Abstract
Heat shock protein (HSP)47 is a collagen-specific molecular chaperone that is essential for the biosynthesis of collagen molecules. It is likely that increased levels of HSP47 contribute to the assembly of procollagen and thereby cause an excessive accumulation of collagens in disease processes associated with fibrosis. Although HSP47 promotes renal fibrosis, the underlying mechanism and associated signaling events have not been clearly delineated. We examined the role of HSP47 in renal fibrosis using a rat unilateral ureteral obstruction model and transforming growth factor (TGF)-β1-treated human proximal tubular epithelial (HK-2) cells. An upregulation of HSP47 in both in vivo and in vitro models was observed, which correlated with the increased synthesis of extracellular matrix (ECM) proteins and expression of tissue-type plasminogen activator inhibitor (PAI)-1. Blockade of HSP47 by short interfering RNA suppressed the expression of ECM proteins and PAI-1. In addition, TGF-β1-induced HSP47 expression in HK-2 cells was attenuated by ERK1/2 and JNK MAPK inhibitors. These data suggest that ERK1/2 and JNK signaling events are involved in modulating the expression of HSP47, the chaperoning effect of which on TGF-β1 would ultimately contribute to renal fibrosis by enhancing the synthesis and deposition of ECM proteins.
Keywords: heat shock protein 47, transforming growth factor-β1, renal proximal tubular cell, fibrosis, extracellular signal-regulated protein kinase 1/2, c-Jun NH2-terminal kinase, mitogen-activated protein kinase
in recent years , numerous studies have focused on delineating the pathogenesis of renal fibrosis, which may be due to the fact that there is a strong correlation between the degree of tubulointerstitial disease and anticipated loss of renal functions. Renal interstitial fibrosis is characterized by an excessive accumulation of the extracellular matrix (ECM), which is partly due to uncontrolled synthesis and partly to incomplete degradation of ECM proteins. Collagens, including types I–VI, are the major components of ECM proteins, and their uncontrolled synthesis and excessive deposition are frequently observed in various renal disease processes affecting humans and experimental animals (32, 35). It is conceivable that to ameliorate fibrosis, measures to regulate the cellular synthesis of collagens need to be thoroughly understood. We speculate that some of the heat shock proteins (HSPs) may modulate the intracellular processing of various types of collagens, and thus their pathobiology with respect to interstitial fibrosis need to be explored.
HSPs are expressed under the influrence of a wide variety of stresses, and they are believed to be important modulators of various physiological and pathological processes (23). HSP47, a 47-kDa stress protein, is localized to the endoplasmic reticulum of cells synthesizing collagens. Interestingly, it is a collagen-specific molecular chaperone that is required for the maturation of various types of collagens while folding the procollagens into their appropriate molecular conformations (15, 17, 36). It is therefore logical to infer that HSP47 is involved in the pathogenesis of chronic renal fibrosis, and results form recent studies have supported this contention (25, 28, 32). However, the signaling mechanism(s) by which HSP47 regulates renal fibrosis is not clear. To address this issue, we first investigated the role of HSP47 in renal fibrosis in an established animal model of tubulointerstitial disease. The experiments were then extended to adult human kidney (HK-2) cells, where the role of HSP47 in transforming growth factor (TGF)-β1-induced ECM de novo synthesis in proximal tubular cells and relevant signaling events were delineated.
MATERIALS AND METHODS
Animal model.
Male Wistar rats weighing 200–250 g were used in these experiments. A unilateral ureteral obstruction (UUO) procedure was performed as previously described (41). Under general anesthesia with isoflurane, rats were subjected to UUO or a sham operation. In UUO animals, the left ureter was identified through a small suprapubic incision, ligated with 5.0 silk at two points, and then severed between the ligatures to prevent retrograde urinary tract infection. The sham operation consisted of a similar suprapubic incision and identification of the left ureter, but ligation of the ureter was not performed. Rats were killed after 2 wk after the ureteral ligation. This experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Xiangya School of Medicine, Central South University.
Morphological experiments.
For the assessment of histopathological changes, kidney tissues fixed with 4% buffered paraformaldehyde were embedded in paraffin, and 3-μm-thick sections were prepared. Sections were then stained with hematoxylin and eosin or Masson's trichrome stains (19). Glomerular and tubulointerstitial damage (score) was assessed by evaluating the degree of tubular atrophy, compression atrophy of glomeruli, interstitial fibrosis, and influx of inflammatory cells. For immunohistochemical experiments, the avidin-biotin complex method was used (34). Kidney sections (3 μm thick) from UUO and control rats were prepared. They were deparaffinized and hydrated in graded series of decreasing concentrations of ethanol. Sections were then overlaid with primary monoclonal antibody directed against HSP47 (1:100, StressGen Biotechnologies, Victoria, BC, Canada), anti-fibronectin (FN) antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), and monoclonal mouse anti-collagen type I antibody (1:100, Abcam Biotechnology, Hong Kong, China). Sections were then stained with avidin-biotin peroxidase following the instructions provided by the vendor.
In vitro cell culture experiments.
An immortalized proximal tubule epithelial cell line derived from normal adult human kidney cells (the HK-2 cell line) was obtained from the American Type Culture Collection (Rockville, MD). Cells were cultured at 37°C in an atmosphere of 5% CO2 in DMEM mixed 1:1 (vol:vol) with F-12 medium (Invitrogen Life Technologies, Carlsbad, CA) and supplemented with 10% FBS. Cells were grown to 70–80% confluency and subjected to serum deprivation for 24 h before any further experimental procedures. Cells were incubated with TGF-β1 (Santa Cruz Biotechnology) at a concentration range of 2.5–10 ng/ml for 12–48 h.
Short interfering RNA transfection.
To analyze the mRNA and protein expression of HSP47, collagen I, collagen IV, FN, and plasminogen activator inhibitor (PAI)-1, HK-2 cells were transfected with short interfering (si)RNA directed against HSP47 (SI02777131 and SI02777138) or control siRNA (SI03650318 and SI04381048, QIAGEN) as per the manufacturer's instructions. Control siRNA included a scrambled sequence that would cause degradation of any known cellular mRNA. For determination of the efficiency of HSP47 knockdown, Western blot analysis for HSP47 was performed. Several concentrations of HSP47 siRNA (1–10 nM) were used to evaluate the optimal gene disruption conditions, as previously described. HiPerFect transfection reagent and siRNA were separately diluted in serum-free medium and incubated at room temperature for 5 min. They were then mixed and incubated at room temperature for 10 min. Aliquots of the transfection mixture were added to the cell culture medium. The medium included DMEM-F-12 supplemented with 10% FBS. After 3 h of transfection, the culture was maintained for another 21 h. Cells were then subjected to serum deprivation for 24 h before TGF-β1 treatment.
Protein expression analyses.
