Abstract
Based on the phenotype of the activin-like kinase-7 (ALK7)-null mouse, activins A and B have been proposed to play distinct roles in regulating pancreatic islet function and glucose homeostasis, with activin A acting to enhance islet function and insulin release while activin B antagonizes these actions. We therefore hypothesized that islets from activin B-null (BBKO) mice would have enhanced glucose-stimulated insulin secretion. In addition, we hypothesized that this enhanced islet function would translate into increased whole body glucose tolerance. We tested these hypotheses by analyzing glucose homeostasis, insulin secretion, and islet function in BBKO mice. No differences were observed in fasting glucose or insulin levels, glucose tolerance, or insulin sensitivity compared with weight-matched young or older males. Similarly, there were no significant differences in insulin secretion comparing islets from WT or BBKO males at either age. However, BBKO islets were more sensitive to activin A, myostatin (MSTN), and follistatin (FST) treatments, so that activin A and FST inhibited and MSTN enhanced glucose stimulated insulin secretion. While mean islet area and the distribution of islet areas were not different between the genotypes, islet mass, islet number, and the proportion of α-cells/islet were significantly reduced in BBKO islets. These results indicate that activin B does not antagonize activin A to influence whole body glucose homeostasis or β-cell function but does influence islet mass and proportion of α-cells/islet. Therefore, loss of activin B signaling alone does not account for the ALK7-null phenotype, but activin B may have important roles in modulating islet mass, islet number, and the cellular composition of islets.
Keywords: myostatin, β-cell, α-cell, follistatin, activin A
although originally discovered for their actions in the reproductive system, activins A and B are now regarded as having numerous roles in tissue fate determination during embryonic development as well as in regulating homeostatic processes in adults (28, 30, 31). A role for activin in regulating pancreatic islet size and function has been proposed based on in vitro and in vivo studies. Acute treatment of cultured human and rat islets with activin A resulted in enhanced insulin secretion either basally or in response to elevated glucose (7, 24), whereas prolonged treatment of mouse islets with activin A inhibited glucose-stimulated insulin release as well as insulin gene expression (22). Activin A also stimulated Pax4 expression, which led to increased proliferation in β-cell lines (6, 27), suggesting that activin might also regulate β-cell replication in islets. Activin A treatment of MIN6 cells increased immature β-cells while decreasing mature cells, an action accompanied by increased MafB gene expression (22). Blockade of activin signaling through receptor deletion or by transgenic overexpression of a dominant negative receptor in β-cells led to hypoplastic islets and impaired glucose tolerance (8, 19, 32). Even when mutations were limited to adults, such as the reversible overexpression of the TGFβ/activin signaling inhibitor Smad7, islets became hypoplastic and diabetes was induced (21). Moreover, increasing the bioactivity of TGFβ superfamily ligands activins A and B, myostatin (MSTN), and GDF11 through inactivation of their antagonist, follistatin like-3 (FSTL3) (17, 20), produced enlarged islets, β-cell hyperplasia, and enhanced glucose tolerance (14). Alternatively, downregulating the Smad3 second messenger for activin and TGFβ signaling improved β-cell function (11), suggesting that regulation of β-cell function by TGFβ ligands is dependent on species, duration of treatment, and ligand and culture conditions. Nevertheless, taken together, these studies indicate that members of the TGFβ superfamily, including those in the activin subfamily, play important roles in regulating islet size and function.
Activins signal through heterotetrameric membrane receptors composed of type II and type I receptors, which in turn phosphorylate Smad2 and -3. Phosphorylated Smads then dimerize with Smad4 and translocate to the nucleus to alter gene transcription (18). Activin A primarily utilizes ActRIIB (Acvr2b) and ALK4 (activin-like kinase-4;Acvr1b) but activin B has been reported to utilize ALK7 (AcvR1c) (26) as well, suggesting that differential type I receptor utilization may direct a subset activin B-specific actions.
Because activin B, but not activin A, utilizes ALK7 for at least some of its actions, ALK7-null mice could be useful to distinguish in vivo actions of activin B from those attributable to activin A. ALK7-null mice were hyperinsulinemic with reduced insulin sensitivity, impaired glucose tolerance, and enlarged islets (3). Moreover, since hyperinsulinemia was the earliest manifestation of this phenotype, followed later by relative insulin resistance, it was proposed that increased insulin secretion and islet size might be the primary defects in these mice (3). In support of this phenotype being due, at least in part, to loss of activin B signaling, young activin B-null (BBKO) mice were hyperinsulinemic to the same degree as observed in ALK7-null mice. Furthermore, in cultured islets, activin A stimulated, while activin B inhibited, glucose-stimulated calcium flux. Based on these findings, it was proposed that activin B, acting via ALK7, opposes the actions of activin A to negatively regulate β-cell function (3).
