Abstract
Activation of A1 adenosine receptors (ARs) protects against renal ischemia-reperfusion (I/R) injury by reducing necrosis, apoptosis, and inflammation. However, extrarenal side effects (bradycardia, hypotension, and sedation) may limit A1AR agonist therapy for ischemic acute kidney injury. Here, we hypothesized that an allosteric enhancer for A1AR (PD-81723) protects against renal I/R injury without the undesirable side effects of systemic A1AR activation by potentiating the cytoprotective effects of renal adenosine generated locally by ischemia. Pretreatment with PD-81723 produced dose-dependent protection against renal I/R injury in A1AR wild-type mice but not in A1AR-deficient mice. Significant reductions in renal tubular necrosis, neutrophil infiltration, and inflammation as well as tubular apoptosis were observed in A1AR wild-type mice treated with PD-81723. Furthermore, PD-81723 decreased apoptotic cell death in human proximal tubule (HK-2) cells in culture, which was attenuated by a specific A1AR antagonist (8-cyclopentyl-1,3-dipropylxanthine). Mechanistically, PD-81723 induced sphingosine kinase (SK)1 mRNA and protein expression in HK-2 cells and in the mouse kidney. Supporting a critical role of SK1 in A1AR allosteric enhancer-mediated renal protection against renal I/R injury, PD-81723 failed to protect SK1-deficient mice against renal I/R injury. Finally, proximal tubule sphingosine-1-phosphate type 1 receptors (S1P1Rs) are critical for PD-81723-induced renal protection, as mice selectively deficient in renal proximal tubule S1P1Rs (S1P1Rflox/flox PEPCKCre/− mice) were not protected against renal I/R injury with PD-81723 treatment. Taken together, our experiments demonstrate potent renal protection with PD-81723 against I/R injury by reducing necrosis, inflammation, and apoptosis through the induction of renal tubular SK1 and activation of proximal tubule S1P1Rs. Our findings imply that selectively enhancing A1AR activation by locally produced renal adenosine may be a clinically useful therapeutic option to attenuate ischemic acute kidney injury without systemic side effects.
Keywords: acute kidney injury, apoptosis, inflammation, necrosis, sphingosine 1-phosphate, sphingosine kinase
acute kidney injury (AKI) is a major clinical problem in the United States and frequently causes prolonged hospitalization, chronic renal failure, and death (24). Renal ischemia-reperfusion (I/R) injury due to surgical renal ischemia or renal hypoperfusion is a leading cause of AKI (20). Ischemic AKI, in particular, is a major clinical problem for patients subjected to major surgical procedures involving the kidney, liver, heart, or aorta, often leading to multiorgan dysfunction and systemic inflammation with extremely high mortality (24). Unfortunately, the severity and incidence of AKI have been increasing without any improvements in therapy or patient survival over the past 50 yr (23). Currently, there are no proven therapies to reduce AKI in the perioperative setting.
We (32, 34) have previously demonstrated that systemic administration of a selective renal A1 adenosine receptor (AR) agonist protected against renal I/R injury in rats and mice with reduced plasma creatinine and dramatically reduced proximal tubular necrosis, inflammation, and apoptosis. Antinecrotic and antiapoptotic effects of renal A1ARs are mediated by activation of ERK, PKB (Akt), and heat shock protein 27 through a pertussis toxin-sensitive G protein pathway (25, 27). Unfortunately, as A1ARs are ubiquitously expressed in many cell types and organs, clinical application of A1AR agonist therapy may be limited by its systemic and undesirable side effects. A1AR agonists produce dose-dependent bradycardia and hypotension as well as central nervous systemic depression and sedation (5). These nonrenal side effects may not be tolerated in patients subjected to ischemic AKI or undergoing major surgical procedures.
The potential side effects of selective A1AR agonists may be mitigated by the use of A1AR-selective allosteric enhancers (5, 26). Allosteric enhancers for A1ARs bind to a site on the receptor that is distinct from the adenosine-binding site (known as the orthosteric site) (5, 7). A1AR allosteric enhancers increase the potency of endogenous adenosine-A1AR interactions, leading to enhanced receptor activity. Since A1AR allosteric enhancers selectively increase the efficacy of endogenous adenosine in tissues (e.g., the ischemic kidney producing increased localized adenosine), systemic administration of A1AR allosteric enhancers may drastically increase renal A1AR activation without producing undesirable systemic side effects.
In this study, we tested the hypothesis that a selective A1AR allosteric enhancer (PD-81723) produces kidney-specific protection against renal I/R injury without producing undesirable systemic side effects (e.g., hypotension or bradycardia) in vivo. We determined whether PD-81723 protects against necrosis and apoptosis in human proximal tubule (HK-2) cells in culture. We also examined the mechanisms of PD-81723-mediated renal tubular protection in vivo and in vitro.
METHODS
Materials.
2-Amino-4,5-dimethyl-3-thienyl-[3-(trifluoromethyl)phenyl] methanone (PD-81723; a selective allosteric enhancer for the A1AR; Fig. 1), 2-chloro-N6-cyclopentyladenosine (CCPA; a selective A1AR agonist), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; a selective antagonist for the A1AR) and 4-{[4-(4-chlorophenyl)-2-thiazolyl]amino}phenol [SKI-II, a selective nonlipid inhibitor of sphingosine kinase (SK)] were purchased from Tocris (Minneapolis, MN). Unless otherwise specified, all other drugs were purchased from Sigma (St. Louis MO).
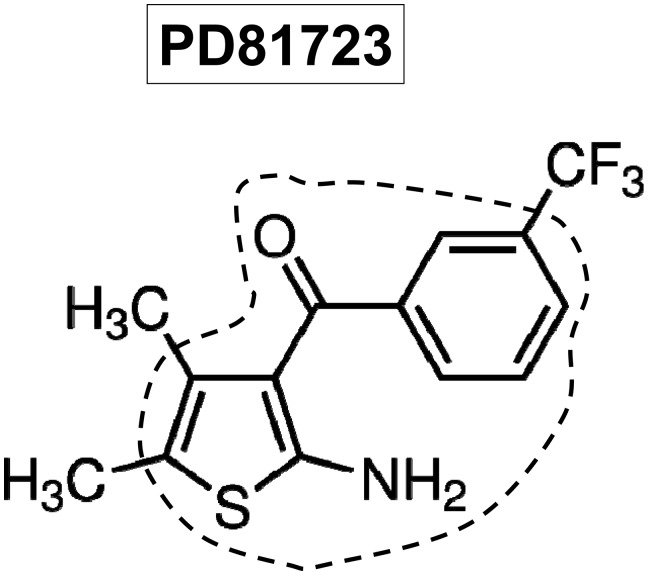
Fig. 1.
Chemical structure of the A1 adenosine receptor (AR) allosteric enhancer 2-amino-4,5-dimethyl-3-thienyl-[3-(trifluoromethyl)phenyl]methanone (PD-81723). Previous structure activity relationship studies have determined that chemicals with a 2-amino-3-benzoylthiophene core (outlined) are excellent A1AR allosteric enhancers.
Murine model of renal I/R injury.
After approval from the Institutional Animal Care and Use Committee, we subjected adult male C57BL/6 (Harlan, Indianapolis, IN) as well as SK1−/− and SK2−/− mice (kindly provided by Dr. R. L. Proia, National Institutes of Health, Bethesda, MD) to 30 min of renal I/R, as previously described (27, 30). To test the renal protective effects of PD-81723, we pretreated mice with vehicle (1% DMSO) or with PD-81723 (0.3–3 mg/kg ip) 15 min before renal ischemia or sham operation. We also tested whether treatment of mice with PD-81723 after the completion of renal ischemia also provides protection against ischemic AKI. Separate cohorts of mice were treated with vehicle or with PD-81723 (3 mg/kg ip) 30 min after reperfusion of the ischemic kidney.
