Abstract
Kidney-specific WNK1 (KS-WNK1) is a variant of full-length WNK1. Previous studies have reported that KS-WNK1 is predominantly expressed in the distal convoluted tubule (DCT) where it regulates sodium-chloride cotransporter. The role of KS-WNK1 in other nephron segments is less clear. Here, we measured the expression of KS-WNK1 transcript in microdissected renal tubules and found that KS-WNK1 was most abundant in the DCT, followed by cortical thick ascending limb (cTAL), connecting tubule, and cortical collecting duct. A high K+ diet enhanced the expression of KS-WNK1 in the DCT and cTAL, selectively. It has been reported that a high-K diet suppresses Na+ reabsorption in TAL. To understand the role of KS-WNK1 in Na+ transport in cTAL and the regulation by dietary K+, we examined Na+ reabsorption using in vitro microperfusion in cTAL isolated from KS-WNK1-knockout mice and wild-type littermates fed either a control-K+ or high-K+ diet. Furosemide-sensitive Na+ reabsorption in cTAL was higher in KS-WNK1-knockout (KO) mice than in wild-type. A high-K+ diet inhibited Na+ reabsorption in cTAL from wild-type mice, but the inhibition was eliminated in KS-WNK1-KO mice. We further examined the role of KS-WNK1 using transgenic mice that overexpress KS-WNK1. Na+ reabsorption in cTAL was lower in transgenic than in wild-type mice. In whole animal clearance studies, a high-K+ diet increased daily urine volume and urinary Na+ and K+ excretion in wild-type mice, which was blunted in KS-WNK1-KO mice. Thus KS-WNK1 inhibits Na+ reabsorption in cTAL and mediates the inhibition of Na+ reabsorption in the segment by a high-K diet.
Keywords: kidney-specific WMK1, potassium adaptation, sodium, thick ascending limb
with-no-lysine (wnk) kinases are a family of serine/threonine kinases comprised of four members (WNK1–4) in mammals. WNK1, the founding member of WNK family, was first identified by Xu et al. (27) in 2000 and found to be ubiquitously expressed in tissues. The main function of WNK kinases is to regulate renal handling of sodium (Na+) and potassium (K+) as is evident by findings that mutations in WNK1 and WNK4 genes cause a syndrome of hereditary hypertension and hyperkalemia, called pseudohypoaldosteronism type II (26). Many studies mostly in vitro, have suggested that WNK1 and 4 modulate the activities of various Na+ transporters and K+ channels in the distal nephron via catalytic and noncatalytic mechanisms leading to increased Na+ absorption and impaired K+ secretion (9, 10, 19, 29). Kidney-specific WNK1 (KS-WNK1) is a shorter alternatively initiated variant of full-length WNK1 (FL-WNK1) predominantly expressed in distal nephron segments (28). The transcription of KS-WNK1 is initiated by an alternative promoter in exon 4A, which resides in intron 4 between exons 4 and 5. Thus the transcript of KS-WNK1 is shorter, containing exon 4A and the remaining exon 5∼28 of full-length WNK1, and the encoded protein lacks the kinase domain encoded by exon 3 and 4 (4). Results of studies (14, 24) based on heterologous cells have suggested that KS-WNK1 functions as an antagonist of full-length WNK1 with respect to regulation of renal Na+ and K+ transporters.
To study the function of KS-WNK1, we have created KS-WNK1 knockout mice and transgenic mice that overexpress KS-WNK1 in the kidney (15, 16). In KS-WNK1 transgenic mice, the total and phosphorylated forms of sodium-chloride cotransporter (NCC) and sodium-potassium-chloride cotransporter (NKCC2) in renal cortex are reduced. These mice display renal Na+ wasting and lower blood pressure under normal Na+ diet (16). Conversely, KS-WNK1 knockout mice have increased expression of NCC and NKCC in renal cortex and hypertension when fed a high Na+ diet (16). Another group (8) has also generated KS-WNK1 knockout mice and reported similar findings of increased NCC abundance and mild expansion of the circulatory volume in the mice as evident by decreases in 24-h urine aldosterone. These two studies are consistent with KS-WNK1 being an inhibitor of NCC in the distal convoluted tubule (DCT). The role of KS-WNK1 on NKCC2, however, remains unsettled despite our studies on KS-WNK1-knockout (KO) and transgenic mice. This is in large part due to previous reports (4, 20) based on in situ hybridization that KS-WNK1 was not detected in thick ascending limb (TAL) and that there have not been direct functional studies of Na+ transport in this nephron segment.
Dietary K+ is an important modulator of Na+ reabsorption in the kidney. It has been reported that dietary K+ loading causes natriuresis in part from suppression of Na+ reabsorption in TAL (25). The underlying mechanism of this K+ adaptive response in TAL is unknown. We (14, 16) have previously demonstrated that a high K+ diet increases the expression of KS-WNK1 in whole kidney and transgenic mice that overexpress KS-WNK1 in the kidney have decreased NKCC2 expression. Here, we study the effect of KS-WNK1 on Na+ transport in TAL and its role in the regulation of sodium transport by dietary K+ using in vitro microperfusion.
METHODS
Animals.
We have created KS-WNK1 knockout mice (KS-WNK1-KO) by deleting exon 4A in 129/sv strain and transgenic mice overexpressing amino acid 1–253 of KS-WNK1 transgenic (KS-WNK1-Tg) in C57BL/6 strain (14, 16). All of the experimental procedures involving these animals were performed in accordance with relevant laws and institutional guidelines approved by the University of Texas Southwestern Medical Center at Dallas Institutional Animal Care and Use Committee.