On the basis of this proposed negative role for activin B, we hypothesized that BBKO mice would have enlarged islets, enhanced islet function, and improved glucose tolerance, since the islet-enhancing functions of activin A would be unopposed. We tested this hypothesis by analyzing glucose homeostasis, insulin sensitivity, islet morphometrics, isolated islet function, and islet gene expression in both young and older BBKO mice. We found no evidence for altered glucose homeostasis, insulin levels, or insulin sensitivity in young or older BBKO mice. However, islet mass was significantly reduced and the proportion of α-cells/islet was significantly decreased in BBKO islets. Our results indicate that loss of activin B signaling does not, by itself, account for the ALK7-null phenotype, suggesting that other related TGFβ superfamily members that utilize ALK7 may have important roles regulating glucose homeostasis. In addition, our results fail to support the concept that activin B antagonizes the actions of activin A on islet function. However, activin B appears to modulate islet mass, islet number, and the proportion of α-cells/islet.
MATERIALS AND METHODS
BBKO mice.
Mice homozygous for deletion of the Inhbb allele were created as previously described (29) and were obtained by breeding heterozygotes with the Inhbb mutation in a mixed 129/Sv-C57Bl6 background (hereafter referred to as BBKO). All studies were carried out according to protocols approved by the Baystate Health Systems/PVLSI IACUC.
Whole body, organ, and fat pad weights.
After mice were weighed, organs and fat pads were dissected, trimmed, and weighed from males ranging in age from 7 to 14 mo (mean = 10). Since body weight increased more slowly with age in BBKO males than in WT littermates (Fig. 1), metabolic experiments were conducted with weight-matched groups to compensate for this weight difference.
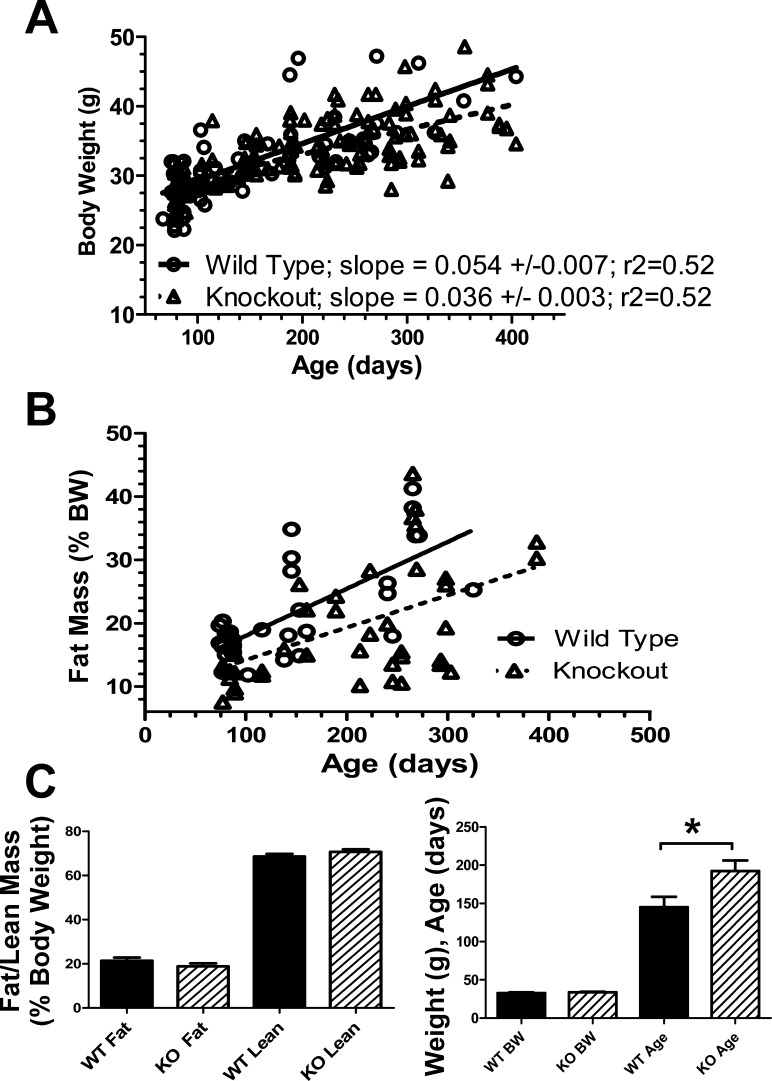
Fig. 1.
Growth and fat analysis of activin B-null (BBKO or KO) mice. A: body weight is plotted vs. age for WT (n = 55) and BBKO (n = 142) mice used in this study. Regression analysis demonstrated that BBKO males grew more slowly than WT littermates, with slopes being significantly different (P < 0.05). We therefore used weight-matched WT and BBKO mice in this study even though the ages were sometimes greater for BBKO mice. B: fat mass increased with age in both WT (n = 35) and BBKO (n = 42) males, but the slopes were not significantly different, indicating that differences in fat mass accumulation were not responsible for the different growth rates. C: mean fat and lean mass, as measured by MRI, are not different for weight-matched WT and BBKO males. D: mice shown in C were weight matched, but mean ages were significantly older for BBKO mice by >30 days.
Whole body composition analysis by quantitative NMR.