Protein was isolated from cultured cell extracts and homogenized in lysis buffer (150 mM NaCl, 10 mM Tris·HCl, 5 mM EDTA, 1 mM EGTA, and 1% Triton X-100) containing a cocktail of protease inhibitors. The protein concentration was measured with a Bradford assay (Bio-Rad, Hercules, CA). After being transfered onto nitrocellulose membranes, blots were probed overnight at 4°C with the following primary antibodies: anti-HSP47 (1:500 dilution), anti-collagen type I (1:1,000), anti-collagen type IV (1:1,000), anti-FN (1:400 dilution), anti-phosphorylated (p-)ERK1/2 (1:300, Santa Cruz Biotechnology), anti-total ERK1/2 (1:300, Santa Cruz Biotechnology), anti-p-JNK (1:300, Santa Cruz Biotechnology), anti-total JNK (1:300, Santa Cruz Biotechnology), anti-β-actin (1:1,000, Santa Cruz Biotechnology), and anti-GAPDH (1:1,000, ProMab Biotechnologies). After an incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies, the horseradish peroxidase reaction product was visualized by an enhanced chemiluminescence system (Kodak Medical X-Ray Processor, Rochester, NY). Swine anti-rabbit IgG or rabbit anti-goat IgG in PBS containing 1% normal goat serum or 1% FCS served as a negative control. β-Actin or GAPDH were used as internal gel loading controls.
mRNA expression analyses.
Total RNA was isolated using a High Pure RNA Isolation kit (Roche) following the manufacturer's instructions. Purified RNA was quantified by absorption spectroscopy at 260 nm. Total RNA (100 μg) was reverse transcribed and subjected to PCR analysis.
For RT-PCR, a Thermo Cycler (Perkin-Elmer) was used to assess HSP47 and ECM gene mRNA expression in HK-2 cells as follows: 94°C for 2 min followed by 40 cycles at 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s with a final extension at 72°C for 10 min. The PCR primer sequences used were as follows: human HSP47, sense 5′-AACTGCGAGCACTCCAAGA-3′ and antisense 5′-ATGAAGCCACGGTTGTCC-3′; human α2-collagen type I, sense 5′-CCAGAGTGGAGCAGTGGTTACTACT-3′ and antisense 5′-TTCTTGGCTGGGATGTTTTCA-3′; human α2-collagen type IV, sense 5′-ACACTGTGGACTTACCAGG-3′ and antisense 5′-CCAGGAAATCCAATGTCACC-3′; human FN, sense 5′-GTGCTGGTGAATGCCCTCT-3′ and antisense 5′-GGTCGCAGCAACAACTTCC-3′; human PAI-1, sense 5′-GTGCTGGTGAATGCCCTCT-3′ and antisense 5′-GGCAGTTCCAGGATGTCGT-3′; and human GAPDH, sense 5′-ACCACAGTCCATGCCATCAC-3′ and antisense 5′-TCCACCACCCTGTTGCTGTA -3′.
Reaction specificity was confirmed by gel electrophoresis of PCR products. Ratios of HSP47 to GAPDH, collagen type I to GAPDH, collagen type IV to GAPDH, FN to GAPDH, and PAI-1 to GAPDH mRNA were calculated for each sample and expressed as means ± SE.
Real-time PCR was used to assess the transcription levels of genes in the kidney of rats, as quantitatively previously described (42). The real-time PCR primer sequences were as follows: rat HSP47, sense 5′-AGAGGTCACCAAGGATGTGGAG-3′ and antisense 5′-TGGGGCATGAGGATGATGAG-3′; rat α2-collagen type I, sense 5′-TGTTCGTGGTTCTCAGGGTAG-3′ and antisense 5′-TTGTCGTAGCAGGGTTCTTTC-3′; rat α2-collagen type IV, sense 5′-ACACTGTGGACTTACCAGG-3′ and antisense 5′-CCAGGAAATCCAATGTCACC-3′; rat FN, sense 5′-GGGATCAAAGGGAAACACAG-3′ and antisense 5′-AGACGGCAAAAGAAAGCAG-3′; rat PAI-1, sense 5′-CTTTATCCTGGGTCTCCCTG-3′ and antisense 5′-TGATGCCTCCCTGACATACA-3′; and rat GAPDH, sense 5′-AGGACCA GGTTGTCTCCTGT-3′ and antisense 5′-TTACTCCTTGGAGGCCATGT-3′.
ELISA procdures.
Total PAI-1 in the cell culture supernatant was measured by specific ELISA methods (R&D Systems, Minneapolis, MN), as previously described (8). A multiwell culture plate was prepared by coating it with a monoclonal mouse PAI-1 antibody and blockade with 4% BSA-PBS overnight at 4°C. The supernatant or PAI-1 standards (recombinant human PAI-1) were poured onto the plate and incubated for 2 h. After a wash, each plate well was successively incubated with the biotinylated secondary antibody, streptavidin-conjugated horseradish peroxidase detection reagent, and substrate solution. Absorbance was measured at 450 nm using a Wallace Victor V plate reader. The mean absorbance of each sample was plotted against PAI-1 standards to quantify the amount of PAI-1 (expressed as pg/ml).
Statistical analyses.
Data were analyzed with a t-test, where the means between two groups were compared, or with one-way ANOVA plus Tukey's post hoc multiple-comparison test to compare mean values across multiple treatment groups. Analysis was performed with standard statistical software (SPSS for Windows, version 15.0). In all cases, P value of <0.05 were considered statistically significant. All data are expressed as means ± SE.
RESULTS
Morphological changes and expression profiles of HSP47 and ECM proteins in kidneys of rats subjected to UUO.