To test whether proximal tubule sphingosine-1-phosphate (S1P) type 1 receptor (S1P1R) activation is critical for PD-81723-mediated renal protection in vivo, we generated mice with proximal tubule-specific deletion of S1P1Rs. We crossed mice carrying the floxed S1P1R gene [S1P1Rff mice, provided by Dr. R. L. Proia, National Institutes of Health (2)] with mice expressing Cre recombinase under the control of phosphoenolpyruvate carboxykinase (PEPCK) promoter [PEPCK-Cre recombinase mice, provided by Dr. Volker Haase, Vanderbilt University, (49)]. S1P1R-floxed mice carrying the PEPCK-Cre gene (S1P1R-null or S1P1Rf/f PEPCKCre/− mice) and control mice [wild-type (WT) or S1P1Rf/f PEPCK−/− mice] were genotyped as previously described (43). We (43) have previously shown that S1P1Rflox/flox PEPCKCre/− mice display drastically reduced (∼10%) S1P1R mRNA in the renal cortex and proximal tubules compared with WT control mice. We also showed that S1P1R mRNA levels in the kidney inner medulla, liver, and lung did not change with proximal tubule S1P1R deletion.
We collected kidneys (cortex and corticomedullary junction) and plasma 6 or 24 h after I/R injury to examine the severity of renal dysfunction (plasma creatinine, renal histology, apoptosis, proinflammatory mRNA expression, and neutrophil infiltration). To test the effects of SK inhibition on PD-81723-mediated renal protection, SKI-II (50 mg/kg sc) was administered 15 min before PD-81723 treatment. SKI-II is an SK-selective inhibitor with minimal effects on other kinases (17). In some mice, we measured systolic blood pressure as well as heart rate to determine the hemodynamic effects of PD-81723 or CCPA administration via an indwelling carotid artery catheter.
HPLC for adenosine.
Mouse kidneys were snap frozen in liquid nitrogen at 5-min intervals immediately after renal ischemia and 30 min after reperfusion. Kidneys were sonicated in 0.6 M perchloric acid and neutralized by the addition of 0.6 M potassium phosphate tribasic. The supernatant was assayed for adenosine by HPLC. Adenosine was quantified on a C18 reverse-phase column with a binary low-pressure gradient elution system with a UV detector set to 254 nm as previously described (14).
Measurement of renal function.
Plasma creatinine was measured as previously described with an enzymatic creatinine reagent kit according to the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA) (50). Unlike the Jaffe method, this method of creatinine measurement largely eliminates the interference from mouse plasma chromagens.
Histological detection of renal necrosis.
Morphological assessment of hematoxylin and eosin staining was performed by an experienced renal pathologist (V. D. D'Agati), who was unaware of the treatment that each animal had received. An established grading scale of necrotic injury (renal injury scores of 0–4) to the proximal tubules was used for the histopathological assessment of I/R-induced damage as outlined by Jablonski et al. (21) and as described in our previous studies (34, 37).
Detection of renal tubular apoptosis.
We detected apoptosis after renal I/R with TUNEL staining as previously described elsewhere (44) using a commercially available in situ cell death detection kit (Roche, Indianapolis, IN) according to the instructions provided by the manufacturer.
Assessment of renal inflammation.
Renal inflammation after I/R injury was determined 1) with detection of neutrophil infiltration with immunohistochemistry 24 h after renal I/R and 2) by measuring mRNA encoding markers of inflammation, including ICAM-1, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-2, and TNF-α. Immunohistochemistry for neutrophils was performed as previously described (11) with a monoclonal antibody against polymorphonuclear neutrophils (clone 7/4). A primary antibody that recognized IgG2a (MCA1212, Serotec, Raleigh, NC) was used as a negative isotype control in all experiments. Semiquantitative RT-PCR assays for proinflammatory mRNAs were performed as previously described (37) (Table 1).
Table 1.
Primers used to amplify mRNAs based on published GenBank sequences for mice
| Sequence |
||||||
|---|---|---|---|---|---|---|
| Primers | Accession Number | Sense | Antisense | Product Size, bp | Cycle Number | Annealing Temperature, °C |
| Mouse TNF-α | X02611 | 5′-TACTGAACTTCGGGGTGATTGGTCC-3′ | 5′-CAGCCTTGTCCCTTGAAGAGAACC-3′ | 290 | 24 | 65 |
| Mouse ICAM-1 | X52264 | 5′-TGTTTCCTGCCTCTGAAGC-3′ | 5′-CTTCGTTTGTGATCCTCCG-3′ | 409 | 21 | 60 |
| Mouse MCP-1 | NM_011333 | 5′-ACCTGCTGCTACTCATTCAC-3′ | 5′-TTGAGGTGGTTGTGGAAAAG-3′ | 312 | 22 | 60 |
| Mouse MIP-2 | X53798 | 5′-CCAAGGGTTGACTTCAAGAAC-3′ | 5′-AGCGAGGCACATCAGGTACG-3′ | 282 | 22 | 60 |
| Mouse SK1 | NM_011451 (variant 1), NM_025367 (variant 2) | 5′-GATGCATGAGGTGGTGAATG-3′ | 5′-GCCCACTGTGAAACGAATC-3′ | 337 | 22 | 64 |
| Mouse SK2 | NM_203280 (variant 1), NM_020011 (variant 2) | 5′-ACTGCTCGCTTCTTCTCTGC-3′ | 5′-ACCATTGAGGGACAGGTCAG-3′ | 437 | 23 | 68 |
| Human SK1 | NM_021972 (variant 1), NM_182965 (variant 2), NM_001142601 (variant 3), NM_001142602 (variant 4) | 5′-ATCTCCTTCACGCTGATGC-3′ | 5′-GTGCAGAGACAGCAGGTTCA-3′ | 330 | 26 | 66 |
| Human SK2 | NM_020126 (variant 1), NM_001204158 (variant 2), NM_001204159 (variant 3), NM_001204160 (variant 4) | 5′-GGAGGAAGCTGTGAAGATGC-3′ | 5′-GCAGGTCAGACACAGAACGA-3′ | 482 | 22 | 66 |
| Mouse and human GAPDH | M32599 | 5′-ACCACAGTCCATGCCATCAC-3′ | 5′-CACCACCCTGTTGCTGTAGCC-3′ | 450 | 15 | 65 |
MCP-1, monocyte chemotactic protein-1; MIP-2, macrophage inflammatory protein-2; SK, sphingosine kinase.
HK-2 cell culture and induction of necrosis and apoptosis.
Necrotic injury in HK-2 cells was induced with exposure to 2 mM H2O2 for 4 h, and lactate dehydrogenase (LDH) released into cell culture media was measured as previously described (33). To induce apoptosis, HK-2 cells were exposed to TNF-α (20 ng/ml) plus cycloheximide (10 μg/ml) for 16 h, as previously described (35). Some HK-2 cells were pretreated with PD-81723 (1–10 μM) 30 min before the induction of necrosis or apoptosis. Separate cohorts of HK-2 cells were treated with PD-81723 (1–10 μM) for 6–16 h to test for the induction of SK1 or SK2 mRNA and protein. Some HK-2 cells were pretreated with 100 nM DPCPX, a selective antagonist for A1ARs, 15 min before PD-81723 treatment.
RT-PCR and immunoblot analyses for SK.
We measured mRNA encoding human SK1 or SK2 6 h after PD-81723 treatment in HK-2 cells as previously described (29). Table 1 shows the primer sequences used in this study. HK-2 cell lysates were also collected for immunoblot analyses of SK1, SK2, and β-actin (an internal protein loading control) as previously described 16 h after PD-81723 treatment (29).
SK activity assay.
SK activity was measured as previously described (28) using a modified protocol according to Vessey et al. (54). To preferentially measure SK1 activity, we supplemented the assay buffer with 0.25 M KCl and 0.5% Triton X-100 (46).
Statistical analysis.
Data were analyzed with Student's t-test when means were compared between two groups or one-way ANOVA plus Tukey's post hoc multiple-comparison test when multiple groups were compared. Two-way ANOVA plus the Bonferroni posttest was used to test the effects of sham operation or renal I/R injury on different mouse strains, treatment groups, and hemodynamic parameters. The ordinal values of the renal injury scores were analyzed by a Mann-Whitney nonparametric test. In all cases, P values of <0.05 were taken to indicate significance. All data are expressed throughout the text as means ± SE.
RESULTS
Adenosine levels after renal ischemia and reperfusion.
