Abstract
Budburst phenology is a key driver of ecosystem structure and functioning, and it is sensitive to global change. Both cold winter temperatures (chilling) and spring warming (forcing) are important for budburst. Future climate warming is expected to have a contrasting effect on chilling and forcing, and subsequently to have a non-linear effect on budburst timing. To clarify the different effects of warming during chilling and forcing phases of budburst phenology in deciduous trees, (i) we conducted a temperature manipulation experiment, with separate winter and spring warming treatments on well irrigated and fertilized saplings of beech, birch and oak, and (ii) we analyzed the observations with five temperature-based budburst models (Thermal Time model, Parallel model, Sequential model, Alternating model, and Unified model). The results show that both winter warming and spring warming significantly advanced budburst date, with the combination of winter plus spring warming accelerating budburst most. As expected, all three species were more sensitive to spring warming than to winter warming. Although the different chilling requirement, the warming sensitivity was not significantly different among the studied species. Model evaluation showed that both one- and two- phase models (without and with chilling, respectively) are able to accurately predict budburst. For beech, the Sequential model reproduced budburst dates best. For oak and birch, both Sequential model and the Thermal Time model yielded good fit with the data but the latter was slightly better in case of high parameter uncertainty. However, for late-flushing species, the Sequential model is likely be the most appropriate to predict budburst data in a future warmer climate.
Introduction
Leaf phenology is a key driver of canopy development, tree growth, ecosystem carbon and water balance, and species distribution [1], [2], [3], [4], [5]. In the Northern hemisphere, a clear advancement of spring tree phenology, paralleling the recent increase in surface temperature, has been well documented [6], [7], [8], [9], [10], [11], [12], [13]. However, this observed correlation between temperature and leaf flushing date cannot be extrapolated to simulate the future phenology changes in a warmer world, because the mechanisms underlying the budburst process are far from fully understood [14], [15].
Winter temperatures (referred from here on as chilling) that determine the release from dormancy in winter [16] and spring warming temperatures (referred to as forcing) that accelerate bud development following the release from dormancy are both acknowledged to influence spring phenology [16], [17], [18], [19]. However, climate warming is expected to have a contrasting effect on chilling and forcing. Warmer springs logically accelerate the accumulation of forcing and hence advance the timing of budburst. Warmer winters may also advance budburst timing if they contribute to forcing, but warmer winters may also reduce the accumulated chilling. This might have no effect on budburst (if the chilling requirement is already fulfilled early in winter), but could also delay it (if the tree chilling requirement remains unfulfilled in warmer conditions) [14], [15], [20], [21], [22]. Thus, future climate warming might not result in a straightforward advancement of budburst because of contrasting effects of warming on the accumulation of chilling and forcing, and the extent of warming in winter and spring is likely to differ [23]. To accurately simulate the budburst process in a changing climate, more information on the impact of warming on chilling and forcing phases, separately, as well as their combined effect on tree phenology, is needed.
Over the years, a wide variety of budburst models have been developed. The simple one phase models that take only the forcing phase into account, are generally accepted to predict the budburst timing well [24], [25]. Nevertheless, very good performance of two-phase models (including both chilling and forcing phases) was recently reported, especially for species flushing later in spring [13], [22], [26]. However, most of these studies used historical phenology observations, and were seldom based on actual climate manipulation experiments in which climate can be forced beyond the currently occurring climatic envelope. Therefore, phenology models may generate high uncertainty when predicting future phenology shifts in response to global warming. To study the budburst response to the anticipated climate warming and to estimate the accuracy of the currently used temperature-based budburst models, a manipulative experiment was designed using climate-controlled greenhouses. In particular, the effect of warming on the chilling and forcing phases was assessed by considering two different warming periods in a factorial design. Three tree species were selected: birch (Betula pendula L., with budburst around mid April in Belgium), oak (Quercus robur L., with budburst in early May) and beech (Fagus sylvatica L., with budburst in mid May [27]). These species were chosen to reflect a different pattern of temperature requirement during the budburst process, particularly between the pioneer birch and the late successional beech. For example, Murray et al [15] reported larger chilling requirement for late flushing species (like beech, 150 chilling days) than early flushing species (like Crataegus monogyna, 80–100 chilling days). The Belgian phenology network dataset [28] indicates chilling requirement of 120 chilling days for beech, 100 chilling das for oak and 50 chilling days for birch (Fu unpublished). We expected the warming-induced budburst advancement to be more pronounced for birch than for beech, as the larger chilling requirement of the latter might be partially unfulfilled in warmer winter conditions. Oak is expected to have an intermediate pattern. Five temperature-driven budburst models were chosen to reproduce budburst timing: the Thermal Time model (TTM) [19], the Parallel model (PM) [29], [30], [31], the Sequential model (SM) [31], [32], [33], the Alternating model (AM) [15], [19], [31], and the Unified model (UM) [34]. We sought to answer the following questions: (i) Does budburst respond differently to winter warming than to spring warming? and (ii) which temperature-driven model is best at reproducing budburst dates in manipulated warming conditions?
Materials and Methods
Warming experiment
The experiment was conducted at the Drie Eiken campus of the University of Antwerp (Belgium, 51°19"N, 4°21"E). The climate in Belgium is characterized by mild winters and cool summers, with a long-term average annual air temperature of 9.6°C, and mean monthly air temperatures between 2.2°C (January) and 17.0°C (July). Annual precipitation averages 776 mm, equally distributed throughout the year.