Balance studies.
For plasma biochemical data in control and high K+ diet, 10 KS-WNK1-KO mice and control littermates (129/sv wild type) (4 female, 6 male, ∼4-mo old) were fed a control K+ (1% KCl, 1 g per 100 g diet) or a high K+ diet (10% KCl, in substitution for equal weight sucrose in the diet; Harlan Teklad, Madison, WI) with free access to tap water for 2 wk before the collection of blood samples. Both control and high K+ diets contain 0.74% NaCl (7.4 g NaCl/Kg food). Mice were anesthetized with isoflurane, and whole blood was drawn by retro-orbital bleeding into Na+-heparin-coated glass capillary tubes. Plasma was recovered immediately by centrifugation. Plasma electrolytes were analyzed using STAT-CCC analyzer (Nova Biomedical, Waltham, MA). Plasma creatinine level was measured by capillary electrophoresis (P/ACE MDQ; Beckman Coulter, Brea, CA) as described previously (16). For balance studies, 10 KS-WNK1-KO mice and control littermates (129/sv wild type; 4 female, 6 male, ∼4 mo old) were fed the indicated diet and water ad libitum and placed in metabolic cages (Hatteras Instruments, Cary, NC) for collection of urine. The concentrations of Na+ and K+ in urine were measured using a microflame-emission photometer (Jenway, Essex, UK). The osmolality of plasma and urine was measured using an osmometer (model 3D3; Advanced Instruments, Norwood, MA). The osmolar-free water clearance (CH2O) was calculated by the equation:
A positive value indicates that the kidneys excrete solute-free water and dilute urine. Conversely, a negative value reflects free water reabsorption and the kidneys excrete concentrated urine.
Preparation of mice for microperfusion and microdissection studies.
These experiments were performed in KS-WNK1-KO and KS-WNK1-Tg mice at 8–10 wk of age and age- and gender-matched wild-type littermates (129/sv strain for KS-WNK1-KO and C57BL/6 strain for KS-WNK1-Tg). The mice were raised in a 12-h day and night cycle and fed a control K+ (1% KCl) or a high K+ diet (10% KCl) and tap water ad libitum for 2 wk before death.
In vitro microperfusion, sodium flux, and transepithelial potential difference.
After the mouse was killed, the kidney was removed quickly, sliced in thin coronal sections and placed in Hanks' solution containing the following (in mM): 137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2 10 Tris(hydroxymethyl)amino methane hydrochloride, 0.25 CaCl2, 2 glutamine, and 2 l-lactate at 4°C. The cortical thick ascending limb (cTAL) of Henle's loop was then dissected free hand without collagenase and transferred to a 1-ml temperature-controlled bathing chamber. Tubules were perfused in vitro as previously described (22).
Isolated cTALs were perfused at a rate of ∼5 nl/min. The perfusate contained the following (in mM): 115 NaCl, 25 NaHCO3, 2.3 Na2HPO4, 10 Na acetate, 1.8 CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, and 5 alanine and had an osmolality equal to that of the bathing solution that contained 6 gm/dl of albumin. There were at least 3 measurements of the perfusion and the collected tubular fluid in each experiment. Na+ transport (JNa) was calculated using the equation: JNa (pmol·min−1·mm−1) = ([Na]perfusate − [Na]collected)(V̇L)/L, where V̇L is collection rate (∼5 nl/min) and L is the tubular length (0.4–0.8 mm). The transepithelial potential difference was determined using the perfusion pipette as a bridge into the tubular lumen and referenced to the bathing solution using a Keithley 6517A programmable electrometer (Cleveland, OH). Furosemide used to inhibit NKCC2 was purchased from Sigma-Aldrich (St. Louis, MO). Na+ concentrations of perfusate and collected drops were measured using a Na+-selective electrode (Sodium Ionophore II-Cocktail A; Fluka) as previously described (2, 17).
Microdissection and RT-PCR.
Slices of kidney were placed into prewarmed collagenase type I (Worthington, Lakewood, NJ; 1.5 mg/ml dissolved in DMEM/F12) solution in 15-ml test tube, which was then shaken vigorously on a titer plate shaker at 37°C for 10–15 min. After digestion, the individual nephron segments were dissected in 4°C Hanks' solution and transferred by adhering the tubules to small glass beads (0.5-mm diameter; Thomas Scientific, Swedesboro, NJ) and then transferring the beads to 1.5 ml tubes containing 0.6 ml RNase inhibitor-containing lysis buffer. Total RNA was immediately extracted using Quick-RNA MicroPrep kit (Zymo Research, Irvine, CA). Reverse transcription was performed using TaqMan reverse transcription reagents (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was carried out on MyiQ single color RT-PCR detection system (Bio-Rad, Hercules, CA). We verified that no amplification was produced when reverse transcription was omitted in each sample. Sequences of primers for RT-PCR analysis were provided in Table 1. Relative mRNA levels of target proteins were standardized with an internal control (GAPDH). For comparison between KS-WNK1 and FL-WNK1, the efficiencies of KS-WNK1 and FL-WNK1 primers in RT-PCR assay were calculated from the results of three serial (4-, 16-, 64-fold) dilutions of cDNA, which encompass our working dilution (∼10-fold dilution) (21). The slope of threshold cycles obtained from KS-WNK1 or FL-WNK1 PCR reactions of serially diluted sample tubular cDNA was used to calculate the corresponding efficiencies (E) according to the equation: . Each sample was assayed in triplicate.