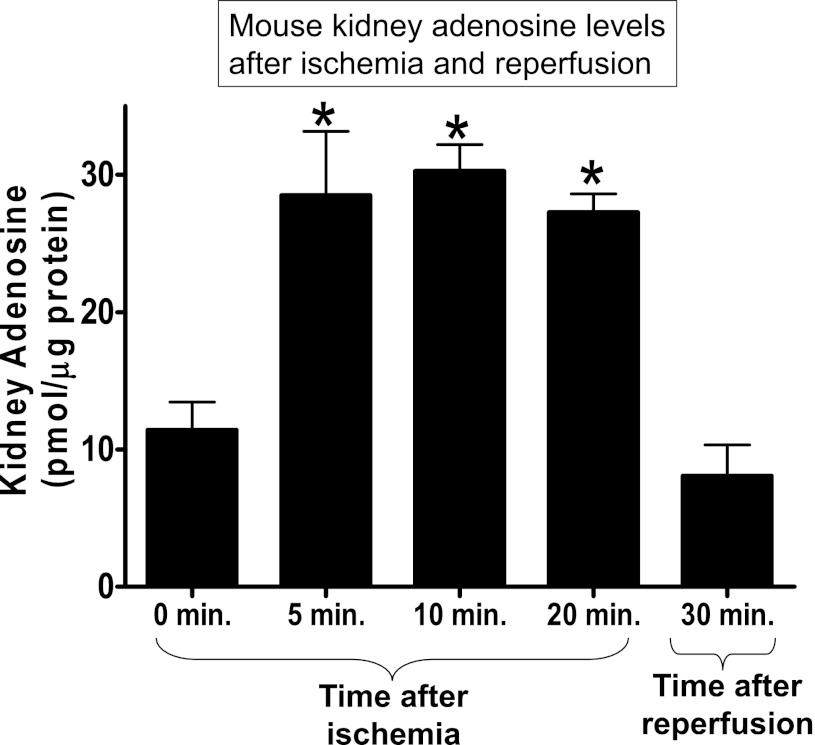
Kidney adenosine levels (in pmol/μg protein) increased within 5 min after renal ischemia compared with preischemic levels (Fig. 2). This increase in renal adenosine levels persisted during 30 min of ischemia. Interestingly, kidney adenosine levels decreased to preischemic levels 30 min after reperfusion of the ischemic kidney. In contrast, we found no changes in cardiac, hepatic, and lung adenosine levels during or after renal ischemia (data not shown).
Fig. 2.
Kidney adenosine levels during renal ischemia and after reperfusion. Kidney adenosine levels were measured by HPLC. Kidneys were snap frozen in liquid nitrogen at the indicated times (n = 3–4 kidneys/time point). Kidney adenosine rapidly increased within 5 min after renal ischemia, and this increase was maintained during ischemia. However, kidney adenosine decreased to preischemic levels 30 min after reperfusion. *P < 0.01 vs. 0 min.
Differential cardiovascular effects of PD-81723 and CCPA.
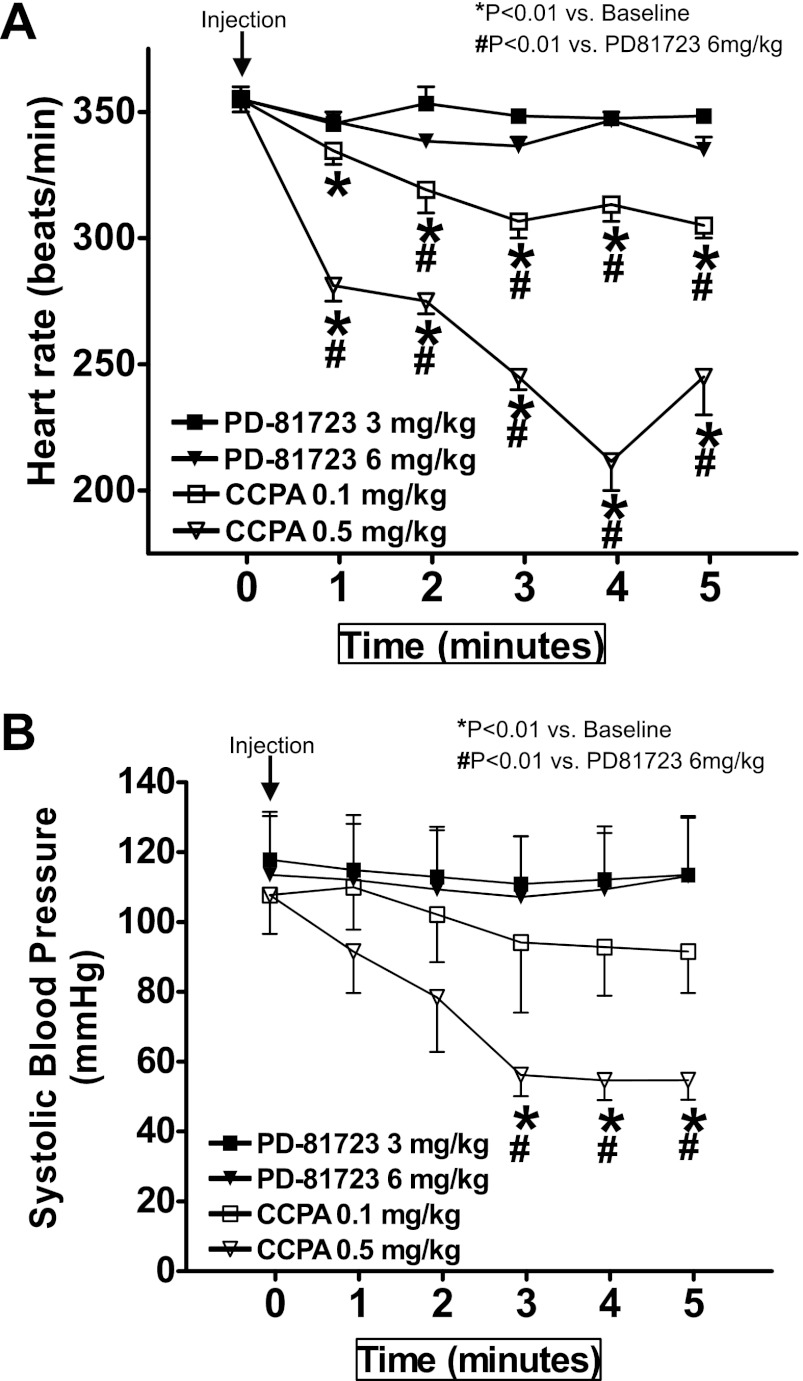
Next, we compared the effects of a selective A1AR allosteric enhancer (PD-81723) and a selective A1AR agonist (CCPA) on mouse heart rate and blood pressure. The results shown in Fig. 3 demonstrate that PD-81723 (3 and 6 mg/kg ip) did not change heart rate or systolic blood pressure in mice. A selective A1AR agonist [CCPA (0.1 and 0.5 mg/kg ip)], in contrast, caused a dose-dependent decrease in heart rate and systolic blood pressure in mice. CCPA at 0.1 mg/kg caused an ∼14% drop in heart rate and an ∼20% decrease in systolic blood pressure. At a higher dose (0.5 mg/kg), the decrease in heart rate and systolic blood pressure with CCPA were significantly greater (Fig. 3).
Fig. 3.
Differential cardiovascular effects of PD-81723 and CCPA. We compared the effects of a selective A1AR allosteric enhancer (PD-81723) and a selective A1AR agonist [2-chloro-N6-cyclopentyladenosine (CCPA)] on mouse heart rate (A) and systolic blood pressure (B). We measured systolic blood pressure as well as heart rate via an indwelling carotid artery catheter. PD-81723 (3 and 6 mg/kg ip) did not significantly change heart or systolic blood pressure in mice (n = 4). In contrast, CCPA (0.1 and 0.5mg/kg ip, n = 4) caused dose-dependent decreases in heart rate and systolic blood pressure in mice. Arrows indicate the time of drug injection. *P < 0.01 vs. baseline; #P < 0.01 vs. mice injected with 6 mg/kg PD-81723.
Renal protective effects of A1AR allosteric enhancer PD-81723 administration.
We initially tested whether PD-81723 pretreatment protects against renal I/R injury in mice. Plasma creatinine values were similar between sham-operated (anesthesia, laparotomy, right nephrectomy, and recovery) vehicle (1% DMSO)-treated (creatinine: 0.48 ± 0.03 mg/dl, n = 3), and PD-81723-treated (creatinine: 0.43 ± 0.03 mg/dl, n = 3) mice. Plasma creatinine significantly increased in vehicle-treated mice subjected to 30 min of renal I/R compared with sham-operated mice (Fig. 4). Pretreatment with PD-81723 (15 min before renal ischemia) significantly and dose dependently attenuated the increases in plasma creatinine in mice (Fig. 4). Further verifying that PD-81723 mediates renal protection via enhanced activation of A1AR, mice deficient in A1ARs [A1AR knockout (A1KO) mice] were not protected against renal I/R with PD-81723 treatment. We also tested whether PD-81723 treatment after renal reperfusion (after completion of renal ischemia) protected against renal I/R injury. We determined that unlike pretreatment, PD-81723 given 30 min after I/R (creatinine: 2.2 ± 0.2 mg/dl, n = 4) failed to protect the kidney.