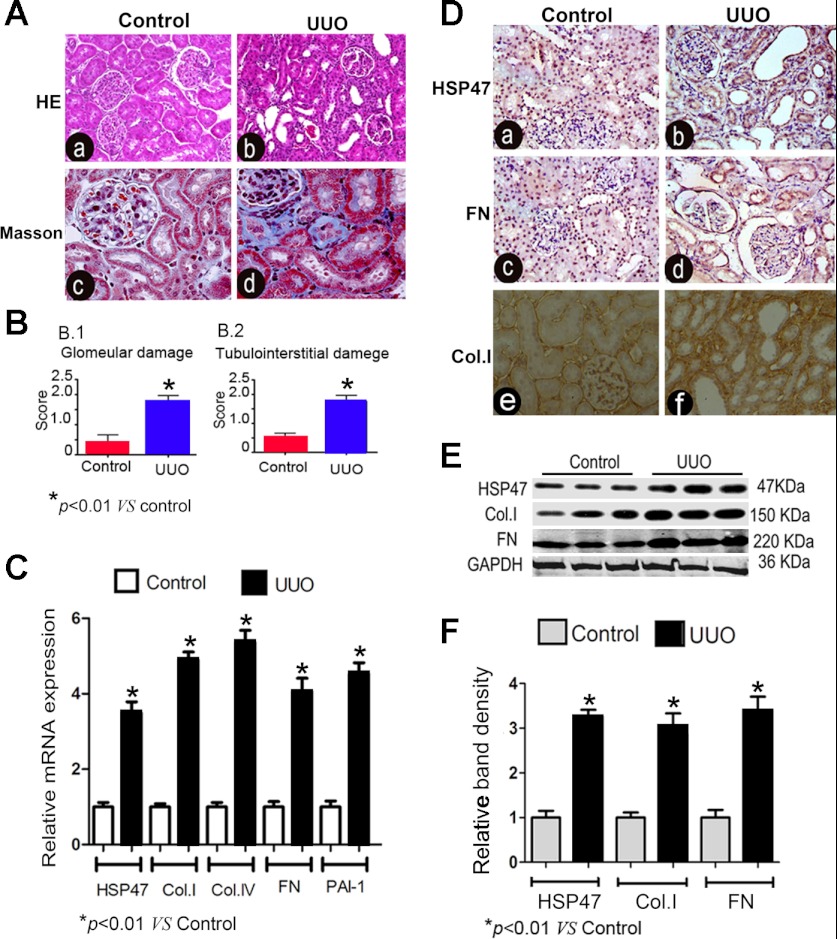
Examination of renal tissues stained with hematoxylin and eosin and with Masson's trichrome revealed notable tubular atrophy, tubular dilatation, interstitial fibrosis, and inflammatory cell infiltration in kidneys of UUO rats (Fig. 1A,b and d). The glomerular damage (score) was ∼10-fold higher compared with that of control kidneys, and tubulointerstitial damage (score) was also markedly increased in UUO rats (Fig. 1B). By real-time PCR, the mRNA expressions of HSP47, collagen type 1, collagen type IV, FN, and PAI-1 were remarkably increased in the kidney of UUO rats on day 14 compared with those of control rats (Fig. 1C). Protein expression of HSP47 in the kidney of UUO rats was evaluated by immunohistochemical procedures. In control rats, the expression of HSP47 was quite low, and it was localized mainly in the renal tubular-interstitial area. However, an increased expression of HSP47 was noted in the tubular-interstitial compartment with an expanded interstitial matrix in the kidneys of UUO rats on day 14, and a similar increase was seen for the expression of FN (Fig. 1D,b and d). In addition, collagen type I was weakly positive in the tubulointerstitium of control kidneys, whereas it increased notably in UUO rats (Fig. 1D,f). Immunoblot analyses also showed an upregulation of HSP47 with a parallel increased expression of FN and collagen type I in kidneys of UUO rats (Fig. 1E). Densitometric analyses confirmed the two- to threefold increase of HSP47 and ECM protein expression in the kidneys of UUO rats compared with control rats (Fig. 1F).
Fig. 1.
Morphological changes and expression profiles of heat shock protein (HSP)47 and extracellular matrix (ECM) proteins in the kidneys of rats subjected to unilateral ureteral obstruction (UUO). A: kidney sections stained with hematoxylin and eosin (HE; a and b) and Masson's trichrome (c and d) showed notable tubular dilatation and expansion of the interstitial compartment due to fibrosis in UUO rats (b and d) compared with control rats (a and c). B: semiquantitative score indicating marked glomerular damage (1) and tubulointerstitial damage (2) in the renal cortex of rats with UUO. C: real-time PCR analyses revealed significant increases in the mRNA expression of HSP47, collagen type I (Col.I), collagen type IV (Col.IV), fibronectin (FN), and plasminogen activator inhibitor (PAI)-1 in the kidney cortexes of UUO rats compared with sham-operated control rats (n = 6, P < 0.01). D: significant increases in the expression of HSP47 and FN in tubular and interstitial compartments of the kidneys in UUO rats (b and d) compared with control rats (a and c) as assessed by immunohistochemistry. Also, Col.I expression was noted to be remarkably increased in the kidney of rats subjected to UUO (F) compared with control rats (E). E: Western blot analyses revealed increased protein expression of HSP47, Col.I, and FN in the kidneys of the UUO group. F: densitometric analysis of Western blot bands of HSP47, Col.1, and FN confirmed the increased expression of the respective proteins (n = 4). *P < 0.01 vs. the control group.
TGF-β1 induced increased expression of HSP47, collagen type I, collagen type IV, FN, and PAI-1 in HK-2 cells.
The above in vivo experiments indicated that the expression of HSP47, collagen type I, and FN was notably increased after UUO. To delineate the mechanisms involved in the increased expression of these proteins, in vitro experiments using proximal tubular cells (HK-2 cells) were performed with the premise that the profibrogenic cytokine TGF-β1 may be responsible for the changes obsereved in rats with UUO.
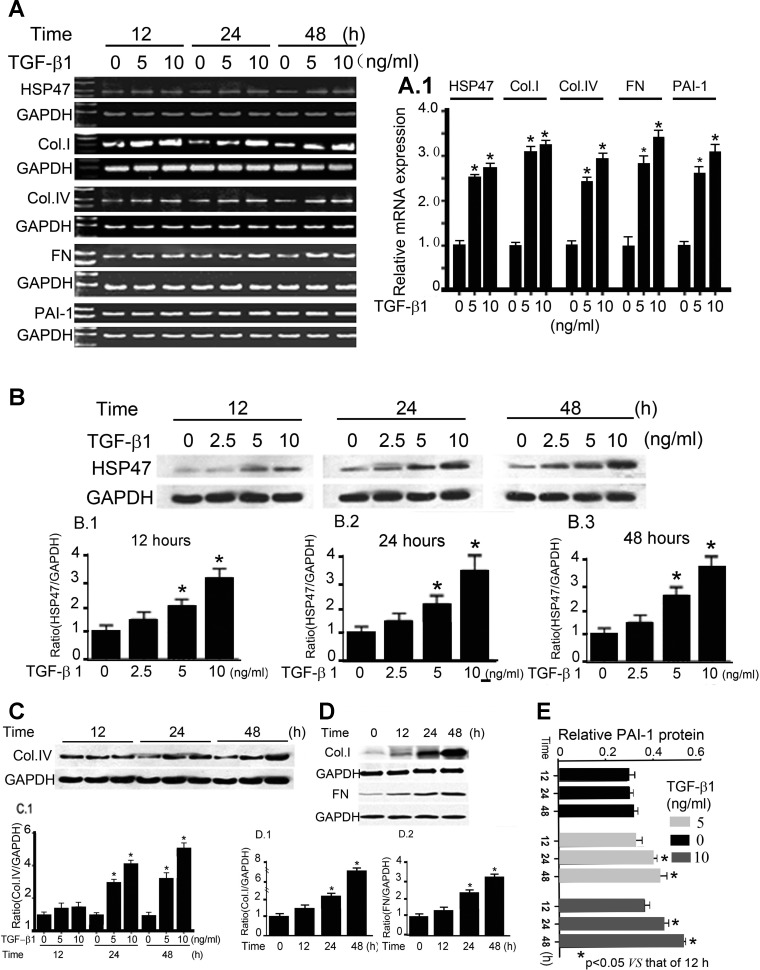
To assess whether the changes were induced by TGF-β1, TGF-β1 was added into the culture medium with increasing concentrations of the cytokine (0, 5, and 10 ng/ml), and HK-2 cells were maintained for 12–48 h. After this, the expression of HSP47, collagen type I, collagen type IV, FN, and PAI-1 in response to TGF-β1 in HK-2 cells was assessed after 12–48 h. RT-PCR analyses and densitometeric tracings demonstrated dose- and time-dependent increases of HSP47, collagen type I, collagen type IV, FN, and PAI-1 (Fig. 2A). Maximal increases of collagen type I, collagen type IV, FN, and PAI-1 in response to TGF-β1 were observed when 10 ng/ml cytokine was included in the culture medium for 48 h (Fig. 2, A and A,1). ELISA methods also confirmed the time- and dose-dependent increases in the protein expression of PAI-1 with TGF-β1 (0–10 ng/ml) treatment for 12–48 h (Fig. 2E). Furthermore, Western blot analyses revealed TGF-β1-induced increases of HSP47 protein in HK-2 cells treated with varying concentrations of the profibrogenic cytokine (0, 2.5, 5, and 10 ng/ml) for 12–48 h (Fig. 2B). Similarly, there were increases in the protein expression of collagen type I, collagen type IV, and FN in HK-2 cells treated with TGF-β1 (Fig. 2, C and D). The concomitant increase HSP47 and ECM protein expression after TGF-β1 treatment suggests that this cytokine may mediate a profibrogenic effect by modulating the biology of HSP47 and thus could play a role in the evolution of renal interstitial fibrosis.
