low-salt feeding.
We observed that administration of candesartan decreased blood pressure in both dietary salt groups under inactin anesthesia, an effect that was more dramatic in rats on a low-salt diet. Using telemetry, Klinger et al. (21) also reported that the AT1 receptor antagonist losartan reduced blood pressure whether on a normal- or low-salt diet (21). It is possible that the reduction of renal ETB receptor binding and urinary ET-1 excretion in rats on a low-salt diet and treated with candesartan may result from the concomitant decrease in blood pressure. However, the blood pressure effect of candesartan on the ET system is not apparent in rats on a normal-salt diet. We demonstrated previously that acute reduction of blood pressure did not significantly attenuate urinary ET-1 excretion compared with normal blood pressure (33). Furthermore, Dumont et al. (10) reported that the AT1 receptor antagonist losartan reduced blood pressure and urinary ET-1 excretion in uremic rats; however, treatment with conventional triple therapy, which reduced blood pressure to a comparable level as the losartan-treated group, did not change urinary ET-1 excretion. While these results suggest that urinary ET-1 excretion, which reflects renal ET-1 production, is dependent on the AT1 receptor pathway, we still cannot exclude the blood pressure-lowering effect of AT1 receptor antagonism having some influence in our study.
Activation of ETB receptors leads to an increase in nitric oxide (NO) production, which inhibits sodium channel activity including ENaC (8). In our study, low-salt feeding reduced ETB receptor function in the renal medulla. Moreover, the blockade of AT1 receptors during low-salt feeding restores renal medullary ETB excretory function. While changes in ETB receptor expression do not account for changes in ETB receptor function, the restoration of ETB receptor function by candesartan may be explained by a variety of potential postreceptor mechanisms related to ETB receptor signaling pathways. ANG II could reduce NO synthase (NOS) activity (42), increase superoxide production (12, 18), and stimulate ENaC expression and activity (4, 25, 28). It is possible that the inhibition of ANG II activity in the kidney by AT1 blockade could oppose any or all of these effects to reduce ENaC activity, as well as increase the ETB/NOS1 signaling, thus leading to enhancing ETB-dependent natriuresis under these conditions. Further studies will need to elucidate the postreceptor pathways that are influenced under various dietary salt intakes.
Perspectives
The current study highlights the physiological importance of ANG II and ET system interactions in the regulation of sodium balance. In a clinical setting, RAS-modulating therapies are among the most widely prescribed treatments to lower blood pressure in hypertensive patients and are concomitant with the recommendation to reduce salt consumption. Because animal models of salt-sensitive hypertension, including Dahl-salt (37) or ANG II hypertensive rats (20), have an impairment of the pronatriuretic actions of the ET system, it will be important to determine whether any of the beneficial effects of AT1 receptor blockade in hypertension is a function of improved renal medullary ETB receptor function.
GRANTS
This work was supported by National Institutes of Health Grants HL60653, HL69999, and HL95499, a grant from the Cardiovascular Discovery Institute of the Georgia Health Sciences University, and an American Heart Association predoctoral fellowship (W. Kittikulsuth).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: W.K., J.S.P., and D.M.P. provided conception and design of research; W.K. performed experiments; W.K. and J.S.P. analyzed data; W.K., J.S.P., and D.M.P. interpreted results of experiments; W.K. prepared figures; W.K. drafted manuscript; W.K., J.S.P., and D.M.P. edited and revised manuscript; W.K., J.S.P., and D.M.P. approved final version of manuscript.
REFERENCES
- 1. Abassi ZA, Tate JE, Golomb E, Keiser HR. Role of neutral endopeptidase in the metabolism of endothelin. Hypertension 20: 89–95, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Allcock GH, Venema RC, Pollock DM. ETA receptor blockade attenuates the hypertension but not renal dysfunction in DOCA-salt rats. Am J Physiol Regul Integr Comp Physiol 275: R245–R252, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Banes-Berceli AK, Al-Azawi H, Proctor D, Qu H, Femminineo D, Hill-Pyror C, Webb RC, Brands MW. Angiotensin II utilizes Janus kinase 2 in hypertension, but not in the physiological control of blood pressure, during low-salt intake. Am J Physiol Regul Integr Comp Physiol 301: R1169–R1176, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 41: 1143–1150, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Boesen EI, Krishnan KR, Pollock JS, Pollock DM. ETA activation mediates angiotensin II-induced infiltration of renal cortical T cells. J Am Soc Nephrol 22: 2187–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boesen EI, Pollock DM. Acute increases of renal medullary osmolality stimulate endothelin release from the kidney. Am J Physiol Renal Physiol 292: F185–F191, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Boesen EI, Williams DL, Pollock JS, Pollock DM. Immunosuppression with mycophenolate mofetil attenuates the development of hypertension and albuminuria in deoxycorticosterone acetate-salt hypertensive rats. Clin Exp Pharmacol Physiol 37: 1016–1022, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol 302: C188–C194, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dumont Y, D'Amours M, Lebel M, Lariviere R. Blood pressure-independent effect of angiotensin AT1 receptor blockade on renal endothelin-1 production in hypertensive uremic rats. J Hypertens 19: 1479–1487, 2001 [DOI] [PubMed] [Google Scholar]