Fig. 4.
Renal protection with PD-81723 pretreatment. Plasma creatinine levels from A1AR wild-type (WT) or A1AR knockout (KO) mice subjected to sham surgery or to renal ischemia-reperfusion (I/R) are shown. A1WT mice treated with 0.3–3 mg/kg PD-81723 15 min before renal ischemia were protected against renal I/R injury (n = 6 mice/group). PD-81723 significantly and dose dependently attenuated the increases in plasma creatinine after renal I/R in A1WT mice. In contrast, PD-81723 failed to protect against renal injury in A1KO mice. Values are means ± SE. *P < 0.05 vs. vehicle-treated A1WT or A1KO mice subjected to sham surgery; #P < 0.05 vs. vehicle-treated A1WT mice subjected to renal I/R.
In addition to plasma creatinine, we also examined renal histology after I/R. The results shown in Fig. 5A demonstrate severe necrotic renal injury in vehicle-treated A1AR WT (A1WT) mice subjected to I/R 24 h after injury. Compared with sham-operated vehicle-treated A1WT mice (not shown), the kidneys of vehicle-treated A1WT mice subjected to renal I/R showed significant tubular necrosis (proteinaceous casts with increased congestion; Fig. 5A). Consistent with the plasma creatinine data, mice treated with PD-81723 15 min before renal ischemia had reduced renal necrosis and tubular injury (Fig. 5A). In contrast, PD-81723-treated A1KO mice showed similar degrees of renal tubular necrosis and cast formation compared with vehicle-treated A1KO mice. The Jablonski scale (21) renal injury score was used to grade renal tubular necrosis 24 h after renal I/R (Fig. 5B). Thirty minutes of renal ischemia and 24 h of reperfusion resulted in severe acute tubular necrosis in vehicle-treated A1WT or A1KO mice. Consistent with the renal histology data, PD-81723 reduced the renal injury score in A1WT mice but not in A1KO mice.
Fig. 5.
PD-81723 reduces kidney necrosis (A and B), apoptosis (C), neutrophil infiltration (D), and proinflammatory gene expression (E) in A1WT mice after renal I/R. A, C, and D: representative photomicrographs for hematoxylin and eosin (H&E) staining (A; magnification: ×200), TUNEL staining (representing apoptotic nuclei; C; magnification: ×200), and immunohistochemistry for neutrophil infiltration (D; magnification: ×400) of kidney sections of mice. Mice were pretreated with vehicle (1% DMSO) or with 3 mg/kg PD-81723 and subjected to 30 min of renal ischemia and 24 h of reperfusion. Photographs are representative of 4–5 independent experiments. B: summary of Jablonski scale renal injury scores (graded from H&E staining, scale: 0–4) for mice subjected to renal I/R. Vehicle-treated mice showed severe renal tubular necrosis, apoptosis, and neutrophil infiltration after I/R. Preischemic PD-81723 treatment significantly attenuated renal I/R injury in A1WT mice but did not protect A1KO mice. Values are means ± SE. #P < 0.05 vs. vehicle-treated A1WT mice subjected to renal I/R. E: representative gel images (top) of RT-PCR and densitometric quantification of relative band intensities normalized to GAPDH (bottom) of proinflammatory markers [ICAM-1, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-2, and TNF-α] from renal cortices of A1WT mouse kidneys (n = 4–5 mice/group). Mice were treated with vehicle or with PD-81723 (3 mg/kg) and subjected to sham operation or to renal I/R (6 h of reperfusion). PD-81723 selectively reduced expression of TNF-α and MCP-1 without affecting MIP-2 or ICAM-1 expression. Values are means ± SE. *P < 0.05 vs. the vehicle-treated sham-operated group; #P < 0.05 vs. the vehicle-treat I/R group. Data were analyzed with one-way ANOVA plus a Tukey post hoc multiple-comparison test.
PD-81723 treatment reduces renal apoptosis after I/R.
TUNEL staining detected apoptotic renal cells in the kidneys of mice subjected to renal I/R where proximal tubule cell apoptosis was predominant. Renal ischemia and 24 h of reperfusion resulted in significant apoptosis in the kidneys of vehicle-treated A1WT mice. PD-81723 pretreatment (3 mg/kg ip 15 min before renal ischemia) reduced the number of apoptotic TUNEL-positive cells in the A1WT mouse kidney without decreasing apoptosis in the A1KO mouse kidney (Fig. 5C; magnification: ×200).
PD-81723 treatment reduces kidney neutrophil infiltration after I/R.
Figure 5D shows representative images of neutrophil immunohistochemistry of the kidney (magnification: ×400) from mice subjected to 30 min of renal ischemia and 24 h of reperfusion. There was significant neutrophil infiltration in the kidneys of vehicle-treated A1WT and A1KO mice subjected to 24 h of renal I/R. Stained neutrophils appeared as dark brown (Fig. 5D). In sham-operated mice, we were unable to detect any neutrophils in the kidney (data not shown). A1WT mice treated with PD-81723 (3 mg/kg ip) 15 min before renal ischemia had reduced neutrophil infiltration in the kidney after I/R. Again, PD-81723 failed to reduce neutrophil infiltration in A1KO mice.
PD-81723 reduces kidney TNF-α and MCP-1 expression after renal I/R.
Renal I/R injury resulted in significantly increased renal proinflammatory ICAM-1, MCP-1, MIP-2, and TNF-α mRNA expression in the kidney within 6 h compared with sham-operated mice (Fig. 5E). PD-81723 pretreatment (3 mg/kg ip 15 min before renal ischemia) selectively reduced the renal expression of TNF-α and MCP-1 compared with vehicle-treated A1WT mice without affecting ICAM-1 or MIP-2 expression after renal I/R.
Acute PD-81723 reduces human renal proximal tubule cell apoptosis without affecting necrosis.
Acute PD-81723 pretreatment protected against HK-2 cell apoptosis. HK-2 cells pretreated with 10 μM PD-81723 were protected against TNF-α plus cycloheximide-induced apoptosis, as evidenced by reduced poly(ADP-ribose) polymerase (PARP) and caspase-3 fragmentation (Fig. 6, A and B) as well as decreased DNA laddering (Fig. 6C). We also tested whether PD-81723 prevented HK-2 cell apoptosis via activation of A1ARs. DPCPX (100 nM) significantly attenuated PARP and caspase-3 fragmentation (Fig. 6, A and B) and prevented the reduction in DNA laddering (Fig. 6C) in HK-2 cells.
Fig. 6.
PD-81723 reduces apoptosis in human renal proximal tubule (HK-2) cells. HK-2 cell apoptosis was induced by TNF-α (20 ng/ml) and cycloheximide (CHX; 10 μg/ml). A: representative poly(ADP-ribose) polymerase (PARP) and caspase-3 fragmentation as indexes of HK-2 cell apoptosis (representative images from 6 experiments). PD-81723 (10 μM) or vehicle (1% DMSO) was applied 30 min before the induction of apoptosis, and cells were harvested 16 h later. The selective A1AR antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 100 nM) prevented this reduction in HK-2 cell apoptosis. B: densitometric quantifications of cleaved PARP (n = 6) and caspase-3 (n = 6) in HK-2 cells. Values are means ± SE. *P < 0.05 vs. vehicle; #P < 0.05 vs. PD-81723. C: representative gel image demonstrating DNA laddering as another index of apoptosis in HK-2 cells. PD-81723 (10 μM) applied 30 min before the induction of apoptosis reduced laddering in HK-2 cells. DPCPX (100 nM) again prevented this reduction in HK-2 cell apoptosis. Photographs are representative of 4 independent experiments.