Table 1.
Primer sequences for RT-PCR
| Target | Sequence (5′-3′) | Orientation |
|---|---|---|
| FL-WNK1 | GTCTGGACACCGAAACCACT | Sense |
| CGAACAATGTTGGGATGTTG | Antisense | |
| KS-WNK1 | AGAAACTACTAGTAGCAAAATCCCTGTC | Sense |
| GCTTCACTCCCTCATTTATACAATCC | Antisense | |
| NKCC2 | CCAGAGCGTTGTCTAAAGCA | Sense |
| TGGGCAGCTGTCATCACTTA | Antisense | |
| NCC | GGGTTTGTGTCATGAGGATG | Sense |
| CTCGTCCGATCGTGGTAGA | Antisense | |
| AQP2 | CTGGCTGTCAATGCTCTCCAC | Sense |
| TTGTCACTGCGGCGCTCATC | Antisense | |
| GAPDH | CGTCCCGTAGACAAAATGGT | Sense |
| TCAATGAAGGGGTCGTTGAT | Antisense |
FL- and KS-WNK1, full-length and kidney-specific WNK1; NKCC2, sodium-potassium-chloride cotransporter; NCC, sodium-chloride cotransporter; AQP2, aquaporin-2.
Statistical analysis.
All results are expressed as means ± SE. Statistical comparisons between two groups of data were made using two-tailed unpaired Student's t-test. Multiple comparisons were determined using one-way ANOVA followed by Tukey's multiple comparison tests. P value <0.05 was considered to be statistically significant.
RESULTS
Characterization of WNK1 isoform mRNA expression in renal tubules in control and high potassium diet.
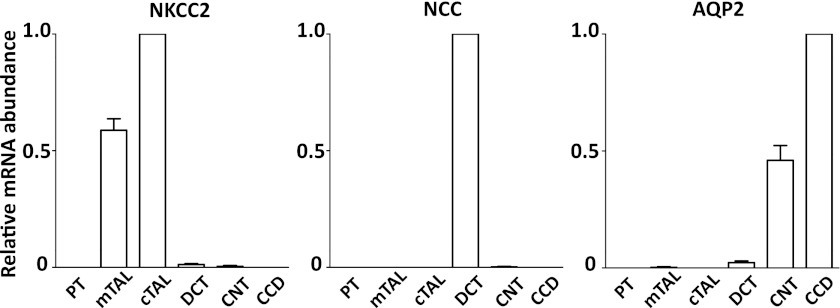
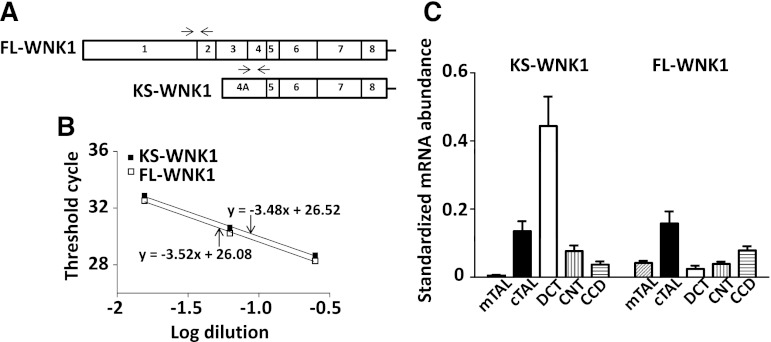
The relative expression of WNK1 isoforms including FL-WNK1 and KS-WNK1 in individual renal tubules has not been studied. We measured relative mRNA abundance of KS-WNK1 and FL-WNK1 in renal tubules including the medullary thick ascending limb (mTAL) and cTAL, DCT, connecting tubule (CNT), and cortical collecting duct (CCD) using quantitative RT-PCR. The purity of these samples was confirmed by measuring the mRNA abundance of nephron segment-specific markers (NKCC2 for TAL, NCC for DCT, and aquaporin-2 for CNT and CCD; Fig. 1). To allow comparison of abundance of KS-WNK1 and FL-WNK1, we first determined efficiencies of PCR primers used in KS-WNK1 and FL-WNK1 RT-PCR assays (position of primers shown in Fig. 2A). The calculated efficiencies of KS-WNK1 and FL-WNK1 RT-PCR assays were 1.94 and 1.92, respectively (Fig. 2B), supporting the legitimacy of direct comparison between two RT-PCR assays (21).
Fig. 1.
Purity of dissected tubules confirmed by measuring mRNA level of tubule specific marker. Selective expression of sodium-potassium-chloride cotransporter (NKCC2) in medullary and cortical thick ascending limb (mTAL and cTAL), of sodium-chloride cotransporter (NCC) in distal convoluted tubule (DCT), and of aquaporin-2 (AQP2) in connecting tubule (CNT) and cortical collecting duct (CCD); n = 10 tubules. PT, proximal tubule. for each.
Fig. 2.
Quantitative comparison of WNK1 isoform expression in distal nephron. A: schematic representation of beginning exons of WNK1 gene showing the positions of primers (arrows) used for RT-PCR analysis of full-length WNK1 (FL-WNK1) and kidney-specific WNK1 (KS-WNK1) in this study. B: similar efficiencies of KS-WNK1 and FL-WNK1 RT-PCR assays. Wild-type cTAL cDNA was serially diluted ¼ (4-, 16-, and 64-fold dilution), and 3 μl of each dilution were used in the KS-WNK1 and FL-WNK1 PCR assays. Threshold cycle was measured and plotted against the log of the dilution. Slope of liner regression was used to calculate the efficiency of RT-PCR. C: mRNA levels of KS-WNK1 and FL-WNK1 in each individual tubular segments under the control K+ diet. WNK1 isoforms in each sample were normalized to the mRNA level of its own housekeeping GAPDH gene; n = 10 tubules for each.