Fig. 2.
Transforming growth factor (TGF)-β1-induced increased expression of HSP47, Col.1, Col.IV, FN, and PAI-1 in HK-2 cells. HK-2 cells were maintained for 12–48 h and treated with various concentrations of TGF-β1 (0–10 ng/ml), and the expression of HSP47, Col.I, Col.IV, FN, and PAI-1 was evaluated. A: RT-PCR analyses revealed increased mRNA expression of HSP47, Col.I, Col.IV, FN, and PAI-1 with treatment with TGF-β1. No discernible changes in GAPDH expression were observed. A,1: mRNA expression of HSP47, Col.I, Col.IV, FN, and PAI-1 relative to GAPDH, as shown in A. Values are expressed as means ± SE; n = 4. *P < 0.01 vs. the control group. B: Western blots (top) and the respective densitometric analyses of the bands (1–3) showing the dose- and time-dependent increases in the expression of HSP47 in HK-2 cells after TGF-β1 treatment. Values are expressed as means ± SE; n = 5. *P < 0.01 compared with the control group. C and D: TGF-β1 treatment also increased the protein expression of Col.IV, Col.I, and FN in HK-2 cells in a dose- and time-dependent manner. The bar graphs in C,1, D,1, and D,2 represent densitometric analyses of the top Western blots. Values were normalized against the GAPDH control and are expressed as means ± SE; n = 5. *P < 0.01. E: ELISA methods revealed that TGF-β1 increases PAI-1 expression in a dose- and time-dependent manner in HK-2 cells. Values are expressed as means ± SE; n = 6. *P < 0.01 vs. 12-h TGF-β1 treatment.
Attenuation of TGF-β1-induced expression of collagen type I, collagen type IV, FN, and PAI-1 by transfection of HK-2 cells with HSP47 siRNA.
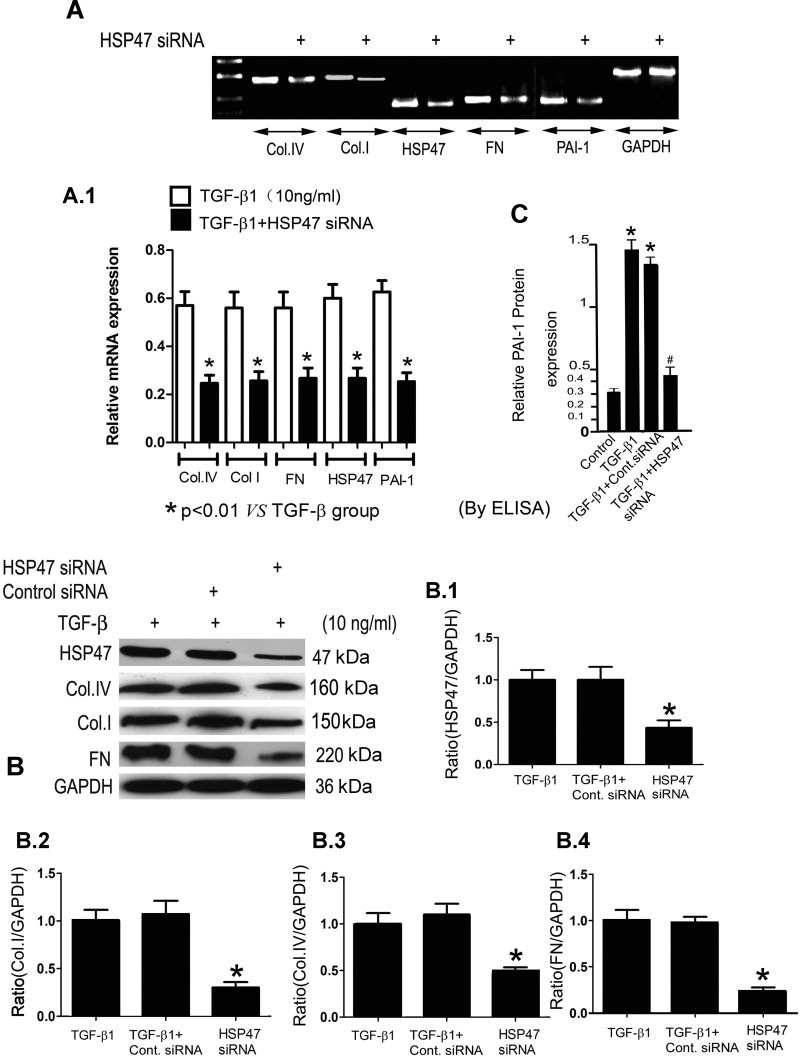
To determine whether the increase of HSP47 expression was specifically related to TGF-β1-induced ECM protein de novo synthesis, HK-2 cells were transfected with HSP47 siRNA before the inclusion of TGF-β1 in the culture media. HSP47 siRNA resulted in a significant reduction in the TGF-β1-induced mRNA expression of HSP47, collagen type I, collagen type IV, FN, and PAI-1 in HK-2 cells, as analyzed by RT-PCR (Fig. 3, A and A,1). Also, similar reduced protein expression of HSP47, collagen type I, collagen type IV, and FN was observed by Western blot procedures in HK-2 cells treated with TGF-β1 (Fig. 3B,1–4). Transfection of scrambled siRNA (control) had no discernible effect on the expression of various ECM proteins (Fig. 3B,1–4). In addition, as assessed by ELISA, HSP47 siRNA inhibited PAI-1 protein expression in HK-2 cells induced by TGF-β1 (Fig. 3C). These data thus support the contention that HSP47 specifically modulates TGF-β1-induced ECM protein synthesis in HK-2 cells.