One-meter-high saplings of Betula pendula L., Quercus robur L. and Fagus sylvatica L. of a local provenance were used in this experiment. Up to the start of our manipulation experiment, all nursery-grown saplings were subjected to uniform conditions, i.e. equal fertilization, irrigation and light conditions. Saplings were transplanted to plastic pots (diameter 25 cm, depth 30 cm) in December of 2009. The pots were filled with a loamy sand top soil with a pH of 5.5 and 1.9% soil organic carbon (determined by the Belgian National Soil Service, Belgium). The soil organic carbon was determined by the dichromate method (ISO 14235), and the pH of the soil was measured using standard methods (ISO 10390). Before moving into the greenhouses, the saplings were fertilized again. The composition of the slow release fertilizer was 13-10–20 for nitrogen, phosphate and potassium (all in %). The recommended fertilizer dose was 100 g m− 2; therefore 20 g of fertilizer was added to each sapling (pot area = 0.2 m2). For this experiment we used 8 climate-controlled experimental chambers. The interior surface area was 150×150 cm, the height at the north side 150 cm and at the south side 120 cm. Each chamber has a roof fitting the upper opening. The four sides were made of polyethylene film (200 mm thick), whereas the roof of polycarbonate plate (4 mm thick). All sides and roof were sunlit, colorless and UV transparent. The chambers can be artificially warmed in a controlled manner up to 9°C, using a centralized heating system of continuous (day and night) warming above fluctuating ambient air temperature [35]. Four chambers were maintained at ambient temperature for the entire duration of the experiment (from December 1st 2009 till budburst in spring 2010), whereas another four chambers were continuously warmed by 6°C. Six saplings of beech, oak and birch were placed in each chamber (totaling 48 saplings per species). Half of the saplings were left in the same chamber for the duration of the experiment. Saplings that remained in the control chambers constituted the W0S0 treatment, whereas saplings that remained in the warmed chambers constituted the W6S6 treatment. The other three saplings per greenhouse were moved from the control chambers to the warmed chambers (W0S6) or vice versa (W6S0) when the ambient mean daily temperature was above 5°C for five-days continuously i.e. on February 22nd in 2010. A forcing temperature threshold of 5°C is commonly used in temperate regions [11], [15], [31]. The moving of these saplings provide the distinct warming treatments, i.e. only winter warming (W6S0) and only spring warming (W0S6). Overall, we considered four treatments (W0S0, W6S0, W0S6, W6S6), with level of replication of (n) of four, i.e. four chambers per treatment. Each replicate value is the average of three within-chamber observations (three saplings of each species per chamber). In total, we had 12 saplings per treatment. The saplings were watered once or twice per week. The saplings were watered as soon as the topsoil appeared dry. Because the warmed chamber resulted in faster evapotranspiration, these saplings were irrigated more frequently than that in control. In this way, the soil retained sufficient water, and would not confound the temperature effect on the budburst process, as Morin et al [21] reported that the soil water content did not affect the leaf unfolding phenology of oak seedlings. Eight temperature sensors (Siemens, type QFA66, Germany) were used to continuously monitor (logging time 30 minutes) the inside air temperature of each chamber, and also the outside reference temperature was monitored. The chambers provided a stable warming treatment (regressions of chamber temperature vs. field temperature were highly significant (P<0.001) and with average R2 above 0.95) and actual warming was within ±5% of the prescribed value [35].
Phenology measurement
Phenological observations were made on the terminal bud of each sapling, according to the following phenology scale: (1) undeveloped bud: bud still in winter dormancy; (2) swollen bud: green or elongated bud or bud with broken scales, i.e. with the leaf tip becoming visible but still forming a single bud tip; (3) bud break: leaf bases still hidden in bud scales but leaf tips detached from the bud axis, and (4) leaf unfolded: the entire leaf blade and the leaf stalk are visible. Monitoring started on February 1 and was repeated every 3 days (within stages 1 and 2) and every 2 days (within stages 3 and 4) always at the same time (2:00–3:00 PM). In this study, we used the starting date of stage 3 to determine budburst date as shown in Figure 1.
Figure 1. Frequency distribution of budburst dates of the experimental trees for the three studied species.
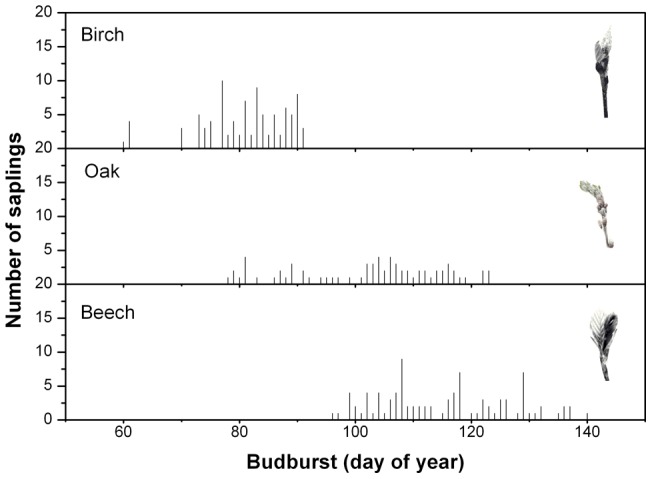
Frequency distribution of budburst dates of the experimental trees for the three studied species. The number of saplings for a given budburst date is presented on the Y-axis. The small photos on the upper right part of each panel visually show the stage of leaf development when we considered budburst to have occurred.