Under control K+ (1% KCl) diet, the expression of KS-WNK1 was most abundant in the DCT but was also present in the cTAL, CNT, and CCD (Fig. 2C, left). The KS-WNK1 transcript was almost undetectable in mTAL and proximal tubule (not shown for proximal tubule). As for FL-WNK1, its mRNA expression was more evenly distributed along the nephron in lower abundance compared with KS-WNK1 (Fig. 2C, right). The ratio of KS-WNK1 to FL-WNK1 mRNA is ∼18-fold in the DCT but closer to 1 in other segments (0.85, 2, and 0.5 for cTAL, CNT, and CCD, respectively).
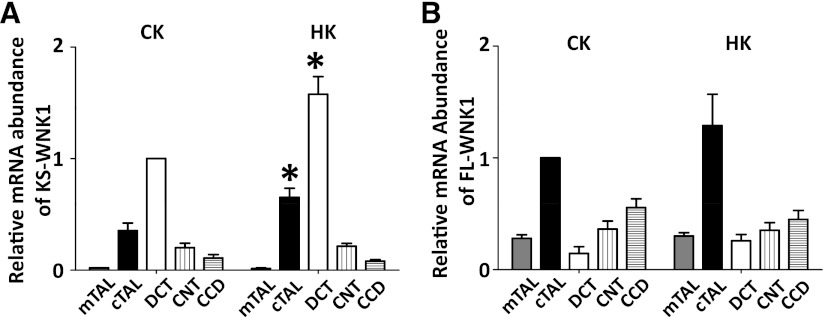
To test the effect of a high K+ (10% KCl) diet on KS-WNK1 and FL-WNK1 mRNA expression, mice were fed either a control or high K+ diet for 2 wk before isolation of renal tubules. A high K+ diet increased KS-WNK1 mRNA expression in cTAL and DCT (90 and 60% increase, respectively) but not in other segments (Fig. 3A). In contrast, a high K+ diet had no effect on FL-WNK1 mRNA (Fig. 3B).
Fig. 3.
High K+ diet upregulates KS-WNK1 mRNA expression in cortical thick ascending limb and distal convoluted tubule. Effect of dietary K+ on the normalized mRNA levels of KS-WNK1 (A) and FL-WNK1 (B). A: normalized mRNA level of KS-WNK1 in DCT in control K+ diet is set as 1. B: FL-WNK1 in cTAL in control K+ diet is set as 1. *P < 0.05, high K+ (HK) vs. control K+ (CK) diet; n = 10 tubules for each.
KS-WNK1 mediates high potassium diet-induced suppression of sodium reabsorption in the cTAL.
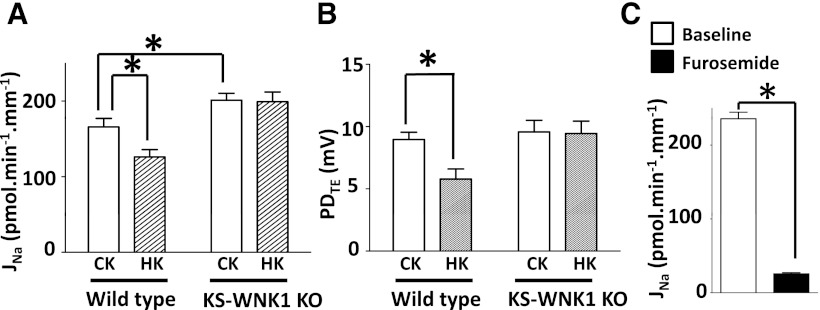
We (16) have previously shown that overexpression of KS-WNK1 decreases protein abundance of total and phosphorylated NKCC2 in the TAL and knockout of KS-WNK1 has opposite effects. Here we studied the physiological relevance of these findings by measuring Na+ reabsorption in isolated cTAL using in vitro microperfusion. In cTALs isolated from wild-type mice, Na+ reabsorption occurred at a rate 175 ± 12 pmol·min−1·mm−1 (Fig. 4A). This reabsorbed flux agrees with previous reports in cTAL (18). Addition of furosemide (100 μM) to perfusate caused ∼90% reduction of Na+ reabsorption in cTAL (Fig. 4C), indicating that it is mediated by NKCC2. A high K+ diet suppressed Na+ reabsorption in cTAL by ∼24% (Fig. 4A). In KS-WNK1-KO mice, the baseline Na+ reabsorption in the cTAL under control K+ diet was ∼20% higher than control (Fig. 4A). High K+ diet did not significantly suppress Na+ reabsorption in the cTAL of KS-WNK1-KO mice (Fig. 4A, right). Na+ reabsorption in TAL via NKCC2 generates a lumen-positive transepithelial potential difference (PDTE ). A similar pattern was observed in measurements of PDTE (Fig. 4B).
Fig. 4.