Fig. 3.
HSP47 short interfering (si)RNA inhibits TGF-β1-induced ECM gene and protein expression in HK-2 cells. HK-2 cells were transiently transfected with human HSP47 siRNA or control siRNA for 24 h and then treated with 10 ng/ml TGF-β1 for an additional 48 h. A and A,1: RT-PCR and densitometric analyses indicated that treatment with HSP47 siRNA significantly decreased the mRNA expression of Col.I, Col.IV, FN, HSP47, and PAI-1, whereas GAPDH was unaffected. B: similarly, immunoblot procedures revealed reductions in the protein expression of HSP47 (1), Col.I (2), Col.IV (3), and FN (4) after HSP47 siRNA transfection. No changes were observed in cells treated with control siRNA, and GAPDH served as a loading control. Densitometric analyses are expressed as means ± SE; n = 4. *P < 0.01. C: ELISA methods showed that PAI-1 expression was increased in HK-2 cells induced by TGF-β1, whereas the effect was abolished with treatment with HSP47 siRNA. Values are expressed as means ± SE; n = 6. *P < 0.01 vs. the control group; #P < 0.01 vs. the TGF-β1-treated group.
Induction of HSP47 expression by TGF-β1 involves ERK1/2 and JNK MAPK signaling pathways.
ERK1/2 and JNK are two main members of MAPK family that apparently play an important role in regulating stress responses and the expression of HSPs (11, 27, 37, 40). Furthermore, both ERK1/2 and JNK have been shown to be involved in the pathogenesis of interstitial fibrosis stemming from a wide variety of mechanisms in different renal disease processes (12, 20, 22). This led us to investigate whether TGF-β1-induced HSP47 expression is regulated by ERK1/2 and JNK MAPKs.
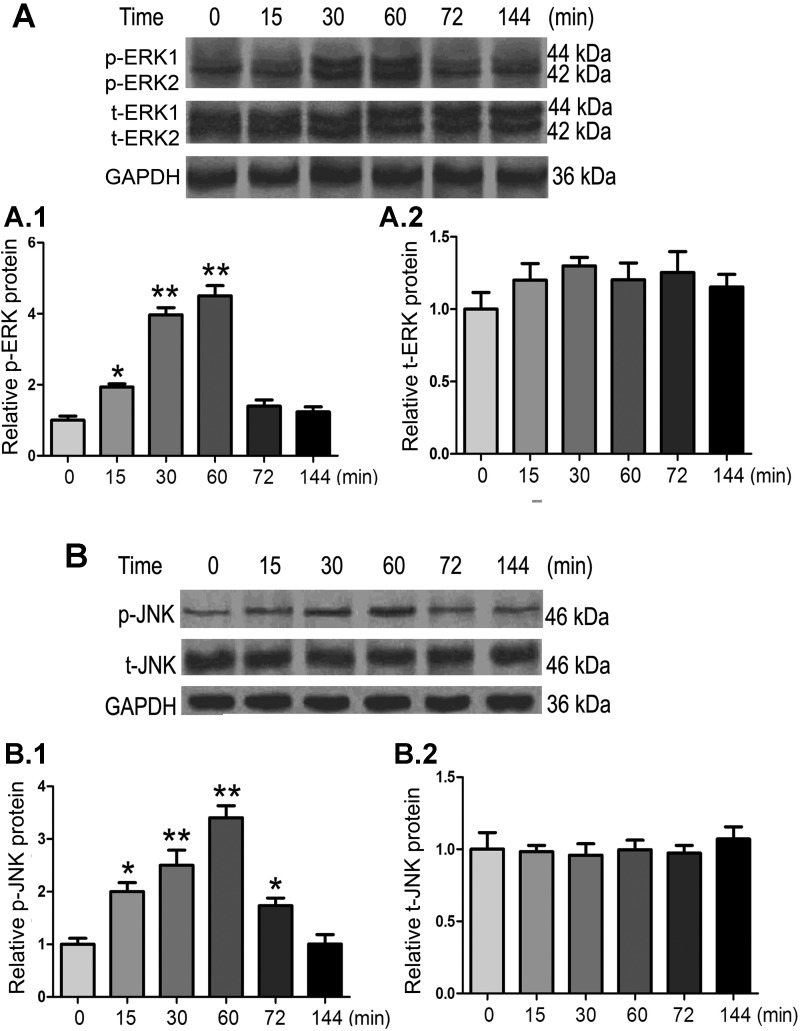
First, the phosphorylation status of ERK1/2 and JNK was investigated to assess if TGF-β1 activates the JNK and ERK1/2 pathway. The results of time course experiments revealed transient increases in the phosphorylation of ERK1/2 and JNK in HK-2 cells treated with TGF-β1 (10 ng/ml). An ∼4-fold increase in ERK1/2 phosphorylation and an ∼3.5-fold increase in JNK phosphorylation in HK-2 cells in response to 10 ng/ml TGF-β1 treatment was observed at 60 min (Fig. 4, A,1 and B,1). Total expressions of ERK and JNK were not significantly altered (Fig. 4, A,2 and B,2).
Fig. 4.
Effect of TGF-β1 on the phosphorylation of ERK1/2 and JNK MAPK in HK-2 cells. Cells were exposed to TGF-β1 (10 ng/ml) for varying periods of time, cellular extracts were subjected to SDS-PAGE, and their protein blots were probed with various antibodies. A and B: TGF-β1 treatment increased ERK1/2 (A) and JNK (B) phosphorylation (p-ERK1/2 and p-JNK) in a dose-dependent manner with a peak effect around 60 min after exposure to the cytokine. No changes were observed in total (t-)ERK1/2 and JNK. The bar graphs (1 and 2) represent densities of the corresponding bands detected by immunoblot procedures relative to GAPDH. Values are expressed as means ± SE; n = 5. *P < 0.05 and **P < 0.01 compared with the control group.