- 11. d'Uscio LV, Shaw S, Barton M, Luscher TF. Losartan but not verapamil inhibits angiotensin II-induced tissue endothelin-1 increase: role of blood pressure and endothelial function. Hypertension 31: 1305–1310, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Dutta UK, Lane J, Roberts LJ, 2nd, Majid DS. Superoxide formation and interaction with nitric oxide modulate systemic arterial pressure and renal function in salt-depleted dogs. Exp Biol Med (Maywood) 231: 269–276, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Ferri C, Desideri G, Baldoncini R, Bellini C, Valenti M, Santucci A, De Mattia G. Angiotensin II increases the release of endothelin-1 from human cultured endothelial cells but does not regulate its circulating levels. Clin Sci (Lond) 96: 261–270, 1999 [PubMed] [Google Scholar]
- 14. Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol 291: F1274–F1280, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol 285: F664–F673, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Herrera M, Garvin JL. A high-salt diet stimulates thick ascending limb eNOS expression by raising medullary osmolality and increasing release of endothelin-1. Am J Physiol Renal Physiol 288: F58–F64, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Hocherl K, Kammerl MC, Schumacher K, Endemann D, Grobecker HF, Kurtz A. Role of prostanoids in regulation of the renin-angiotensin-aldosterone system by salt intake. Am J Physiol Renal Physiol 283: F294–F301, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Huang A, Yan C, Suematsu N, Cuevas A, Yang YM, Kertowidjojo E, Hintze TH, Kaley G, Sun D. Impaired flow-induced dilation of coronary arterioles of dogs fed a low-salt diet: roles of ANG II, PKC, and NAD(P)H oxidase. Am J Physiol Heart Circ Physiol 299: H1476–H1483, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Effects of dietary salt changes on renal renin-angiotensin system in rats. Am J Physiol Renal Physiol 283: F995–F1002, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Kittikulsuth W, Pollock JS, Pollock DM. Sex differences in renal medullary endothelin receptor function in angiotensin II hypertensive rats. Hypertension 58: 212–218, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klinger F, Grimm R, Steinbach A, Tanneberger M, Kunert-Keil C, Rettig R, Grisk O. Low NaCl intake elevates renal medullary endothelin-1 and endothelin A (ETA) receptor mRNA but not the sensitivity of renal Na+ excretion to ETA receptor blockade in rats. Acta Physiol (Oxf) 192: 429–442, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kohan DE. Endothelins in the normal and diseased kidney. Am J Kidney Dis 29: 2–26, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Lara LS, McCormack M, Semprum-Prieto LC, Shenouda S, Majid DS, Kobori H, Navar LG, Prieto MC. AT1 receptor-mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II-salt hypertension. Am J Physiol Renal Physiol 302: F85–F94, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol 279: F252–F258, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Lyon-Roberts B, Strait KA, van Peursem E, Kittikulsuth W, Pollock JS, Pollock DM, Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 300: F650–F656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol 294: F1205–F1211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nielsen J, Kwon TH, Masilamani S, Beutler K, Hager H, Nielsen S, Knepper MA. Sodium transporter abundance profiling in kidney: effect of spironolactone. Am J Physiol Renal Physiol 283: F923–F933, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Pandit MM, Strait KA, Matsuda T, Kohan DE. Na delivery and ENaC mediate flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 302: F1325–F1330, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ETB receptor-mediated NO release. Am J Physiol Renal Physiol 279: F326–F333, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Sasser JM, Pollock JS, Pollock DM. Renal endothelin in chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol 283: R243–R248, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension 51: 1605–1610, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schneider MP, Inscho EW, Pollock DM. Attenuated vasoconstrictor responses to endothelin in afferent arterioles during a high-salt diet. Am J Physiol Renal Physiol 292: F1208–F1214, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Shimizu T, Hata S, Kuroda T, Mihara S, Fujimoto M. Different roles of two types of endothelin receptors in partial ablation-induced chronic renal failure in rats. Eur J Pharmacol 381: 39–49, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Sivritas SH, Ploth DW, Fitzgibbon WR. Blockade of renal medullary bradykinin B2 receptors increases tubular sodium reabsorption in rats fed a normal-salt diet. Am J Physiol Renal Physiol 295: F811–F817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Speed JS, LaMarca B, Berry H, Cockrell K, George EM, Granger JP. Renal medullary endothelin-1 is decreased in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 301: R519–R523, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stow LR, Gumz ML, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Wingo CS. Aldosterone modulates steroid receptor binding to the endothelin-1 gene (edn1). J Biol Chem 284: 30087–30096, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stow LR, Jacobs ME, Wingo CS, Cain BD. Endothelin-1 gene regulation. FASEB J 25: 16–28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Ujiie K, Terada Y, Nonoguchi H, Shinohara M, Tomita K, Marumo F. Messenger RNA expression and synthesis of endothelin-1 along rat nephron segments. J Clin Invest 90: 1043–1048, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vaneckova I, Kramer HJ, Maly J, Backer A, Bokemeyer D, Cervenka L. Lack of a role of neuronal nitric oxide synthase in the regulation of the renal function in rats fed a low-sodium diet. Kidney Blood Press Res 25: 224–231, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Vassileva I, Mountain C, Pollock DM. Functional role of ETB receptors in the renal medulla. Hypertension 41: 1359–1363, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Wong NL, Tsui JK. Angiotensin regulates endothelin-B receptor in rat inner medullary collecting duct. Metabolism 50: 661–666, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Wong WH, Wong BP, Wong EF, Huang MH, Wong NL. Downregulation of endothelin B receptors in cardiomyopathic hamsters. Cardiology 89: 195–201, 1998 [DOI] [PubMed] [Google Scholar]