Models used
Five models were parameterized to predict the budburst date. In the models, the effect of temperature is accounted by calculating (daily) the rate of forcing (Rf) and rate of chilling (Rc), both as functions of the daily air temperature (T). These functions differ among models. Rf and Rc determine the state of forcing (Sf) and the state of chilling (Sc), respectively:
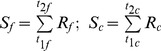 |
(1) |
where t1f and t1c is the initial day of the forcing- and chilling period, respectively, and, similarly, t2f and t2c is the final day of the forcing- and chilling period, respectively. The budburst day (BB) occurs when Sf reaches a forcing threshold F*, whereas, in the models that account for the chilling temperature, the start of forcing is triggered when Sc reaches the chilling threshold C*.
| (2) |
| (3) |
where D is the day of the year. For the model names and model parameters, the terminology follows original works (cited below). For each model, a complete list of model parameters and variable used is reported in table 1.
Table 1. The symbols and units of the variables and parameters used in the equations of the studied models.
| Model Symbole | TTM | SM | PM | AM | UM | Description | Units |
| Variables | |||||||
| CU | Chilling unit | - | |||||
| FU | Forcing unit | - | |||||
| Sc | State of chilling | CU | |||||
| Sf | State of forcing | FU | |||||
| Rc | Rate of chilling | CU | |||||
| Rf | Rate of forcing | FU | |||||
| T | Mean daily air temperature | °C | |||||
| D | Day of the year | day | |||||
| BB | Day of budburst | day | |||||
| F* | (a) | (a) | (a) | (a) | Forcing threshold | FU | |
| Parameters | |||||||
| t1c | Start day of chilling period | day | |||||
| t2c | t2c | End day of chilling period | day | ||||
| t1f | t1f | Start day of forcing period | day | ||||
| Tb | Tb | Critical temperature to induce forcing start | °C | ||||
| Tc | Critical temperature to accumulate chilling units | °C | |||||
| C* | C* | C* | Chilling threshold | CU | |||
| Tmin | Tmin | Minimum temperature for rate of chilling | °C | ||||
| Topt | Topt | Optimal temperature for rate of chilling | °C | ||||
| Tmax | Tmax | Maximum temperature for rate of chilling | °C | ||||
| a, b, c | a, b, c, Km | a, b, c | Ca, Cb, Cc, w, k, Fb, Fc, | Constant | - | ||
(a) Parameter for the model.
Thermal time model (TTM) [19] In the TTM, the forcing period starts on a fixed day (t1f), and Rf is computed when the air temperature is above a critical temperature (Tb) by using a linear relationship as follows (Eqs.4).
| (4) |
The TTM ignores any eventual role of chilling. TTM has 3 parameters (t1f, Tb , F* ).
Sequential model (SM) [30], [31], [32]. The SM model uses a triangular chilling function (Eq 5) and a sigmoid forcing function (Eq 6):
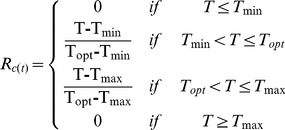 |
(5) |
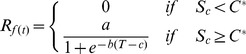 |
(6) |
where Tmin, Tmax, Topt are the minimal, maximal and optimal temperature for chilling period, and a, b, c are fitting parameters. The SM starts accumulating forcing units only when a sufficient amount of chilling has occurred (Eq. 3). SM has eight parameters (Tmin, Tmax, Topt, a, b, c, C* and F*).
Parallel model (PM) [29], [30], [31] The PM assumes that the forcing phase can take place even during the chilling phase. Rc is calculated as in SM (Eq. 6), whereas Rf is calculated with a modification of Eq. 5 of SM:
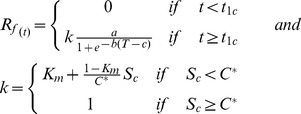 |
(7) |
Where Km is a model parameter and C* is the threshold indicating complete chilling. PM has one more parameter (Km) than SM, thus nine in total.
Alternating model (AM) [15] The AM has the same rate of forcing as the TTM (Eq. 4) but a fixed forcing start on January 1st [15]. The chilling rate equals the number of chilling days, i.e. days with average temperature less than a chilling threshold Tc (Eq. 8), with start of chilling fixed on November 1st [15].
| (8) |
The major difference between AM and the other models is the definition of F* (Eq. 2), which in AM is not a constant parameter but a negative exponential function of the state of chilling (Eq.9) [19], [30]. In this way, flexibility is introduced in modeling the budburst process as the forcing period is controlled by the chilling period.
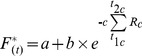 |
(9) |
Where a, b and c are fitting parameters. AM has eight parameters (t1f , t1c, t2c, Tb, Tc, a, b, c).
Unified model (UM [34])
The unified model combines features of the other models and merges the equations for Rc and Rf into one sigmoid equation:
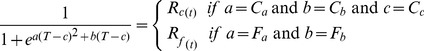 |
(10) |
where Ca, Cb and C c are chilling rate parameters, Fb and Fc are forcing rate parameters. In UM, t1c is set on September 1st [34]. The forcing units start to accumulate when a sufficient amount of chilling has occurred (C*), and F* is calculated with an exponential function of Rc similarly to Eq. 14 from t1c to t2c,
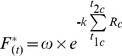 |
(11) |
where k, w and t2c are model parameters. The Unified model has nine parameters (Ca, Cb, Cc, Fb, Fc ,C*, k, w, t2c).