KS-WNK1 mediates chronic K+ load-induced natriuresis in cortical thick ascending limb. Na+ reabsorption (JNa; A) and lumen-positive transepithelial potential difference (PDTE; B) in the cTAL isolated from wild-type or KS-WNK1-knockout (KO) mice fed a control K+ or high K+ diet for 2 wk. (n = 10 tubules for control K+; n = 8 for high K+). C: effect of furosemide on Na+ reabsorption in cTAL of wild-type mouse (n = 8) fed a control K+ diet. *P < 0.05, between indicated groups. Note that KS-WNK1-KO mice and control littermates (used in A and B) are 129/Sv strain and wild-type mice used in C is C57BL/6 strain.
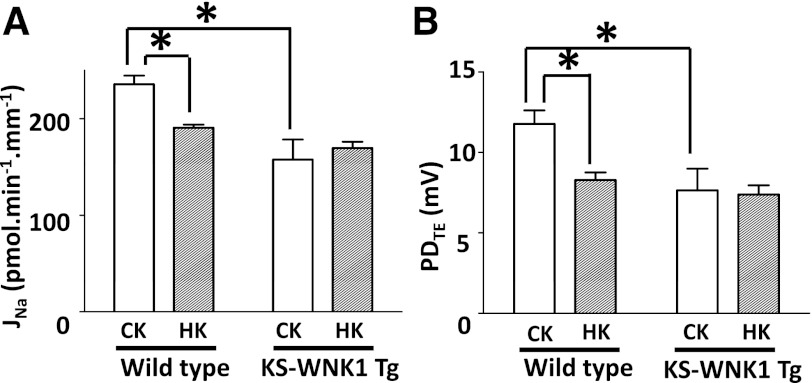
To further support the functional role of KS-WNK1 in cTAL, we measured Na+ flux in cTAL of transgenic mice that overexpress KS-WNK1 in the kidney. Na+ reabsorption in cTAL was ∼33% lower in KS-WNK1-Tg mice than in control littermates under control K+ diets (Fig. 5A). A high K+ diet did not cause further reduction of Na+ reabsorption in cTAL from KS-WNK1-Tg mice. Similar changes were observed in PDTE measurements (Fig. 5B). These functional results, together with our (16) previous biochemical and immunological analysis of NKCC2 in KS-WNK1-KO and KS-WNK1-Tg mice, indicate that KS-WNK1 inhibits NKCC2 in cTAL and that KS-WNK1 mediates the inhibition of Na+ reabsorption in cTAL by a high K+ diet.
Fig. 5.
Overexpressed KS-WNK1 inhibits Na+ reabsorption in cortical thick ascending limb. Na+ reabsorption (A) and lumen-positive PDTE (B) in the cTAL isolated from wild-type or KS-WNK1-Tg mice fed a control K+ or high K+ diet for 2 wk. (n = 8 for each group). *P < 0.05, between indicated groups.
KS-WNK1 knockout blunts the high potassium diet-induced natriuresis.
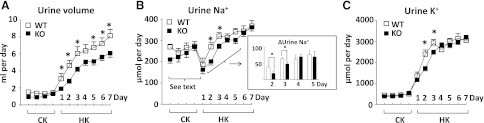
A high K+ diet causes natriuresis by affecting Na+ reabsorption at multiple nephron segments (1, 25). To understand the contribution of KS-WNK1-mediated inhibition of NKCC2 to the overall natriuretic response in renal K+ adaptation, we performed whole animal clearance studies. Mice were fed a control K+ diet for 4 days and then switched to a high K+ diet for 7 days. Daily urine volume and urinary Na+ and K+ excretion during control K+ diet were not different between KS-WNK1-KO and wild-type mice. A change to a high K+ diet resulted in an increase in urine volume in both wild-type and KS-WNK1-KO mice starting from the first day of high K+ diet (Fig. 6A). The high K+-induced diuretic response, however, was blunted in KS-WNK1-KO mice relative to wild-type littermates.
Fig. 6.
KS-WNK1-KO mice have more concentrated urine and blunted natriuretic and kaliuretic responses to a high K+ diet. Daily urine volume (A), urine Na+ excretion (B), and urine K+ excretion (C) in response to a high K+ diet. Wild-type (WT) or KS-WNK1-KO mice (n = 10 for each group) were fed a control K+ diet for 4 days and then switched to a high K+ diet for 7 days. Inset: increases in urine Na+ excretion at days 2–5 above day 1 of high K+ diet (ΔUrine Na+ = urine Na+ excretion on day 2–5 minus urine Na+ excretion on day 1). The drop of urine Na+ excretion on the first day of high K+ diet is due to decreased intake of the high K+ diet (see results for further details). *P < 0.05 wild type vs. KO.
We next examined the natriuretic response to a high K+ diet. Unexpectedly, urinary Na+ excretion in both wild-type and KS-WNK1-KO mice decreased on the first day of high K+ diet (Fig. 6B). This initial drop in urinary Na+ excretion likely reflected decreased intake of the high K+ diet due to palatability of the diet (see Table 2). A similar observation has been made in mice by others (3, 20). After the first day, urinary Na+ excretion increased in both wild-type and KS-WNK1-KO mice reflecting natriuretic response to a high K+ diet. Compared with wild-type, the natriuretic response in KS-WNK1 mice was somewhat blunted on the second and third day of a high K+ diet (Fig. 6B, inset). It should be mentioned that the increase in urinary Na+ excretion during high K+ diet may reflect alterations in the extra-renal Na+ excretion as well as renal Na+ excretion. In the control K+ diet, urinary Na+ excretion was ∼59% of intake (261 μmol over 443 μmol), indicating that ∼41% of ingested Na+ was excreted extra-renally, presumably mostly by the intestine. In the high K+ diet, urinary Na+ excretion slightly exceeded the amount that was ingested [362 μmol excreted vs. 342 μmol Na+ ingested (0.74% × 2.7 g diet)]. We speculate that increased aldosterone secretion in high K+ diet increases intestinal Na+ absorption, which together with the renal natriuretic effect, results in urinary Na+ excretion exceeding that is ingested.