However, acute PD-81723 (1–10 μM) treatment (30 min before H2O2 treatment) had no effect on HK-2 cell necrosis (measured with LDH released) induced with 2 mM H2O2 for 4 h (LDH in the 10 μM PD-81723-treated group: 25.4 ± 2%, n = 4, vs. LDH in the vehicle-treated group: 26.3 ± 3%, n = 4). Interestingly, when HK-2 cells were pretreated with 10 μM PD-81723 4 h before H2O2 treatment, HK-2 cells were protected against necrosis and released significantly less LDH (LDH in the PD-81723 treated group: 30.6 ± 2%, n = 4, vs. LDH in the vehicle-treated group: 43 ± 3%, n = 4, P < 0.05).
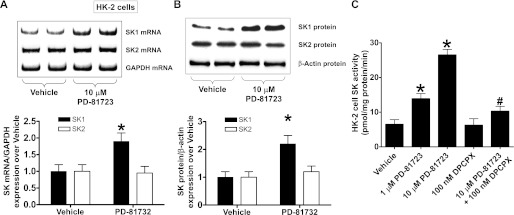
PD-81723 increases SK1 synthesis and induces SK activity in HK-2 cells.
Next, we determined the mechanisms of PD-81723-mediated renal protection. Since we (43) have recently shown that a selective A1AR agonist (CCPA) induced SK1 in HK-2 cells and in the mouse kidney, we tested the hypothesis that PD-81723 may also induce SK1 in renal proximal tubules. HK-2 cells were treated with 10 μM PD-81723 for SK mRNA (6 h) or protein (16 h) analysis. We determined that PD-81723 selectively increased SK1 mRNA and protein expression in HK-2 cells (Fig. 7, A and B). SK2 mRNA or protein expression did not change. Furthermore, PD-81723 treatment for 6 h increased SK activity in HK-2 cells (Fig. 7C). We preferentially measured SK1 activity by adding Triton X-100 in our SK activity assay. A selective A1AR antagonist (100 nM DPPCX) significantly attenuated the PD-81723-induced increases in SK activity (Fig. 7C).
Fig. 7.
PD-81723 induces sphingosine kinase (SK)1 expression and activity in HK-2 cells. A and B: representative images (top) and relative normalized band intensities (bottom) for SK mRNA (by RT-PCR; A) and protein (by immunoblot analysis; B) expression in HK-2 cells treated with vehicle (1% DMSO) or 10 μM PD-81723 (n = 4 per group). HK-2 cells were treated for 6 h (mRNA analysis) or 16 h (protein analysis). Note that PD-81723 increased SK1 mRNA and protein expression in HK-2 cells but did not change SK2 expression. C: SK activity in HK-2 cells treated with vehicle or with 1–10 μM PD-81723 6 h (n = 4 per group). PD-81723 caused a dose-dependent increase in SK activity in HK-2 cells. We preferentially measured SK1 activity by the addition of Triton X-100 in our SK activity assay. A selective A1AR antagonist (100 nM DPPCX) significantly attenuated the PD-81723-induced increase in SK activity. Values are means ± SE. *P < 0.05 vs. vehicle; #P < 0.05 vs. PD-81723.
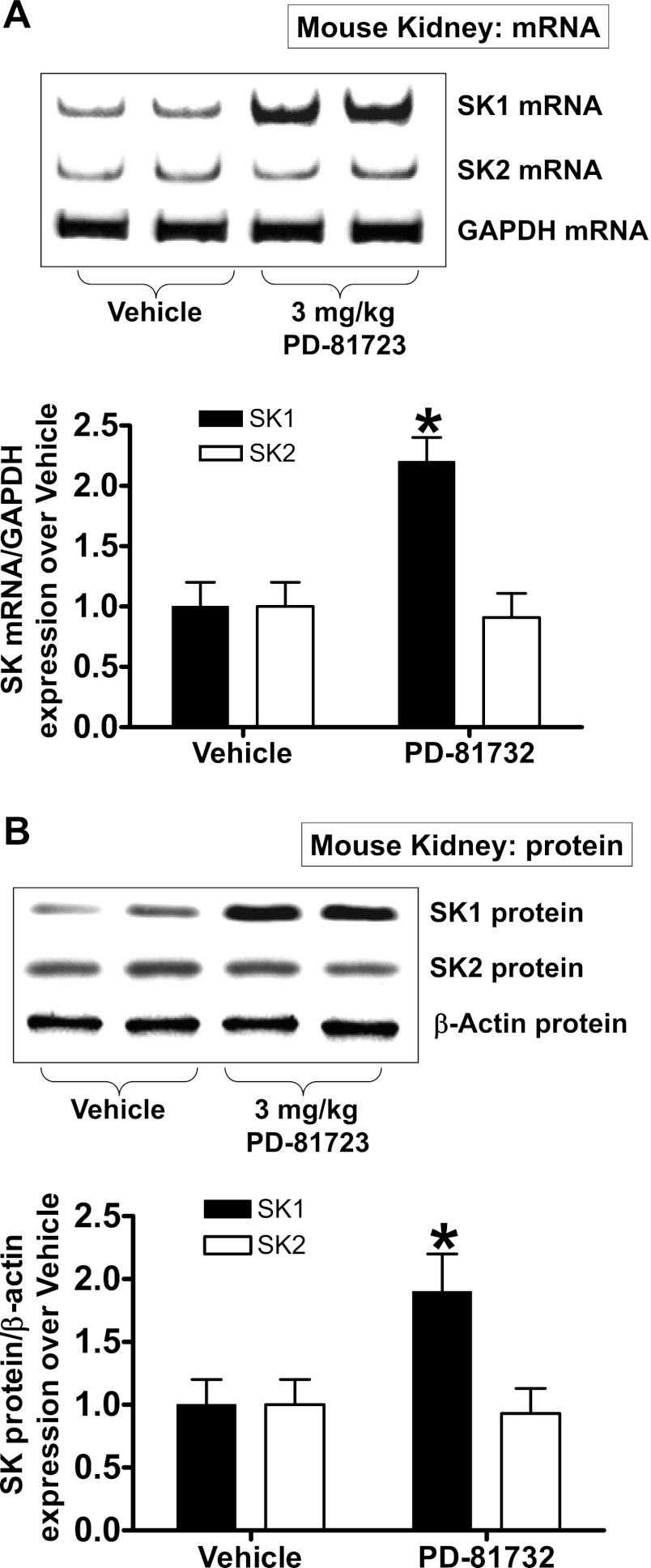
PD-81723 increases SK1 mRNA and protein synthesis in the mouse kidney.
PD-81723 also increased SK1 mRNA and protein expression in the mouse kidney. Figure 8 shows selective SK1 mRNA (A) and protein (B) induction in the kidneys of mice injected with 3 mg/kg ip 6 h after PD-81723 treatment.
Fig. 8.
PD-81723 induces renal SK1 in mice. A and B: representative images (top) and relative normalized band intensities (bottom) for kidney SK mRNA (by RT-PCR; A) and protein (by immunoblot analysis; B) expression after vehicle (1% DMSO) or PD-81723 (3 mg/kg ip) treatment (n = 4 per group). Kidneys were isolated 6 h after treatment. PD-81723 increased SK1 mRNA and protein expression in HK-2 cells but did not change SK2 expression. Values are means ± SE. *P < 0.05 vs. vehicle.
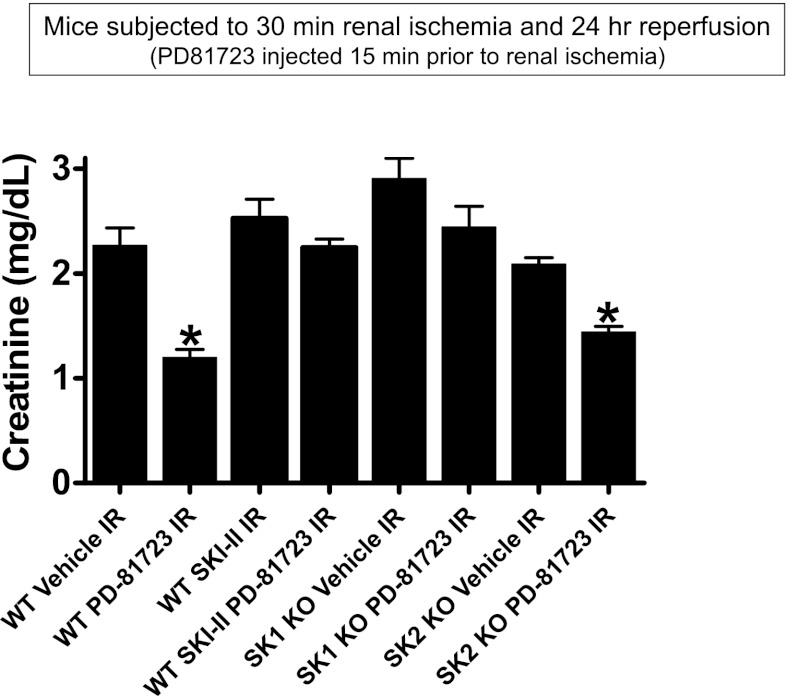
Critical role of SK1 in PD-81723-mediated renal protection against I/R.