Model calibration and validation
The models were parameterized by Bayesian method using the version of Markov Chain Monte Carlo known as the Metropolis-Hastings random walk [36]. This method has already been used for the parameterization of the same phenology models investigated here [37]and a detailed methodological description can be found in [28]. Because of lack of information on the parameters, flat distributions for parameters were defined as the prior parameter distribution [38]. Datasets used for parameterization and validation comprised all the saplings used in the winter-spring warming experiment described above (48 saplings per species) plus additionally 36 sapling per species. The latter saplings were studied in a parallel experiment that used chambers warmed by 2°C in winter in the same experimental platform described above and moved after February 22nd to control conditions (W2S0), +2°C (W2S2) and +6°C (W2S6) in another experimental platform [39]. As a result, a wide range of budburst dates were available for each species (Figure 1), which benefit the budburst modeling work. The models were parameterized and validated against the whole dataset (internal validation) and/or parameterized on a randomly selected half of the dataset and tested against the other half (external validation). The model performances were evaluated by the Root Mean Square Error (RMSE), Model Efficiency (ME) and Akaike's Information Criterion (AIC):
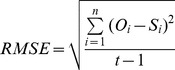 |
(12) |
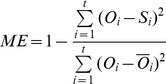 |
(13) |
| (14) |
Where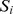 is the model prediction,
is the model prediction, 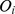 is the experimental data, and t is the number of observations, p is the number of model parameters plus one and
is the experimental data, and t is the number of observations, p is the number of model parameters plus one and 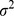 is the residual sum of squares divided by t.
is the residual sum of squares divided by t.
Statistics and calculations
Pearson's correlation coefficient (r) was calculated to determine the correlation between the observed and predicted budburst dates. One way ANOVAs were used to evaluate the significance level of the budburst differences among treatments. All statistical analyses were conducted using SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Budburst dates under warming
A wide range of budburst dates was obtained for each of the three studied species (Figure 1). Independently of the treatment, birch flushed earlier than oak, and oak, on average, exhibited earlier budburst than beech (Figure 1). Compared with the budburst date range of birch (day of year, DOY, 60–95), the budburst date range was wider in oak (47 days: DOY 78–125) and beech (45 days: DOY 95–140) (Figure 1). The continuously warmed W6S6 treatment resulted in the earliest budburst dates, while in the control chambers (W0S0) the latest budburst dates were observed (Figure 2).
Figure 2. Budburst response to different warming treatments.
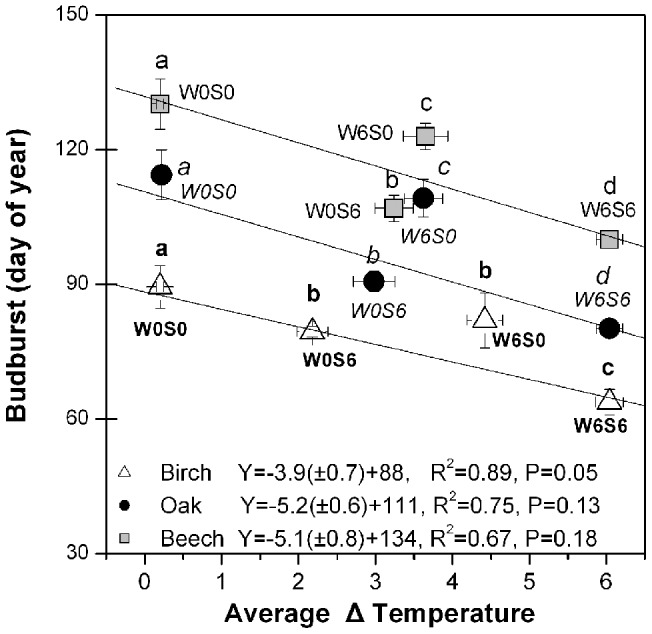
Budburst response to different warming treatments: W0S0: no warming; W6S6: winter and spring warming; W6S0: winter warming only, and W0S6: spring warming only (winter is from December 1 2009 to February 22 2010 and spring from February 22 to budburst date). The delta temperature was calculated as the average difference between treatments and outside controls from December 1st to the day of observed budburst for three warming treatments. Because the period with warming in spring was shorter than the period with warming in winter, the W0S6 treatment experienced less warming on average than the W6S0 treatment. Especially for birch, the earliest flushing species, this difference was pronounced. The different letter close to the symbols denote a significant difference (at P<0.05) among treatments for each species, separately.
Budburst response to winter and spring warming
Warming advanced the timing of budburst in all three study species (Figure 2). However, the observed temperature sensitivity of budburst, estimated here as the number of days advancement in budburst over the average increase in temperature over the period December 1st to bud burst, differed substantially among species (slopes in Figure 2). Oak and beech exhibited the highest sensitivity to warming (>5 days earlier per °C warming), and birch the lowest (3.5 days °C−1), but these species differences in sensitivity were not statistically significant (P = 0.41 for birch and oak; P = 0.53 for birch and beech; P = 0.79 for oak and beech).
Although all warming treatments elicited earlier budburst relative to the control population, and this in all three species, substantial differences in temperature sensitivity were found between trees that were warmed in winter versus spring (Figure 2). In all three species, budburst in the W6S0 treatment (warmed only in winter), occurred later than in the W0S6 treatments (warmed only in spring), despite the former having been warmed for a longer period. Figure 2 clearly shows that all W6S0 treatments are above the average temperature sensitivity line, while W0S6 treatments are well below the line, confirming that budburst in all three species is much more sensitive to spring warming that to winter warming.