Table 2.
Steady-state data for plasma and urine biochemistries and urine Na+, K+, and water excretion in control and high K+ diets
| Control K+ Diet |
High K+ Diet |
|||
|---|---|---|---|---|
| WT (n = 10) | KO (n = 10) | WT (n = 10) | KO (n = 10) | |
| Body weight, g | 22.5 ± 0.9 | 25.1 ± 1.2 | 21.5 ± 0.9 | 24.0 ± 0.9 |
| Water intake, g | 3.9 ± 0.4 | 3.1 ± 0.2 | 10.6 ± 0.9† | 8.7 ± 0.5† |
| Plasma | ||||
| Na+, mmol/l | 144.5 ± 0.9 | 145.5 ± 0.7 | 147.7 ± 0.5‡ | 146.1 ± 0.6 |
| K+, mmol/l | 5.0 ± 0.1 | 4.5 ± 0.1 | 5.0 ± 0.2 | 4.5 ± 0.1 |
| Cl−, mmol/l | 116.4 ± 0.9 | 115.9 ± 0.6 | 117.4 ± 0.6 | 116.1 ± 1.0 |
| Osmolality, mosmol/kgH2O | 290.9 ± 1.0 | 290.7 ± 1.8 | 297.3 ± 2.4‡ | 297.1 ± 1.4‡ |
| Creatinine, mg/dl | 0.06 ± 0.003 | 0.06 ± 0.004 | 0.08 ± 0.002† | 0.06 ± 0.002§ |
| Urine | ||||
| Na+, μmol/day | 261 ± 9 | 243 ± 13 | 368 ± 25† | 362 ± 17† |
| K+, μmol/day | 451 ± 16 | 428 ± 25 | 3,048 ± 202† | 3,197 ± 140† |
| Osmolality, mosmol/kgH2O | 2,207 ± 174 | 2,895 ± 144* | 1,199 ± 62† | 1,606 ± 46† § |
| Total osmoles, mosmol·kgH2O−1·day−1 | 2.8 ± 0.1 | 2.7 ± 0.2 | 8.7 ± 0.4† | 8.5 ± 0.3† |
| Volume, ml/day | 1.4 ± 0.2 | 1.0 ± 0.1 | 8.1 ± 0.7† | 6.1 ± 0.5† § |
| CH2O, μl/min | −5.9 ± 0.1 | −6.7 ± 0.2* | −4.7 ± 0.2‡ | −6.2 ± 0.3§ |
Values in means ± SE. Due to palatability of the high K+ diet, mice consumed significantly less high K+ diet than control K+ diet (daily intake: 2.7 ± 0.5 g vs. 3.5 ± 0.6 g). Daily consumption between wild-type (WT) and knockout (KO) mice yet were not different (not shown). Increase in urinary osmolar excretion during high K+ diet reflects the higher osmolar content in the high K+ diet [substituting 10% )g per g food) KCl for sucrose].
P < 0.05, KO vs. WT in control K+ diet.
P < 0.001, high K+ vs. control K+.
P < 0.05, high K+ vs. control K+.
P < 0.05, KO vs. WT in high K+ diet. CH2O, osmolar-free water clearance.
As expected, urinary K+ excretion increased on a high K+ diet (Fig. 6C). Likely because of the decrease in intake of the high K+ diet (and increased intestinal K+ secretion as well; see below), daily urinary K+ excretion increased only by ∼7-fold rather than the expected 10-fold increase. As for the natriuretic response, the increase in K+ excretion was less in KS-WNK1-KO mice relative to wild-type mice on the second and third day.
The above results support the hypothesis that KS-WNK1 contributes to high K+ diet-mediated diuresis and natriuresis. Serum K+ levels, however, were not different between KS-WNK1-KO and wild-type mice, even on a high K+ diet (Table 2). Thus other mechanisms must fully compensate for the transient K+ excretion impairment caused by loss of KS-WNK1. Of note, the difference in urinary K+ excretion between wild-type and KS-WNK1-KO mice on day 2 and 3 of high K+ diet apparently far exceeds that can be potentially buffered by the intracellular space. We did not detect significant differences in the daily food intake between wild-type and-KO mice (not shown), which was supported by the finding that urinary osmolar excretion was not different (Table 2). Thus increased intestinal K+ excretion may also contribute to the compensatory responses in-KO mice. An increase in aldosterone secretion, as mentioned above, may be a compensatory mechanism for increased intestinal K+ excretion in high K+ diet. However, we have no explanations why increases in the intestinal K+ excretion would be greater in KS-WNK1-KO than in wild-type mice.
Interestingly, urine osmolality is significantly higher in KS-WNK1-KO than in wild-type in control K+ as well as high K+ diets (Table 2). Moreover, the increase of urine volume on the seventh day of a high K+ diet was much higher than the increase of urine osmolar excretion (Table 2; ∼6-fold vs. ∼3-fold). As the total daily urine osmolar excretion was not different between the two groups on either a control or high K+ diet, these results suggest that potassium-induced diuresis has more than an effect on osmotic diuresis, and may be in part due to a decrease in free water reabsorption (−CH2O; −5.9 ± 0.4 μl/min [control K+] vs. −4.7 ± 0.7 μl/min [high K+]; P < 0.05; Table 2). Interestingly, free water reabsorption was higher in KS-WNK1-KO mice than in wild-type mice. These results are consistent with the idea that KS-WNK1 inhibits NKCC2, and increased NKCC2 activity in WNK1-KO mice enhances urinary concentration ability.