We next tested whether PD-81723 requires the induction of SK1 for renal protection. PD-81723 (3 mg/kg ip)-induced renal protection was abolished in A1WT mice pretreated with SKI-II (a selective SK inhibitor, 50 mg/kg, sc; Fig. 9). Furthermore, we determined that SK1−/− mice were not protected with PD-81723 treatment, whereas SK2−/− mice were significantly protected against renal I/R with PD-81723 treatment (Fig. 9).
Fig. 9.
Critical role for SK1 in PD-81723-mediated renal protection against I/R. Plasma creatinine levels in vehicle (1% DMSO)-treated or PD-81723 (3 mg/kg)-treated mice subjected to sham surgery (n = 4 mice/group) or to renal I/R (n = 6 mice/group) are shown. WT mice treated with a potent SK inhibitor (SKI-II; 50 mg/kg sc 15 min before ischemia) were not protected against renal I/R injury with PD-81723 treatment. Moreover, mice deficient in SK1 were not protected against renal I/R injury with PD-87123. In contrast, SK2-deficient mice were protected against renal I/R with PD-81723 treatment. Values are means ± SE. *P < 0.05 vs. vehicle-treated mice to subjected to renal I/R.
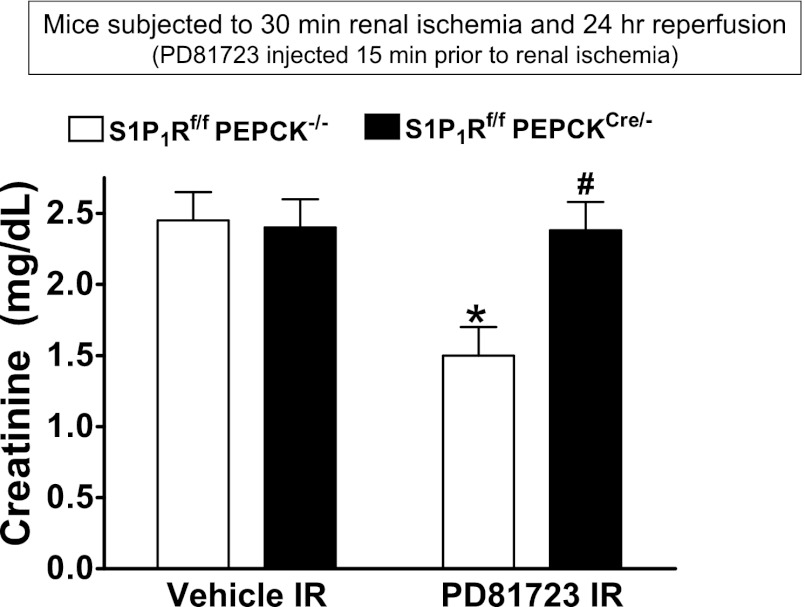
Critical role of renal proximal tubule S1P1Rs in PD-81723-mediated renal protection.
To test whether proximal tubule S1P1R activation is critical for PD-81723-mediated renal protection, we generated mice with proximal tubule-specific deletion of S1P1Rs by crossing mice carrying the floxed S1P1R gene with PEPCKCre recombinase mice (2, 49). Figure 10 shows plasma creatinine from WT (S1P1Rf/f PEPCK−/−) mice and proximal tubule-specific S1P1R-null (S1P1Rf/f PEPCKCre/−) mice subjected to 30 min of renal ischemia and 24 h of reperfusion. Treatment with PD-81723 (3 mg/kg ip 15 min before renal ischemia) protected WT mice but failed to protect proximal tubule-specific S1P1R-null mice from renal I/R injury.
Fig. 10.
Critical role of renal proximal tubule sphingosine-1-phosphate type 1 receptors (S1P1Rs) in PD-81723-mediated protection against renal I/R injury. Plasma creatinine from WT (S1P1Rf/f PEPCK−/−) mice or from proximal tubule-specific S1P1R-null (S1P1Rf/f PEPCKCre/−) mice subjected to 30 min of renal ischemia and 24 h of reperfusion are shown. Treatment with an A1AR agonist (PD-81723, 3 mg/kg ip 15 min before renal ischemia) protected S1P1Rf/f PEPCK−/− mice but failed to reduce plasma creatinine levels in S1P1Rf/f PEPCKCre/− mice subjected to renal I/R. *P < 0.05 vs. vehicle-treated S1P1Rf/f PEPCK−/− mice; #P < 0.05 vs. PD-81723-treated S1P1Rf/f PEPCK−/− mice.
DISCUSSION
The major new findings of our study are that 1) a selective allosteric enhancer for A1ARs (PD-81723) dose dependently attenuated renal I/R injury in vivo; 2) PD-81723 also attenuated HK-2 cell apoptosis in vitro; 3) this in vivo and in vitro protection afforded by PD-81723 was directly mediated via activation of A1ARs; 4) mechanistically, PD-81723 selectively induced SK1 in HK-2 cells and in mouse kidneys; and 5) mice deficient in SK1 were not protected against renal I/R injury with PD-81723 treatment.
Renal I/R injury creates a cascade of renal tubular necrosis, apoptosis, and inflammation (8). We (25, 32, 34) have previously demonstrated that renal A1AR activation protected against I/R injury in mice and rats. The A1AR-mediated protection against ischemic AKI was characterized by significant reductions in renal necrosis, apoptosis, and inflammation (34). Although A1AR agonist-mediated protection against ischemic AKI has significant therapeutic potential, the nonrenal A1AR effects (e.g., A1AR effects on the heart and brain) may produce undesirable systemic side effects, limiting its clinical applications (5). The present study demonstrates powerful and dose-dependent protective effects of a selective A1AR allosteric enhancer, PD-81723, against renal I/R injury in mice. Specifically, we showed that PD-81723 attenuated renal histological injury (Jablonski renal injury scores) and apoptosis (TUNEL staining and PARP/caspase-3 fragmentation). Furthermore, we demonstrated a significantly reduced influx of proinflammatory neutrophils after renal I/R in PD-81723-treated mouse kidneys. Therefore, exogenous administration of PD-81723 provides powerful renal protection against ischemic AKI by targeting all three pathways of cell death in vivo. Few studies have explored the tissue protective role of A1AR allosteric enhancers. Mizumura et al. (40) also showed that PD-81723 reduced the preconditioning threshold in the heart. Furthermore, hippocampal injury and behavioral recovery were improved by PD-81723 after cerebral ischemia (39). The renal protective effects of PD-81723 are mediated by A1ARs, as a selective A1AR antagonist (DPCPX) attenuated the induction of SK1 and mice deficient in A1ARs were not protected with PD-81723.
We aimed to test A1AR-based therapeutic strategies to improve renal function after I/R injury without the potential systemic side effects (e.g., bradycardia, hypotension, and sedation) that may limit A1AR agonist-based therapeutic strategies. The selective A1AR allosteric enhancer PD-81723 acts to increase agonist binding to A1ARs and increases A1AR-mediated effects in many cell types (5, 7). PD-81723 increases radiolabeled adenosine ligand binding and coupling in rat, guinea pig, and human tissues. We postulated that locally increased renal adenosine levels after renal ischemia coupled with an A1AR allosteric enhancer will allow for enhanced renal A1AR activation. Anticipating higher localized renal adenosine levels after renal I/R, we tested the hypothesis that a selective A1AR allosteric enhancer (PD-81723) will produce kidney-specific protection against renal I/R injury without undesirable systemic side effects in vivo. We demonstrated, in this study, that in vivo renal protection with PD-81723 occurred without significant effects on heart rate or blood pressure (potential systemic effects of A1AR activation in the heart) in mice.