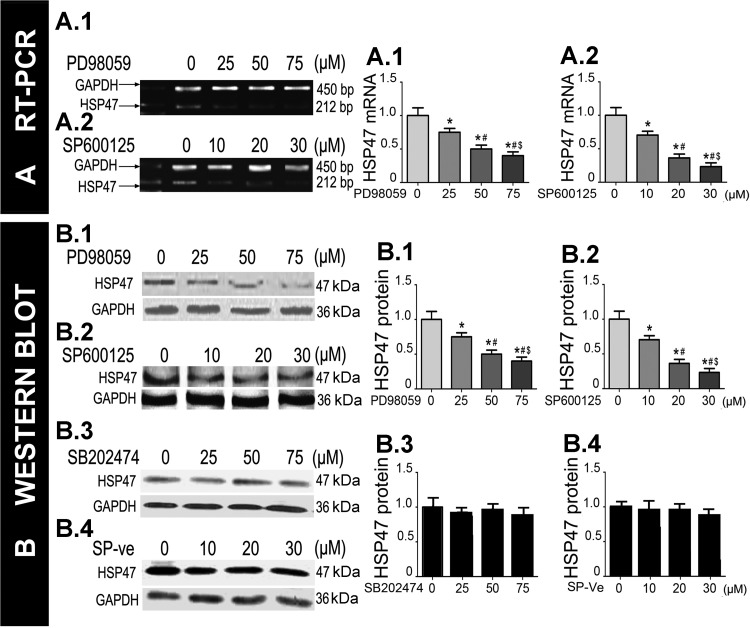
To ensure that the TGF-β1-induced increase in HSP47 expression was directly related to ERK1/2 and JNK activation, HK-2 cells were individually pretreated with the ERK1/2 inhibitor PD-98059 (25–75 μM) or the JNK inhibitor SP-600125 (10–30 μM) for 60 min followed by treatment with TGF-β1 (10 ng/ml) for 24 h. Compared with the treatment with TGF-β1 alone, RT-PCR analysis revealed that PD-98059 and SP-600125 attenuated the mRNA expression of HSP47 in a dose-dependent manner in HK-2 cells (Fig. 5A,1a and 2a). Similar results were seen for the protein expression of HSP47 by Western blot analyses after the treatment with ERK1/2 and JNK inhibitors (Fig. 5B,1a and 2a). HSP47 expression was unaffected by the treatment with SB-202474, an inactive congener of PD-98059, or with the inactive analog of SP-600125 (SP-ve; Fig. 5B,3a and 4a), suggesting that the effects of inhibitors of ERK1/2 and JNK are specific. These results indicate that the ERK1/2 and JNK pathways, most likely, are responsible for modulating HSP47 expression in HK-2 cells treated with TGF-β1.
Fig. 5.
Effect of ERK1/2 and JNK inhibitors on HSP47 expression after treatment of HK-2 cells with TGF-β1. HK-2 cells were pretreated with varying doses of PD-98059 (ERK1/2 inhibitor) or SP-600125 (JNK inhibitor) for 60 min and then treated with TGF-β1 (10 ng/ml) for 24 h. A: RT-PCR analyses revealed that the expression of HSP47 was markedly decreased in a dose-dependent manner in cells treated with PD-98059 (1) or SP-600125 (2). B,1 and 2: Western blot analyses confirmed the results determined by RT-PCR analyses. B,3 and 4: there were no changes in cells treated with SB-202474, a negative control for PD-98059, or with SP-ve, an inactive analog of SP-600125. The bar graphs (A,1a and 2a, and B,1a, 2a, 3a, and 4a) represent density analyses of the bands from RT-PCR and Western blots. Values are expressed as means ± SE; n = 6. *P < 0.05 vs. the control group (0 μM); #P < 0.05 vs. 25 μM PD-98059 or 10 μM SP-600125; $P < 0.05 vs. 50 μM PD-98058 or 20 μM SP-00125.
DISCUSSION
Reports in the literature relevant to the mechanism(s) of HSP47 in renal fibrosis have focused mainly on its regulation of collagens. Studies have shown that there is a close association between increased expression of HSP47 and excessive accumulation of collagens in various human and experimental diseases affecting the kidney, such as murine anti-thymocyte serum-induced glomerulonephritis, streptozotocin-induced diabetic nephropathy, UUO, human diabetic nephropathy, IgA nephropathy, and hypertensive nephrosclerosis (7, 24, 26, 30, 31). There are other reports that have indicated that suppression of HSP47 expression with antisense HSP47 oligodeoxynucleotides or HSP47 siRNA not only reduce the accumulation of collagens to delay the progression of fibrosis in experimental animal models but also abrogate α-smooth muscle actin expression, reduce the influx of macrophages, macrophage infiltration in blood vessels, and macrophage chemoattractant protein-1 expression in rats (28, 39). The reports also indicated that in UUO kidneys, the upregulation of HSP47 mRNA preceded the increased expression of collagen type I mRNA and tubulointerstitial fibrosis. Also, in angiotensin-converting enzyme inhibitor-treated mice, alterations in HSP47 mRNA expression preceded the changes in collagen type I mRNA. The sequence of changes in HSP47 mRNA, collagen type I mRNA, and interstitial fibrosis strongly suggest that overexpression of HSP47 is an upstream event, and it may play a role in regulating the pathogenesis of renal fibrosis (25). However, these studies did not describe the changes in other ECM components, such as FN and laminin. These studies indicated that the role of HSP47 may be limited to regulating collagen synthesis alone to promote renal fibrosis. We contend that HSP47, an upstream regulator in the progression of renal fibosis, in addition to modulating the expression of collagen, has the potential to regulate the expression of other ECM proteins, such as FN and laminin, and also other inflammatory cytokines.
UUO has been widely used to study various pathogenetic mechanisms that lead to tubulointerstitial fibrosis (18). Previously, we (42) reported that low-dose administration of paclitaxel in a rat model of UUO significantly reduces tubulointerstitial fibrosis. To attest to our contention that the other ECM proteins are involved in the pathogenesis of renal tubulointerstitial fibrosis and the phosphorylation of signaling molecules induced by HSP47 are essential for this process, we used the UUO model for the experiments described in this investigation. In accordance with our contention, our data indicated that increases in the mRNA and protein expression of collagen type I, collagen type IV, FN, and PAI-1 correlated with the increased expression of their molecular chaperone, HSP47, in the kidneys of UUO rats. Next, we investigated if the overexpression of HSP47 promotes excessive collagen synthesis, which could explain the pathogenesis of renal tubulointerstitial fibrosis.
To delineate the mechanism by which HSP47 increases the expression of collagen, in vitro experiments using HK-2 cells treated with TGF-β1 were esed. TGF-β1 is known to induce fibrosis in kidneys and also has been described to increase ECM expression (2, 13). We demonstrated that TGF-β1 increased the expression of collagen type I, collagen type IV, FN, and PAI-1 in HK-2 cells. Normally, HSP47 is expressed at low levels in the absence of TGF-β1 in HK-2 cells. Treatment with TGF-β1 significantly increased protein and mRNA levels of HSP47. The question that arises here is what is the specific role of HSP47 in TGF-β1-induced ECM synthesis in HK-2 cells? To address this question, gene-specific inhibition of inducible HSP47 by means of siRNA was carried out. First, we established that HSP47 induction can be effectively and specifically silenced by the use of HSP47 siRNA. After the successful downregulation of HSP47 with siRNA in vitro, a remarkable decrease in the de novo synthesis of collagen type types I and IV was observed, meaning thereby that their secretion into the extracellular space would be reflective of their accumulation in the interstitial compartment in vivo. From these findings, one may infer that suppression of HSP47 may be a therapeutic tool to ameliorate fibrosis in the kidney. In addition, our data suggest that HSP47 siRNA also effectively inhibited FN expression. The probable reason that more than one ECM component is affected by HSP47 siRNA may be that various ECM proteins form an interlacing network of assembly in the tubulointerstium compartment whose expression is modulated in an interdependent manner (6, 8). This may mean that changes in the expression of one ECM protein would be reflected in the expression of others at the same time. However, it is also conceivable that HSP47 may regulate FN expression independent of that of the collagens.