Model comparison
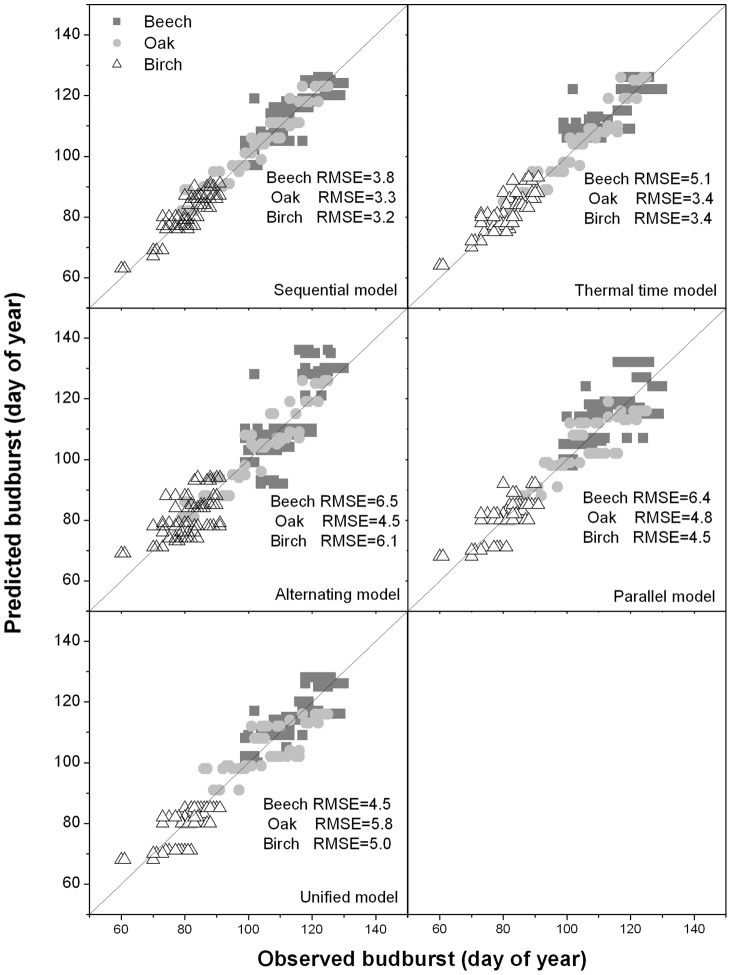
All models yielded low values of RMSE (less than seven days) when fitted on the whole observation dataset (Figure 3). The best-fit parameters values of each model (those with the maximum likelihood as determined with Bayesian calibration) are provided in appendix table S1. For all models, cross-validation showed low values of RMSE and high values of ME in both internal and external validations across the studied models (Table 2). This indicates that all models can be used to reproduce the budburst date, although caution should be taken when applying PM to beech and AM to beech and birch (which showed RMSE = 5–7 days and ME = 0.3–0.5) (Table 2). The Sequential model (SM) performed best across the three studied species (lowest RMSE and highest of all models; Table 2). However, the Sequential model has more parameters and therefore the AIC supports the TTM as best model for oak and birch (AIC = 57.5 for both species), while SM is still selected for beech (AIC = 72.7).
Figure 3. Comparison of the observed budburst dates with the predicted values for five models.
Comparison of the observed budburst dates with the predicted values for five models fitted on the whole observation dataset. Data are for three species. The diagonal line is the 1∶1 line, whereas RMSE is the Root Mean Square Error.
Table 2. Root mean square error (RMSE), model efficiency (ME) and Akaike's Information Criterion (AIC) of model validation for the five models and the three study species.
| Models | Internal dataset | External dataset | whole dataset | ||||||||||||
| beech | oak | birch | beech | oak | birch | beech | oak | birch | |||||||
| RMSE | ME | RMSE | ME | RMSE | ME | RMSE | ME | RMSE | ME | RMSE | ME | AIC | |||
| TTM | 4.1 | 0.75 | 3.1 | 0.94 | 3.0 | 0.82 | 5.6 | 0.59 | 3.7 | 0.88 | 3.7 | 0.77 | 75.0 | 57.5 | 57.5 |
| SM | 3.4 | 0.82 | 2.9 | 0.95 | 2.9 | 0.84 | 4.0 | 0.79 | 3.7 | 0.91 | 3.4 | 0.80 | 72.7 | 67.2 | 65.0 |
| PM | 6.3 | 0.41 | 4.7 | 0.85 | 4.4 | 0.62 | 6.5 | 0.47 | 5.0 | 0.85 | 4.9 | 0.66 | 98.6 | 87.5 | 81.5 |
| AM | 6.5 | 0.41 | 4.0 | 0.9 | 5.3 | 0.48 | 6.5 | 0.46 | 4.7 | 0.85 | 6.2 | 0.33 | 88.1 | 72.2 | 85.6 |
| UM | 4.2 | 0.74 | 5.8 | 0.79 | 4.5 | 0.61 | 4.8 | 0.7 | 5.8 | 0.79 | 5.5 | 0.56 | 81.2 | 91.1 | 86.4 |
‘Internal dataset’ indicates that calibration and validation were done on the same dataset, whereas ‘external dataset’ indicate that calibration and validation were done on independent datasets. The AIC were calculated on the whole dataset. TTM is Thermal Time model, SM is sequential model, PM is Parallel model, AM is Alternating model and UM is Unified model. The smallest RMSE and highest ME values for each species are in bold.
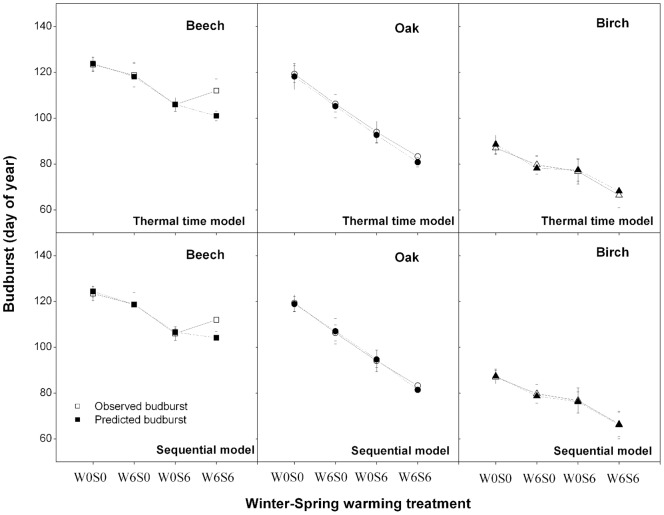
As the SM and the TTM were the best models, the performances of these two models were checked for the different warming treatments, separately (Figure 4). The warming treatments resulted in small differences in model performance. Although the SM performed slightly better, both SM and TTM successfully reproduced the budburst date of birch and oak across all treatments. For beech, both TTM and SM successfully predicted budburst data in W0S0, W0S6 and W6S0, but yielded earlier budburst than observed in W6S6. This difference was small for SM (3 days), but relevant for TTM (10 days).