We and others (18, 32, 34, 36, 37, 41, 42) have previously shown differential effects of AR subtype activation against ischemic AKI . Selective A1, A2a, or A2bAR activation produces renal protection, whereas A3AR activation in the kidney was detrimental against ischemic AKI. Therefore, although renal adenosine levels rise rapidly (∼3-fold) during ischemia, this increase may not selectively activate cytoprotective ARs to produce endogenous renal protection. Using an allosteric modulator for A1ARs would use this increase in adenosine for selective activation of cytoprotective renal A1ARs. We also determined that PD-81723 given 30 min after the completion of renal ischemia failed to protect against renal I/R injury. This is consistent with our findings showing that renal adenosine levels fell rapidly after reperfusion to near preischemic levels after reperfusion. Therefore, our data confirm that PD-81723-mediated renal protection must be coupled with increased tissue adenosine in the ischemic organ for protection to occur.
Clinically, pretreatment is an important and valuable option for the frequent clinical scenario where patients are subjected to renal ischemia or hypoperfusion during surgical procedures (e.g., kidney transplantation, open heart surgery, aortovascular surgery, or liver transplantation). During the perioperative period, renal injury can be frequently anticipated before surgery, and a selective A1AR allosteric enhancer can be directly infused into the kidney or intravenously. A1AR allosteric enhancer-based pretreatment therapies may reduce ischemia AKI in a wide range of patients subjected to major surgical procedures.
Upregulation of proinflammatory cytokines and adhesion molecules after renal I/R contributes to the pathophysiology of kidney injury (12). Therefore, we determined whether PD-81723 attenuates ischemic AKI by modulating renal proinflammatory mRNA expression. We showed that, as expected, all of the proinflammatory mRNAs examined (TNF-α, MCP-1, MIP-2, and ICAM-1) showed enhanced expression in the kidney after I/R. We found that PD-81723 selectively reduced the expression of TNF-α and MCP-1 without changing ICAM-1 or MIP-2 expression. TNF-α is a well-known mediator of kidney injury after I/R and upregulates early to mediate neutrophil infiltration after renal I/R (15, 16). TNF-α promotes the migration of inflammatory cells into the kidney by upregulating additional cytokines and adhesion molecules (e.g., MCP-1, ICAM-1, and MIP-2) (15, 16, 48). MCP-1 is also an important mediator in the pathogenesis of AKI (47, 51). Upregulation of MCP-1 leads to neutrophil recruitment during AKI induced by renal I/R or nephrotoxic agents. Indeed, our study showed that renal I/R-induced neutrophil infiltration was significantly attenuated by PD-81723 pretreatment, consistent with targeting of these proinflammatory and neutrophil attracting cytokines. The role of neutrophils in the pathophysiology of organ injury (including in the liver and kidney) is well recognized (13). MCP-1 also acts as a chemoattractant for monocytes and lymphocytes to areas of injury (47, 51). Monocytes and lymphocytes play critical roles in the pathogenesis of renal I/R (3, 12).
Our data showed that PD-81723 produces renal protection by direct induction of renal tubular SK1 via enhanced activation of A1ARs as 1) PD-81723 induced SK1 in HK-2 cells and in mouse kidneys, 2) the PD-81723-mediated induction in HK-2 cell SK activity was blocked with a selective A1AR antagonist (DPCPX), and 3) mice deficient in SK1 were not protected against renal I/R injury with PD-81723. PD-81723-mediated induction of SK1 has never been previously described, and our data may provide a novel mechanistic cytoprotective mechanism triggered by PD-81723 administration. However, our present data cannot rule out the induction of SK1 in other nephron segments with PD-81723 treatment.
SK is a multifunctional lipid kinase that phosphorylates sphingosine to form S1P. Of the two forms of SK, SK1 is a cytosolic enzyme that migrates to the plasma membrane or to the nucleus upon activation (19, 31). SK1 is a well-known mediator of tissue protection (including protection against I/R injury), growth, and survival (38). SK1 overexpression protects against acute lung and kidney injury (45, 56). Furthermore, SK1 activation plays a potent cytoprotective role, as SK1-deficient cardiomyocytes had increased injury after ischemia (55). We and others (4, 22, 28, 29) have previously demonstrated a renal protective role of SK1 as well as S1P1 receptor activation. Overall, activation of SK1 has been shown to produce antinecrotic, anti-inflammatory, and antiapoptotic effects. Our finding that PD-81723 induced SK1 is consistent with our recent finding that a selective A1AR agonist (CCPA) induced SK1 in the mouse kidney and in HK-2 cells (43). It remains to be tested whether PD-81723 induces SK1 in other cell types.
Our experiments also showed that PD-81723 treatment not only induces SK1 but that its renal protective effects are dependent on SK1, as mice deficient in the SK1 enzyme were not protected against renal I/R injury with PD-81723 treatment. In addition, renal proximal tubule S1P1Rs are required for PD-81723-mediated renal protection. S1P is a potent lipid signaling molecule that activates five known S1P receptors to regulate diverse biological effects, including cell growth, survival, and modulation of inflammation (1, 10, 53). S1P1R activation, in particular, has been shown to produce cytoprotective effects, including reducing T lymphocyte-mediated inflammation, strengthening endothelial vascular permeability and producing direct renal tubular protection (2, 4). Indeed, direct S1P1R activation with a selective agonist (4) or enhanced activation of S1P1Rs secondary to increased SK1 activity (28) produces protection against renal I/R injury in mice. Collectively, our data suggest that PD-81723-mediated SK1 induction enhances the synthesis of endogenous S1P in the kidney to activate renal tubular S1P1Rs.
Although protective against HK-2 cell apoptosis, acute PD-81723 treatment failed to attenuate HK-2 cell necrosis in vitro. This is in contrast to the in vivo protective effects of acute PD-81723 administration, where we observed reduced necrosis as well as apoptosis against ischemic AKI. Differences in models of cell death (H2O2 vs. renal I/R) may explain the discrepancies between our in vitro and in vivo effects of PD-81723 against necrosis. However, since 4 h of pretreatment with PD-81723 did produce protection against necrosis, a more likely explanation is that PD-81723-mediated protection against cell death requires the induction of a downstream cytoprotective factor (such as SK1).
One of the limitations of this study is that we used an enzymatic creatinine assay method that overestimates plasma creatinine values, especially when the values are low (e.g., in sham-operated mice). Previous studies by Yuen et al. (57) and Takahashi et al. (52) have shown that enzymatic creatinine assays overestimate plasma creatinine. Indeed, the plasma creatinine values obtained from our sham-operated mice (∼0.4 mg/dl) were higher than the values obtained with either tandem mass spectrometry (∼0.07 mg/dl) or HPLC (∼0.1–0.2 mg/dl) (52, 57). However, mice subjected to renal I/R and treated with vehicle or with an A1AR allosteric enhancer had plasma creatinine values of ∼2.3 and ∼1.2 mg/dl, respectively. The degree of overestimation by enzymatic creatinine assay is reduced at plasma creatinine values above 0.5 mg/dl (57). Furthermore, the degree of overestimation would be similar for both vehicle- and PD-81723-treated mice subjected to renal I/R.
Detailed structure activity relationship studies (6, 9) to develop a potent A1AR allosteric enhancer have been previously reported. The common structural theme that has emerged from these studies is that chemicals with a 2-amino-3-benzoylthiophene core are excellent A1AR allosteric enhancers. Furthermore, Bruns et al. (9) demonstrated that an unsubstituted 2-amino group and an adjacent ketone group are important for the potency of the allosteric enhancers. Subsequent structure activity relationship studies revealed that modification of the benzoyl moiety at the 3-position of the thiopentene ring with chlorine or trifluromethyl chlorine moiety resulted in enhanced allosteric actions.