To explore whether HSP47 plays a role in regulating ECM degradation, we investigated PAI-1 expression after HSP47 siRNA treatment of HK-2 cells. PAI-1 is a powerful fibrosis-promoting molecule by inhibiting fibrinolysis and upregulating ECM genes (5). Our data showed that HSP47 siRNA remarkably decreased PAI-1 expression induced by TGF-β1 treatment of HK-2 cells. The precise mechanism by which HSP47 exerts its modulatory effect on PAI-1 is not clearly defined. The HSP47 is mainly localized in the endoplasmic reticulum, and FN and PAI-1 are both secreted proteins that ought to be processed within the endoplasmic reticulum before being secreted into the exterior. In view of this, we speculate that HSP47 may regulate FN and PAI-1 expression by a certain yet to be characterized mechanism (chaperone?) within the endoplasmic reticulum before being excreted out of the cells to form the assembled matrix extracellulary. Nevertheless, further investigations are needed to delineate the mechanism(s) responsible for the interplay among these molecules.
Another significant aspect of the present study was to investigate various signaling pathways, such as MAPKs, that relate to the biology of HSP47 modulated by TGF-β1. MAPKs are intricately linked to the pathobiology of various kidney diseases, including renal fibrosis. It is also known that there is a considerable cross-talk between TGF-β1 and MAPK signaling pathways in the synthesis and turnover of the ECM by fibroblast-like cells in the kidney. In addition, MAPK signaling is believed to contribute in the TGF-β1-induced transition of tubular epithelial cells into myofibroblasts (10, 16, 21, 29, 33). Moreover, ERK1/2 and JNK can regulate heat shock transcription factor (3, 16, 29), which, in turn, can regulate the synthesis of nearly all of the HSPs (1). Interestingly, MAPK is involved in the synthesis of HSPs in response to various stimuli (4, 38). For instance, ERK1/2 is known to participate in the pathways that relate to TGF-β1-stimulated HSP27 induction in osteoblasts (11). As HSP47 is a member of the HSP family, we speculate that ERK1/2 and JNK would participate in the pathways of TGF-β1-stimulated HSP47 induction in HK-2 cells. In line with this contention, we demonstrated that treatment of HK-2 cells with TGF-β1 activates ERK1/2 and JNK pathways, as evidenced by ERK1/2 and JNK phosphorylation. The kinetics of ERK1/2 and JNK phosphorylation were in agreement with the results of a previous report (14). Further support for the role of these signaling molecules was derived from the experiments in which PD-98059 and SP-600125 were shown to inhibit ERK1/2 and JNK signaling as well as suppression of TGF-β1-stimulated HSP47 expression. The results clearly suggest that TGF-β1-induced HSP47 expression in HK-2 cells in vitro mainly uses ERK1/2 and JNK pathways as mediators. However, because the increased HSP47 expression induced by TGF-β1 could not be completely abrogated by blockade of ERK1/2 or JNK signaling in HK-2 cells, this would suggest that other pathways may also be involved that contribute to the increase of HSP47 expression.
In summary, the data provided in this study suggest that HSP47 mediated TGF-β1-induced ECM accumulation in human proximal tubular cells. Two novel observations made in this investigation are as follows: 1) HSP47 siRNA attenuates TGF-β1-induced collagen type I, collagen type IV, FN, and PAI-1 expression in HK-2 cells, suggesting the functionality of HSP47 in regulating ECM synthesis and degradation in processes related to renal tubulointerstitial fibrosis; and 2) ERK1/2 and JNK pathways are involved in the enhancement of HSP47 expression in HK-2 cells induced by TGF-β1. Overall, our study establishes the role of HSP47 in the pathogenesis of tubulointerstitial fibrosis, and it is anticipated that this chaperone may serve as an important therapeutic tool in future investigations.
GRANTS
This work was supported by Creative Research Group Fund 30871169/C140405 from the National Foundation Committee of Natural Sciences of China, the Furong Scholars Fund from the Hunan Province Education Department, and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-60635.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.-B.X., R.-H.L., L.X., Y.-C.X., J.L., Y.-H.L., M.Z., S.Y., and L.S. performed experiments; H.-B.X., R.-H.L., G.-H.L., Y.-C.X., F.-Y.L., J.L., J.-l.L., M.Z., S.Y., and L.S. analyzed data; H.-B.X., R.-H.L., and J.L. interpreted results of experiments; H.-B.X. and L.S. drafted manuscript; R.-H.L., L.X., and Y.-H.L. prepared figures; R.-H.L. and Y.S.K. approved final version of manuscript; G.-H.L., L.X., F.-Y.L., Q.-K.C., Y.S.K., and L.S. edited and revised manuscript; L.S. conception and design of research.