Figure 4. Modeled against observed budburst dates for different warming treatments.
Modeled against observed budburst dates for different warming treatments (W0S0: no warming; W6S6: winter and spring warming; W6S0: winter warming only, and W0S6: spring warming only; winter is from December 1 2009 to February 22 2010 and spring from February 22 to budburst date) when using the Sequential model and the Thermal time model for each species. The models were fitted on the whole observation dataset. The observed budburst dates are represented by open symbols and the predicted budburst dates are represented by solid symbols.
Discussion
Budburst response to warming
The experimentally manipulated separate warming in winter and spring demonstrated that both winter and spring warming significantly hasten budburst. The strong effect of spring warming on budburst advancement was expected. On the other hand, for all study species, we did not observe a delayed budburst response (rather advancement) to winter warming compared to the control treatment, but did observed later budburst dates in winter warming treatment than in spring warming. We propose two hypotheses to explain the positive effect of winter warming on budburst. First, the chilling requirement of the investigated species was met even under warming conditions. In fact, chilling occurred even in the warmed treatments (data not shown), and some additional chilling might have occurred between bud set [40] and the start of the warming experiment on December 1st. Second, the delaying effect due to unfulfilled chilling was offset and outweighed by a positive effect of winter warming. The positive effect of winter warming (assumed in both hypotheses) might be indirect and related to an earlier start of the forcing phase in warmed saplings (thus before February 22nd).
Our expectations on budburst advancement to be more pronounced for birch than for beech and oak were not confirmed as the budburst temperature sensitivities were non-significantly different among the three study species. In addition, the fact that the advancing effect of strong winter warming, i.e. the W6S6 treatment, on budburst is less for beech, indicate that photoperiod might also play a role in determining the switching between chilling and forcing in late successional species, whose phenology is well known to be affected by photoperiod [41], [42], [43], [44], [45]. The investigated saplings were well fertilized and irrigated throughout the experiment, in order to eliminate a confounding influence of soil water and nutrient availabilities. The future warming may likely lead to changes of precipitation [46], thus altering soil water conditions. Therefore, spring growth conditions may be affected by soil moisture as well as warmer temperatures in future warming conditions.
Predicting future phenology shifts
The model comparison results suggest that temperature-based models can successfully reproduce the date of budburst, and confirmed that temperature is still the main driver of the budburst process under future warming climate. In this study, the one-phase Thermal Time model (without chilling requirement) performed as good as the Sequential model and better than other two-phase models, suggesting that the chilling phase does not play a decisive role in the budburst process of these species under the investigated environmental conditions in this study. The poor performance of the PM, AM and UM model may be link to the fact that these models have many parameters, introducing a risk of over-parameterization. This result is consistent with other model comparison studies using historical phenology datasets [24], [25], [37]. This finding further suggests that budburst is mainly controlled by the forcing phase (i.e. budburst can be modeled with warmth units accumulated since a fixed starting date), and negative effects in response to the reduced chilling are only marginally important. However, caution should be raised. First, the chilling requirement for Betula pendula L. and also for Quercus robur L. is probably much smaller than the chilling requirement of Fagus sylvatica L.[15]. Therefore sufficient chilling may have occurred between bud set and the start of the temperature manipulation [40]. Under these conditions, the similar performances for the TTM and SM are not surprising. On the other hand, the fact that SM performed markedly better than TTM for W6S6 in beech (and to a lesser extent in oak too), indicates that chilling did influence budburst in the late-flushing successional species. A better performance of two-phase models for late-flushing / late-successional species was also pointed out in other studies [13], [26].
In this study, we found that both the SM and TTM were unable to accurately predict the budburst date in the W6S6 treatment, although the SM model approximated the observations relatively well. A hypothesis to explain this result may be a possible effect of photoperiod in constraining the advancement of spring phenology, as both SM and TTM predict earlier budburst than observed, but only in the warmest treatments that exhibited budburst earliest. Thus, the determining factor in budburst advancement may have shifted from temperature requirements to a light signal under extreme warming conditions (e.g. +6°C) [30], [43]. The short photoperiod in late winter and early spring may prevent budburst to protect the trees from frost damage. The important role of photoperiod (especially for late-flushing and late-successional species such as beech) and light quality (red to far-red light) in the budburst process is well established [30], [47]. and photoperiod has indeed been shown to affect the phenology of some species, especially for late-flushing and late-successional species such as beech [41], [48].
Previous comparisons of the different models suggested that no model is superior for all species and should be put forward as a consensus model [22], [28], [49], [50]. In this study, we found that SM is the best model for beech, and both SM and TTM were very good models for oak and birch, but with the TTM slightly better for the latter two species in case parameter uncertainty is high. The SM model therefore might be the most appropriate model to predict budburst data in a future warmer climate, especially for late-flushing species.