In summary, our findings of PD-81723-mediated SK1 induction leading to reduced renal injury represent a potential new direction in A1AR allosteric enhancer therapy for ischemia AKI. Our experiments may lead to new therapeutic approaches with a drug that can reduce all three pathways of renal cell death (necrosis, apoptosis, and inflammation) to lessen the clinical perils from AKI and have implications in organ protection strategies beyond the kidney. These therapeutic approaches may be able to protect the kidney against impending but anticipated renal I/R injury during the perioperative period.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK-058547 and GM-06708.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.W.P. and H.T.L. conception and design of research; S.W.P., J.Y.K., A.H., K.M.B., M.K., and H.T.L. performed experiments; S.W.P., J.Y.K., A.H., K.M.B., M.K., V.D.D., and H.T.L. analyzed data; S.W.P., J.Y.K., A.H., K.M.B., M.K., V.D.D., and H.T.L. interpreted results of experiments; S.W.P., J.Y.K., A.H., K.M.B., M.K., and H.T.L. prepared figures; S.W.P. and H.T.L. edited and revised manuscript; S.W.P., J.Y.K., A.H., K.M.B., M.K., V.D.D., and H.T.L. approved final version of manuscript; H.T.L. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank R. L. Proia (National Institute of Diabetes and Digestive and Kidney Diseases) for providing the SK1−/− and SK2−/− mice.
REFERENCES
- 1.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 279: 15396–15401, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102: 3665–3667, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, Soloski MJ, Rabb H. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol 177: 3380–3387, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baraldi PG, Iaconinoto MA, Moorman AR, Carrion MD, Cara CL, Preti D, Lopez OC, Fruttarolo F, Tabrizi MA, Romagnoli R. Allosteric enhancers for A1 adenosine receptor. Mini Rev Med Chem 7: 559–569, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Baraldi PG, Romagnoli R, Pavani MG, Nunez MC, Tabrizi MA, Shryock JC, Leung E, Moorman AR, Uluoglu C, Iannotta V, Merighi S, Borea PA. Synthesis and biological effects of novel 2-amino-3-naphthoylthiophenes as allosteric enhancers of the A1 adenosine receptor. J Med Chem 46: 794–809, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Linden J. The allosteric enhancer, PD 81,723, stabilizes human A1 adenosine receptor coupling to G proteins. Biochim Biophys Acta 1265: 15–21, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bruns RF, Fergus JH, Coughenour LL, Courtland GG, Pugsley TA, Dodd JH, Tinney FJ. Structure-activity relationships for enhancement of adenosine A1 receptor binding by 2-amino-3-benzoylthiophenes. Mol Pharmacol 38: 950–958, 1990 [PubMed] [Google Scholar]
- 10.Chae SS, Proia RL, Hla T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat 73: 141–150, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Chen SW, Kim M, Kim M, Song JH, Park SW, Wells D, Brown K, Belleroche JD, D'Agati VD, Lee HT. Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion. Kidney Int 75: 499–510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daemen MA, de Vries B, Buurman WA. Apoptosis and inflammation in renal reperfusion injury. Transplantation 73: 1693–1700, 2002 [DOI] [PubMed] [Google Scholar]
- 13.De Greef KE, Ysebaert DK, Ghielli M, Vercauteren S, Nouwen EJ, Eyskens EJ, De Broe ME. Neutrophils and acute ischemia-reperfusion injury. J Nephrol 11: 110–122, 1998 [PubMed] [Google Scholar]
- 14.Delabar U, Kloor D, Luippold G, Muhlbauer B. Simultaneous determination of adenosine, S-adenosylhomocysteine and S-adenosylmethionine in biological samples using solid-phase extraction and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 724: 231–238, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Donnahoo KK, Meldrum DR, Shenkar R, Chung CS, Abraham E, Harken AH. Early renal ischemia, with or without reperfusion, activates NFκB and increases TNF-α bioactivity in the kidney. J Urol 163: 1328–1332, 2000 [PubMed] [Google Scholar]
- 16.Donnahoo KK, Meng X, Ayala A, Cain MP, Harken AH, Meldrum DR. Early kidney TNF-α expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol Regul Integr Comp Physiol 277: R922–R929, 1999 [DOI] [PubMed] [Google Scholar]
- 17.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res 63: 5962–5969, 2003 [PubMed] [Google Scholar]
- 18.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5: e137, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta 1758: 2016–2026, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Ikeda M, Prachasilchai W, Burne-Taney MJ, Rabb H, Yokota-Ikeda N. Ischemic acute tubular necrosis models and drug discovery: a focus on cellular inflammation. Drug Discov Today 11: 364–370, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Jablonski P, Howden BO, Rae DA, Birrel CS, Marshall VC, Tange J. An experimental model for assessment of renal recovery from warm ischemia. Transplantation 35: 198–204, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Jo SK, Bajwa A, Ye H, Vergis AL, Awad AS, Kharel Y, Lynch KR, Okusa MD. Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int 75: 167–175, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol 2: 356–365, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol 22: 193–208, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Joo JD, Kim M, Horst P, Kim J, D'Agati VD, Emala CW, Sr, Lee HT. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol 293: F1847–F1857, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kiesman WF, Elzein E, Zablocki J. A1 adenosine receptor antagonists, agonists, and allosteric enhancers. Handb Exp Pharmacol: 25–58, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Kim M, Chen SW, Park SW, Kim M, D'Agati VD, Yang J, Lee HT. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int 75: 809–823, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M, Kim M, Kim N, D'Agati VD, Emala CW, Sr, Lee HT. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol 293: F1827–F1835, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kim M, Kim M, Park SW, Pitson SM, Lee HT. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol 31: 353–362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim M, Park SW, Kim M, Chen SW, Gerthoffer WT, D'Agati VD, Lee HT. Selective renal overexpression of human heat shock protein 27 reduces renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 299: F347–F358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leclercq TM, Pitson SM. Cellular signalling by sphingosine kinase and sphingosine 1-phosphate. IUBMB Life 58: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A1 and A3 receptors. Am J Physiol Renal Physiol 278: F380–F387, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Lee HT, Emala CW. Adenosine attenuates oxidant injury in human kidney proximal tubular cells via A1 and A2a adenosine receptor activation. Am J Physiol Renal Physiol 282: F844–F852, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15: 102–111, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Lee HT, Kim M, Jan M, Penn RB, Emala CW. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int 71: 1249–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Lee HT, Ota-Setlik A, Xu H, D'Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Renal Physiol 284: F267–F273, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol 286: F298–F306, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol 71: 493–511, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Meno JR, Higashi H, Cambray AJ, Zhou J, D'Ambrosio R, Winn HR. Hippocampal injury and neurobehavioral deficits are improved by PD 81,723 following hyperglycemic cerebral ischemia. Exp Neurol 183: 188–196, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Mizumura T, Auchampach JA, Linden J, Bruns RF, Gross GJ. PD 81,723, an allosteric enhancer of the A1 adenosine receptor, lowers the threshold for ischemic preconditioning in dogs. Circ Res 79: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Okusa MD. A2A adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol 282: F10–F18, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol Renal Physiol 277: F404–F412, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Park SW, Kim M, Brown KM, Haase VH, Lee HT. Proximal tubule sphingosine kinase-1 has a critical role in A1 adenosine receptor-mediated renal protection from ischemia. Kidney Int; doi:10.1038/ki.2012.224. [DOI] [PMC free article] [PubMed]
- 44.Park SW, Kim M, Chen SW, Brown KM, D'Agati VD, Lee HT. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P1 receptor activation. Lab Invest 90: 1209–1224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SW, Kim M, Kim M, D'Agati VD, Lee HT. Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int 80: 1315–1327, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Pitman MR, Pham DH, Pitson SM. Isoform-selective assays for sphingosine kinase activity. Methods Mol Biol 874: 21–31, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int 68: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Ramesh G, Reeves WB. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest 17: 381–387, 1965 [DOI] [PubMed] [Google Scholar]
- 51.Sung FL, Zhu TY, Au-Yeung KK, Siow YL, Ok Enhanced MCP-1 expression during ischemia/reperfusion injury is mediated by oxidative stress and NF-κB. Kidney Int 62: 1160–1170, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int 71: 266–271, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J 397: 461–471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vessey DA, Kelley M, Karliner JS. A rapid radioassay for sphingosine kinase. Anal Biochem 337: 136–142, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Vessey DA, Kelley M, Li L, Huang Y, Zhou HZ, Zhu BQ, Karliner JS. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit 12: BR318–BR324, 2006 [PubMed] [Google Scholar]
- 56.Wadgaonkar R, Patel V, Grinkina N, Romano C, Liu J, Zhao Y, Sammani S, Garcia JG, Natarajan V. Differential regulation of sphingosine kinases 1 and 2 in lung injury. Am J Physiol Lung Cell Mol Physiol 296: L603–L613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuen PS, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol 286: F1116–F1119, 2004 [DOI] [PubMed] [Google Scholar]