DISCUSSION
Using quantitative measurement of mRNA and in vitro microperfusion, we show in this study that KS-WNK1 is expressed in cTAL and plays an important role in inhibition of NKCC2-mediated Na+ reabsorption in the segment. Moreover, KS-WNK1 mediates the high K+ diet-induced inhibition of Na+ reabsorption in cTAL and is important for urinary concentration.
Since the first discovery of KS-WNK1 in 2002 (28), two studies (4, 20) using in situ hybridization of KS-WNK1 mRNA have shown that the expression of KS-WNK1 is exclusively in the renal cortex and that the DCT has strikingly high KS-WNK1 expression. Using a more quantitative and sensitive method, quantitative RT-PCR, we show that while KS-WNK1 transcript is most abundant in DCT, and it is also present in cTAL, CNT, and CCD; the abundance in these segments relative to DCT are 30, 17, and 8%, respectively. We confirm that KS-WNK1 is barely detected in mTAL and the proximal tubule. The physiological importance of KS-WNK1 in Na+ reabsorption in cTAL is demonstrated by microperfusion studies.
Transepithelial Na+ reabsorption in cTAL is mediated in large part by the transcellular route, which requires Na+ entry across the apical membrane predominantly through NKCC2 and exit via the basolateral Na+-K+-ATPase. NKCC2-mediated Na+ reabsorption generates a lumen-positive PDTE, partly due to apical recycling of K+. The Na+/H+ exchanger also contributes to the apical Na+ entry, but the role is relatively minor and it does not generate lumen-positive PDTE (23). Several pieces of evidence indicate that the effect of KS-WNK1 on Na+ reabsorption in cTAL is at least in large part through inhibition of NKCC2. First, it is inhibited by the NKCC2 inhibitor furosemide. Second, the effect on Na+ flux parallels that on lumen-positive PDTE. Third, and perhaps more importantly, we (16) have previously shown that KS-WNK1 negatively regulates NKCC2 abundance by Western blot analysis and immunofluorescent staining. The additional effect of KS-WNK1 on other transporters in cTAL, nonetheless, is possible. In this study, we did not directly examine the mechanism by which KS-WNK1 inhibits NKCC2. However, results of studies based on Xenopus oocytes and cultured cells (14, 19, 24) suggest that KS-WNK1 probably antagonizes the activation of NKCC2 by FL-WNK1.
The other significant finding of our study is that KS-WNK1 appears to mediate high K+-induced inhibition of Na+ reabsorption in cTAL. It is known that a high K+ diet inhibits Na+ reabsorption in the proximal tubule and TAL (1, 11, 25). We (14) have previously shown that a high K+ diet upregulate KS-WNK1 mRNA in the whole kidney lysate. Here, we further show that high K+ diet upregulates KS-WNK1 mRNA in cTAL as well as DCT but not in other segments. In microperfused cTAL, a high K+ diet inhibits Na+ reabsorption, and the inhibition is obliterated in KS-WNK1-KO mice. Thus KS-WNK1 is important for inhibition of Na+ reabsorption in cTAL by a high K+ diet. One important physiological role of a high K+-diet induced natriuresis and diuresis is to maximize renal K+ excretion during high K+ intake. The renal K+ excretion occurs predominantly by secretion via K+ secretory channels in CNT and early CCD that requires lumen-negative PDTE generated by ENaC-mediated Na+ reabsorption. Several factors limit K+ secretion via this mechanism. First, continuing K+ secretion on top of water reabsorption accompanying Na+ reabsorption in the CNT and CCD raises the luminal K+ concentration to an extent that it favors reabsorption rather secretion in late CCD and the downstream outer medullary collecting duct. Second, Na+ reabsorption in the more proximal segments reduces delivery to distal segments. Inhibition of Na+ reabsorption in the proximal tubule and TAL by high a K+ diet would increase Na+ and fluid delivery to the CNT and CCD to overcome the above limiting factors. Moreover, increased flow to distal nephron segments also stimulates flow-activated maxi-K channels adding to K+ secretion. To our surprise, we found that urinary K+ excretion was only transiently decreased in KS-WNK1-KO mice in the second and third days of a high K+ diet. Serum K+ levels and urinary K+ excretion at day 4 of a high K+ diet and onward were not different between KO and wild-type mice. Apparently, other factors, such as inhibition of Na+ reabsorption in the proximal tubule by high K+ diets, compensate for the deficiency caused by loss of KS-WNK1.