Parameterization of phenology models is troublesome. Fitting many parameters with relatively few data is an inherently difficult process and, although model predictions might be correct, some model parameter values might be not fully realistic. Furthermore, complex phenology models might be over-parameterized. For instance, some parameters of the Unified model can be correlated or not relevant (corresponding to low model sensitivity) [28]. This lowers the quality of the parameterization procedure and maybe explains why simpler models were found to have a better fit than the parameter-rich models. Improvements on model parameterization might comprise treating correlated parameters as clusters, model insensitive parameters as constants and, in particular, determining experimentally the model parameters with biological meaning.
Supporting Information
The best-fit parameters values of each model. The best-fit parameters values of each model, those with the maximum likelihood as determined with Bayesian calibration.
(DOCX)
Acknowledgments
Yongshuo H. Fu is holding a research grant from the China Scholarship Council. The authors gratefully acknowledge Dr. Karine Vandermeiren (CODA-CERVA) for experimental assistance. We are grateful to prof. Jacqueline Mohan and the two anonymous reviewers for their valuable comments. We are also grateful to Dr. Maarten Op de Beeck and Dr. Raphael Bequet for field assistance. This research has been financially supported by the research project GHG-Europe-Greenhouse gas management in European Land use systems in EU contract No. 244122.
Funding Statement
The project GHG-Europe-Greenhouse gas management in European Land use systems in EU (http://www.ghg-europe.eu/index.php?id=84) contract no. 244122 and the China Scholarship Council (http://en.csc.edu.cn) provided dissertation funding (fees and living stipend) for Yongshuo H. Fu. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Baldocchi D, Wilson K (2001) Modeling CO2 and water vapor exchange of a temperate broadleaved forest across hourly to decadal time scales. Ecological Modelling 142: 155–184. [Google Scholar]
- 2. Piao SL, Ciais P, Friedlingstein P, Peylin P, Reichstein M, et al. (2008) Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451: 49–U43. [DOI] [PubMed] [Google Scholar]
- 3. Bertin RI (2008) Plant phenology and distribution in relation to recent climate change. Journal of the Torrey Botanical Society 135: 126–146. [Google Scholar]
- 4. Chuine I (2010) Why does phenology drive species distribution? Philosophical Transactions of the Royal Society B-Biological Sciences 365: 3149–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dijkstra JA, Westerman EL, Harris LG (2011) The effects of climate change on species composition, succession and phenology: a case study. Global Change Biology 17: 2360–2369. [Google Scholar]
- 6. Schwartz M, Reiter B (2000) Changes in North American spring. International Journal of Climatology 20: 929–932. [Google Scholar]
- 7. Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397: 659–659. [Google Scholar]
- 8. Menzel A, Estrella N, Fabian P (2001) Spatial and temporal variability of the phenological seasons in Germany from 1951 to 1996. Global Change Biology 7: 657–666. [Google Scholar]
- 9. Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, et al. (2006) European phenological response to climate change matches the warming pattern. Global Change Biology 12: 1969–1976. [Google Scholar]
- 10. Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421: 37–42. [DOI] [PubMed] [Google Scholar]
- 11. Hänninen H, Kramer K (2007) A framework for modelling the annual cycle of trees in boreal and temperate regions. Silva Fennica 41: 167–205. [Google Scholar]
- 12. Cleland E, Chuine I, Menzel A, Mooney H, Schwartz M (2007) Shifting plant phenology in response to global change. Trends in Ecology & Evolution 22: 357–365. [DOI] [PubMed] [Google Scholar]
- 13. Morin X, Lechowicz MJ, Augspurger C, O' Keefe J, Viner D, et al. (2009) Leaf phenology in 22 North American tree species during the 21st century. Global Change Biology 15: 961–975. [Google Scholar]
- 14. Chuine I, Morin X, Bugmann H (2010) Warming, Photoperiods, and Tree Phenology. Science 329: 277–278. [DOI] [PubMed] [Google Scholar]
- 15. Murray MB, Cannell MGR, Smith RI (1989) Date of Budburst of 15 Tree Species in Britain Following Climatic Warming. Journal of Applied Ecology 26: 693–700. [Google Scholar]
- 16.Romberger J (1963) Meristems, growth, and development in woody plants. Washington, D.C. 214 p. [Google Scholar]
- 17.Schwartz MD (2003) Phenology: An integrative environmental science; Kratochwil A, Lieth H, editors. Dordrecht: Kluwer Academic Publishers. 564 p. [Google Scholar]
- 18. Boyer WD (1973) Air Temperature, Heat Sums, and Pollen Shedding Phenology of Longleaf Pine. Ecology 54: 420–426. [Google Scholar]
- 19. Cannell MGR, Smith RI (1983) Thermal Time, Chill Days and Prediction of Budburst in Picea-Sitchensis. Journal of Applied Ecology 20: 951–963. [Google Scholar]
- 20. Harrington C, Gould P, St Clair J (2010) Modeling the effects of winter environment on dormancy release of Douglas-fir. Forest Ecology and Management 259: 798–808. [Google Scholar]
- 21. Morin X, Roy J, Sonie L, Chuine I (2010) Changes in leaf phenology of three European oak species in response to experimental climate change. New Phytologist 186: 900–910. [DOI] [PubMed] [Google Scholar]
- 22. Vitasse Y, Francois C, Delpierre N, Dufrene E, Kremer A, et al. (2011) Assessing the effects of climate change on the phenology of European temperate trees. Agricultural and Forest Meteorology 151: 969–980. [Google Scholar]