REFERENCES
- 1. Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem 80: 1089– 1115, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Bottinger EP, Bitzer M. TGF-β signaling in renal disease. J Am Soc Nephrol 13: 2600– 2610, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem 271: 30847– 30857, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Chen YC, Tsai SH, Shen SC, Lin JK, Lee WR. Alternative activation of extracellular signal-regulated protein kinases in curcumin and arsenite-induced HSP70 gene expression in human colorectal carcinoma cells. Eur J Cell Biol 80: 213– 221, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Courey AJ, Horowitz JC, Kim KK, Koh TJ, Novak ML, Subbotina N, Warnock M, Xue B, Cunningham AK, Lin Y, Goldklang MP, Simon RH, Lawrence DA, Sisson TH. The vitronectin-binding function of PAI-1 exacerbates lung fibrosis in mice. Blood 118: 2313– 2321, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chernousov MA, Stahl RC, Carey DJ. Schwann cells use a novel collagen-dependent mechanism for fibronectin fibril assembly. J Cell Sci 111: 2763– 2777, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Diange Liu, Razzaque MS, Cheng M, Taguchi T. The renal expression of heat shock protein 47 and collagens in acute and chronic experimental diabetes in rats. Histochem J 33: 621– 628, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Dzamba BJ, Wu H, Jaenisch R, Peters DM. Fibronectin binding site in type I collagen regulates fibronectin fibril formation. J Cell Biol 121: 1165– 1172, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feldman G, Kiely B, Martin N, Ryan G, McMorrow T, Ryan MP. Role for TGF-β in cyclosporine-induced modulation of renal epithelial barrier function. J Am Soc Nephrol 18: 1662– 1671, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Frank Y, Flanc RS, Tesch GH, Han Y, Atkins RC, Bennett BL, Friedman GC, Fan JH, Nikolic-Paterson DJ. A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J Am Soc Nephrol 18: 472– 484, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Hatakeyama D, Kozawa O, Niwa M, Matsuno H, Ito H, Kato K, Tatematsu N, Shibata T, Uematsu T. Upregulation by retinoic acid of transforming growth factor-beta-stimulated heat shock protein 27 induction in osteoblasts: involvement of mitogen-activated protein kinases. Biochim Biophys Acta 1589: 15– 30, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-β1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int 56: 1710– 1720, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Hewitson TD. Renal tubulointerstitial fibrosis: common but never simple. Am J Physiol Renal Physiol 296: F1239– F1244, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Yan JD, Yang S, Zhang J, Zhu TH. BMP6 reverses TGF-β1-induced changes in HK-2 cells: implications for the treatment of renal fibrosis. Acta Pharmacologica Sinica 30: 994– 1000, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. John JS, Nikitakis N, Siavash H. HSP47a novel collagen binding serpin chaperone, autoantigen and therapeutic target. Front Biosci 10: 107– 118, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Jynho KA, Nueda A, Meng YH, Dynan WS, Mivechi NF. Analysis of the phosphorylation of human heat shock transcription factor-1 by MAP kinase family members. J Cell Biochem 67: 43– 54, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Nagata K. Expression and function of heat shock protein 47: a collagen- specific molecular chaperone in the endoplasmic reticulum. Matrix Biol 16: 379– 386, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861– F875, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Meng L, Qu L, Tang J, Cai SQ, Wang H, Li X. A combination of Chinese herbs, Astragalus membranaceus var. mongholicus and Angelica sinensis, enhanced nitric oxide production in obstructed rat kidney. Vasc Pharmacol 47: 174– 183, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Ma FY, Flanc RS, Tesch GH, Han Y, Atkins RC, Bennett BL, Friedman GC, Fan JH, Nikolic-Paterson DJ. A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J Am Soc Nephrol1 8: 472– 484, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Ma FY, Sachchithananthan M, Flanc RS, Nikolic-Paterson DJ. Mitogen activated protein kinases in renal fibrosis. Front Biosci 1: 171– 87, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Masaki T, Stambe C, Hill PA, Dowling J, Atkins RC, Nikolic-Paterson DJ. Activation of the extracellular-signal regulated protein kinase pathway in human glomerulopathies. J Am Soc Nephrol 15: 1835– 1843, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Invest 95: 3– 12, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohammed SR, Taguchi T. Collagen-binding heat shock protein 47 expression anti-thymocyte serum (ATS)-induced glomerulonephritis. J Pathol 183: 24– 29, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Moriyama T, Kawada N, Ando A, Yamauchi A, Horio M, Nagata K, Imai E, Hori M. Up-regulation of HSP47 in the mouse kidneys with unilateral ureteral obstruction. Kidney Int 54: 110– 119, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Mukae H, Nakayama S, Sakamoto N, Kakugawa T, Yoshioka S, Soda H, Oku H, Urata Y, Kondo T, Kubota H, Nagata K, Kohno S. Pirfenidone inhibits the expression of HSP47 in TGF-β1-stimulated human lung fibroblasts. Life Sci 82: 210– 217, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Natsume H, Adachi S, Takai S, Tokuda H, Matsushima-Nishiwaki R, Minamitani C, Yamauchi J, Kato K, Mizutani J, Kozawa O, Otsuka T. (−)-Epigallocatechin gallate attenuates the induction of HSP27 stimulated by sphingosine 1-phosphate via suppression of phosphatidylinositol 3-kinase/Akt pathway in osteoblasts. Int J Mol Med 24: 197– 203, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Nishino T, Miyazaki M, Abe K, Furusu A, Mishima Y, Harada T, Ozono Y, Koji T, Kohno S. Antisense oligonucleotides against collagen-binding stress protein HSP47 suppress peritoneal fibrosis in rats. Kidney Int 64: 887– 896, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Park J, Liu AY. JNK phosphorylates the HSF1 transcriptional activation domain: role of JNK in the regulation of the heat shock response. J Cell Biochem 82: 326– 338, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Razzaque MS, Azouz A, Shinagawa T, Taguchi T. Factors regulating the progression of hypertensive nephrosclerosis. Contrib Nephrol 139: 173– 186, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Razzaque MS, Kumatori A, Harada T, Taguchi T. Coexpression of collagens and collagen-binding heat shock protein 47 in human diabetic nephropathy and IgA nephropathy. Nephron 80: 434– 443, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Razzaque MS, Le VT, Taguchi T. Heat shock protein 47 and renal fibrogenesis. Contrib Nephrol 148: 57– 69, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Santibanez JF. JNK mediates TGF-β1-induced epithelial mesenchymal transdifferentiation of mouse transformed keratinocytes. FEBS Lett 580: 5385– 5391 2006 [DOI] [PubMed] [Google Scholar]
- 34. Sun L, Sahai A, Chugh SS, Pan X, Wallner EI, Danesh FR, Lomasney JW, Kanwar YS. High glucose stimulates synthesis of fibronectin via a novel protein kinase C, Rap1b, and B-Raf signaling pathway. J Biol Chem 277: 41725– 41735, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Taguchi T, Razzaque MS. Cellular and molecular events leading to renal tubulo- interstitial fibrosis. Clin Electron Microsc Soc Japan 35: 68– 80, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Taguchi T, Razzaque MS. The collagen-specific molecular chaperone HSP47: is there a role in fibrosis? Trends Mol Med 13: 45– 53, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol 67: 1815– 1829, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Adler V, Schaffer A, Kim J, Dolan L, Ronai Z. UV irradiation and heat shock mediate JNK activation via alternate pathways. J Biol Chem 270: 26071– 26077, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Wang Y, Li C, Wang X, Zhang J, Chang Z. Heat shock response inhibits IL-18 expression through the JNK pathway in murine peritoneal macrophages. Biochem Biophys Res Commun 296: 742– 748, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Xia Z, Abe K, Furusu A, Miyazaki M, Obata Y, Tabata Y, Koji T, Kohno S. Suppression of renal tubulointerstitial fibrosis by small interfering RNA targeting heat shock protein 47. Am J Nephrol 28: 34– 46, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Xie P, Sun L, Nayak B, Haruna Y, Liu FY, Kashihara N, Kanwar YS. C/EBP-β modulates transcription of tubulointerstitial nephritis antigen in obstructive uropathy. J Am Soc Nephrol 20: 807– 819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang D, Sun L, Xian W, Liu F, Ling G, Xiao L, Liu Y, Peng Y, Haruna Y, Kanwar YS. Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF- β/Smad activity. Lab Invest 90: 436– 447, 2010 [DOI] [PubMed] [Google Scholar]