It is well known that Na+ reabsorption by medullary TAL is critical for establishment of hypertonic medulla essential for the urinary concentration mechanism (6). Water restriction increases NKCC2 abundance in TAL and vasopressin stimulates trafficking and insertion of NKCC2 to the apical membrane (5, 7). Bartter's mutations in NKCC2 and pharmacological inhibition of NKCC2 impair the urinary concentration mechanism (12, 13). The role of cTAL in the urinary concentration is less clear. Our results demonstrate that KS-WNK1 plays a role in the urinary concentration mechanism. KS-WNK1-KO mice have a higher baseline urinary osmolality and free water reabsorption. We speculate that this is due to increased NKCC2 activity in the cortical TAL of KS-WNK1-KO mice. The role of NKCC2 in medullary TAL is unlikely because KS-WNK1 is not expressed there. The possibilities that KS-WNK1 regulates aquaporin-2 and urea transporters also exist and require further investigation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-41612, DK-59530, and DK-079328. C. J. Cheng is supported by a scholarship grant from the Ministry of Defense, Taiwan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.-J.C., M.B., and C.-L.H. conception and design of research; C.-J.C. and T.T. performed experiments; C.-J.C., T.T., and C.-L.H. analyzed data; C.-J.C., T.T., M.B., and C.-L.H. interpreted results of experiments; C.-J.C. prepared figures; C.-J.C. drafted manuscript; M.B. and C.-L.H. edited and revised manuscript; C.-L.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jessica Lucas for measuring creatinine concentrations of plasma and urine samples. This work was performed by C. J. Cheng in partial fulfillment of the requirements of the Ph.D. degree at the University of Texas Southwestern Medical Center.
REFERENCES
- 1. Brandis M, Keyes J Windhager EE. Potassium induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol 222: 421–427, 1972 [DOI] [PubMed] [Google Scholar]
- 2. Cheng CJ, Lozano G, Baum M. Prenatal programming of rat cortical collecting tubule sodium transport. Am J Physiol Renal Physiol 302: F674–F678, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dekel B, Nakhoul F, Abassi Z, Aviv R, Winaver J, Szylman P. Complete adaptation to chronic potassium loading after adrenalectomy: possible humoral mechanisms. J Lab Clin Med 129: 453–461, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol 23: 9208–9221, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ecelbarger CA, Kim GH, Wade JB, Knepper MA. Regulation of the abundance of renal sodium transporters and channels by vasopressin. Exp Neurol 171: 227–234, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Gimenez I, Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem 278: 26946–26951, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Hadchouel J, Soukaseum C, Büsst C, Zhou XO, Baudrie V, Zürrer T, Cambillau M, Elghozi JL, Lifton RP, Loffing J, Jeunemaitre X. Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension. Proc Natl Acad Sci USA 107: 18109–18114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest 117: 1078–1087, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang CL, Yang SS, Lin SH. Mechanism of regulation of renal ion transport by WNK kinases. Curr Opin Nephrol Hypertens 17: 519–525, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Jung JY, Kim S, Lee JW, Jung ES, Heo NJ, Son MJ, Oh YK, Na KY, Han JS, Joo KW. Effects of potassium on expression of renal sodium transporters in salt-sensitive hypertensive rats induced by uninephrectomy. Am J Physiol Renal Physiol 300: F1422–F1430, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Kemter E, Rathkolb B, Bankir L, Schrewe A, Hans W, Landbrecht C, Klaften M, Ivandic B, Fuchs H, Gailus-Durner V, Hrabé de Angelis M, Wolf E, Wanke R, Aigner B. Mutation of the Na+-K+-2Cl− cotransporter NKCC2 in mice is associated with severe polyuria and a urea-selective concentrating defect without hyperreninemia. Am J Physiol Renal Physiol 298: F1405–F1415, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Kim GH, Choi NW, Jung JY, Song JH, Lee CH, Kang CM, Knepper MA. Treating lithium-induced nephrogenic diabetes insipidus with a COX-2 inhibitor improves polyuria via upregulation of AQP2 and NKCC2. Am J Physiol Renal Physiol 294: F702–F709, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Z, Wang HR, Huang CL. Regulation of ROMK channel and K+ homeostasis by kidney-specific WNK1 kinase. J Biol Chem 284: 12198–12206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Z, Xie J, Wu T, Truong T, Auchus RJ, Huang CL. Downregulation of NCC and NKCC2 cotransporters by kidney-specific WNK1 revealed by gene disruption and transgenic mouse models. Hum Mol Genet 20: 855–866, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maddrell SH, O'Donnell MJ, Caffrey R. The regulation of haemolymph potassium activity during initiation and maintenance of diuresis in fed Rhodnius prolixus. J Exp Biol 177: 273–285, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Mandon B, Siga E, Chabardes D, Firsov D, Roinel N, De Rouffignac C. Insulin stimulates Na+, Cl−, Ca2+, and Mg2+ transports in TAL of mouse nephron: cross-potentiation with AVP. Am J Physiol Renal Fluid Electrolyte Physiol 265: F361–F369, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685–42693, 2005 [DOI] [PubMed] [Google Scholar]
- 20. O'Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol 17: 2402–2413, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: 2002–2007, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quigley R, Chakravarty S, Baum M. Antidiuretic hormone resistance in the neonatal cortical collecting tubule is mediated in part by elevated phosphodiesterase activity. Am J Physiol Renal Physiol 286: F317–F322, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shirley DG, Walter SJ, Unwin RJ, Giebisch G. Contribution of Na+-H+ exchange to sodium reabsorption in the loop of henle: a microperfusion study in rats. J Physiol 513: 243–249, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Subramanya AR, Yang CL, Zhu X, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol 290: F619–F624, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Unwin R, Capasso G, Giebisch G. Potassium and sodium transport along the loop of Henle: effects of altered dietary potassium intake. Kidney Int 46: 1092–1099, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Wilson FH, Disse-Nicode'me S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Xu Q, Modrek B, Lee C. Genome-wide detection of tissue-specific alternative splicing in the human transcriptome. Nucleic Acids Res 30: 3754–3766, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]