- 23.IPCC2007 (2007) Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry KL, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, editors. Cambridge: Cambridge University Press. [Google Scholar]
- 24. Linkosalo T, Lappalainen HK, Hari P (2008) A comparison of phenological models of leaf bud burst and flowering of boreal trees using independent observations. Tree Physiology 28: 1873–1882. [DOI] [PubMed] [Google Scholar]
- 25. Leinonen I, Kramer K (2002) Applications of phenological models to predict the future carbon sequestration potential of boreal forests. Climatic Change 55: 99–113. [Google Scholar]
- 26. Thompson R, Clark RM (2008) Is spring starting earlier? Holocene 18: 95–104. [Google Scholar]
- 27. Campioli M, Caroline Vincke, Mathieu Jonard, Vincent Kint, Demarée G, et al. (2012) Current status and predicted impact of climate change on forest production and biogeochemistry in the temperate oceanic European zone: review and prospects for Belgium as a case study. Journal of forest research 17: 1–18. [Google Scholar]
- 28. Fu YSH, Campioli M, Demarée G, Deckmyn A, Hamdi R, et al. (2012) Bayesian calibration of the Unified budburst model in six temperate tree species. International journal of biometeorology 56: 153–164. [DOI] [PubMed] [Google Scholar]
- 29. Landsberg JJ (1974) Apple Fruit Bud Development and Growth - Analysis and an Empirical Model. Annals of Botany 38: 1013–1023. [Google Scholar]
- 30. Hänninen H (1990) Modelling bud dormancy release in trees from cool and temperate regions, . Acta For Fenn 213: 1–47. [Google Scholar]
- 31. Kramer K (1994) Selecting a Model to Predict the Onset of Growth of Fagus-Sylvatica. Journal of Applied Ecology 31: 172–181. [Google Scholar]
- 32. Sarvas R (1974) Investigations on the annual cycle of development of forest trees. II. Autumn dormancy and winter dormancy. Commun Inst For Fenn 84: 1–101. [Google Scholar]
- 33. Hänninen H (1987) Efftects of temperature on dormancy release in woody plants: implications of prevailing models. Silva Fennica 21: 279–299. [Google Scholar]
- 34. Chuine I (2000) A unified model for budburst of trees. Journal of Theoretical Biology 207: 337–347. [DOI] [PubMed] [Google Scholar]
- 35. Naudts K, van den Berge J, Janssens AI, Nijs I, Ceulemans R (2011) Does an extreme drought event alter the response of grassland communities to a changing climate? Environmental and experimental botany 70: 51–157. [Google Scholar]
- 36.Robert CP, Casella G (2004) Monte Carlo Statistical Methods. New York. 645 p. [Google Scholar]
- 37. Fu YSH, Campioli M, Deckmyn G, Janssens IA (2012) Bayesian comparison of six different temperature-based budburst models for four temperate tree species. Ecological Modelling 230: 92–100. [Google Scholar]
- 38. Van Oijen M, Rougier J, Smith R (2005) Bayesian calibration of process-based forest models: bridging the gap between models and data. Tree Physiology 25: 915–927. [DOI] [PubMed] [Google Scholar]
- 39. D'Haese D, Vandermeiren K, Caubergs RJ, Guisez Y, De Temmerman L, et al. (2004) Non-photochemical quenching kinetics during the dark to light transition in relation to the formation of antheraxanthin and zeaxanthin. Journal of Theoretical Biology 227: 175–186. [DOI] [PubMed] [Google Scholar]
- 40. Søgaard G, Johnsen Ø, Nilsen J, Junttila O (2008) Climatic control of bud burst in young seedlings of nine provenances of Norway spruce. Tree Physiology 28: 311–320. [DOI] [PubMed] [Google Scholar]
- 41. Myking T, Heide OM (1995) Dormancy Release and Chilling Requirement of Buds of Latitudinal Ecotypes of Betula-Pendula and B-Pubescens. Tree Physiology 15: 697–704. [DOI] [PubMed] [Google Scholar]
- 42. Linkosalo T, Hakkinen R, Hänninen H (2006) Models of the spring phenology of boreal and temperate trees: is there something missing? Tree Physiology 26: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 43. Körner C, Basler D (2010) Phenology Under Global Warming. Science 327: 1461–1462. [DOI] [PubMed] [Google Scholar]
- 44. Partanen J, Leinonen I, Repo T (2001) Effect of accumulated duration of the light period on bud burst in Norway spruce(picea abies) of varying ages. Silva Fennica 35: 111–117. [Google Scholar]
- 45. Caffarra A, Donnelly A, Chuine I, Jones MB (2011) Modelling the timing of Betula pubescens budburst. I. Temperature and photoperiod: a conceptual model. Climate Research 46: 147–157. [Google Scholar]
- 46.IPCC (2007) Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry KL, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, editors. Cambridge: Cambridge University Press. [Google Scholar]
- 47. Linkosalo T, Lechowicz MJ (2006) Linkosalo T, Lechowicz MJ. Twilight far-red treatment advances leaf bud burst of silver birch (Betula pendula). Tree Physiology 26: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 48. Heide O (1993) Daylength and Thermal Time Responses of Budburst during Dormancy Release in Some Northern Deciduous Trees. Physiologia Plantarum 88: 531–540. [DOI] [PubMed] [Google Scholar]
- 49. Hunter AF, Lechowicz MJ (1992) Predicting the Timing of Budburst in Temperate Trees. Journal of Applied Ecology 29: 597–604. [Google Scholar]
- 50. Chuine I, Cour P, Rousseau DD (1998) Fitting models predicting dates of flowering of temperate-zone trees using simulated annealing. Plant Cell and Environment 21: 455–466. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The best-fit parameters values of each model. The best-fit parameters values of each model, those with the maximum likelihood as determined with Bayesian calibration.
(DOCX)