An Echo MRI-100 (Echo Model Systems, Houston, TX) was used to analyze body composition by quantitative (q)NMR on males ranging in age from 2.5 to 13 mo.
Analysis of serum glucose and insulin.
Glucose measurements were taken using a hand-held glucometer (OneTouch Ultra; Lifescan, Milpitas, CA), and insulin levels were assessed using ultrasensitive ELISA with mouse insulin standard (Crystal Chem, Downers Grove, IL). Random glucose measurements and serum (for insulin assay) were collected during the day, and fasting measurements and serum were collected in the morning after an overnight fast (>16 h). Young males were ages 3–5 mo and older males were 7–13 mo for insulin and glucose measurements.
Glucose and insulin tolerance tests (GTT and ITT).
For glucose tolerance tests (GTT), animals were fasted overnight (>16 h) and injected with glucose (2 g d-glucose/kg body wt ip), and glucose measurements were taken at 15, 30, 45, 60, 90, and 120 min postinjection. A serum sample was also collected 15 min postinjection. For insulin tolerance tests (ITT), mice were fasted for 4 h and then injected with insulin (Eli Lilly, Indianapolis, IN) at a concentration of 0.9 U/kg body wt ip, and glucose measurements were taken at the same intervals as in the GTT. For GTT and ITT studies, young males were 3–5 mo old, and older mice were 6–8.5 mo old.
Islet analysis.
Weight-matched males that were 9 ± 3 mo of age were utilized, five of each genotype for islet area analysis and seven of each genotype for islet composition analysis. Pancreata were collected from mice and fixed in 4% paraformaldehyde overnight and then transferred into 70% ethanol and processed for paraffin embedding. Sections (4 μm) were stained with hematoxylin and eosin, and all islets from three sections >100 μm apart and representing a large portion of the pancreas that contained a major duct were analyzed. For histomorphometry, 629 WT and 402 BBKO islets were measured on three sections per pancreas for a total of 15 sections per genotype. Islet areas were determined using Spot software and summed for the entire section. Fractional islet area was calculated by dividing this islet area sum by total pancreas area for that section determined with the same microscope and software as used for islet area. To calculate islet mass, fractional islet area was multiplied by pancreas weight. For islet composition studies, immunofluorescence was used on sections adjacent to those used for islet area and were stained for insulin with a guinea pig anti-insulin antibody (Linco Research, St. Charles, MO), and glucagon was visualized using a mouse anti-glucagon antibody (Sigma, St. Louis, MO). The secondary antibodies used were an anti-guinea pig-TRITC and anti-mouse-FITC (Jackson Immunoresearch Laboratories, West Grove, PA). Pictures were captured using Metavue software and the total number of α- and β-cells counted and expressed as a percentage of total cells in the islets.
Islet isolation.
Pancreata were injected with collagenase P (1.2–1.4 mg/ml; Roche Diagnostics, Indianapolis, IN) and removed from donor animals. After digestion, islets were purified using a two-layer gradient comprising Histopaque 1.077 (Sigma) and Hanks' balanced salt solution (Mediatech, Manassas, VA) and then hand-picked. Islets were cultured in a 5% CO2 incubator with RPMI 1640 solution with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin (Mediatech). Four separate islet isolations were analyzed. Young males were 2–3 mo old, and older males were 9 mo of age.
Glucose-stimulated insulin secretion assay.
After overnight culture in RPMI to recover from isolation, islets were equilibrated in low-glucose solution (2.8 mM) for 1 h. Triplicate samples of 10 size-matched islets were placed in 12-μm Millicell Cell Culture PCF inserts (Millipore, Burlington, MA) in 24-well plates. Fresh low-glucose solution (with or without treatments) was added for 2 h, and then the insert with the islets was moved into high-glucose solution (16.7 mM) (with or without treatments) for 2 h. Insulin secretion was determined using ELISA (Mercodia, Winston-Salem, NC), and raw data were transformed as fold difference from the average control in low glucose so that experimental replicates could be combined. The stimulation index was calculated from the raw data and a value >1 indicated functional islets. Human activin A and mouse MSTN (1 nM) were purchased from R&D Systems (Minneapolis, MN). Human FST 315 (5 nM) was produced in the Schneyer laboratory as previously described (5).
Islet gene expression.
For each experiment, pools of 100–150 islets from each genotype at 2–3 or 9 mo were cultured for 24 h to recover from isolation. Islet RNA was then extracted with Nucleospin RNA II columns (Macherey-Nagel, Germany), and 0.5–1 μg RNA was reverse transcribed as previously described (9). Gene expression was determined by SYBR qPCR as described (9), using primers listed in Table 1. For quantitation of islet mRNA, a standard was created using pooled islet RNA that was serially diluted. This standard was run with all qPCR assays including the normalization target RPL19, and only assays in which efficiency was 100 ± 10% were used so they were all parallel. Results from each gene target were normalized to RPL19 to control for variation in RNA amount or quality that was reverse transcribed. Since all PCR results were calculated in terms of the same standard they could be compared for relative expression levels. Results are means of three separate islet isolations.
Table 1.
Primer sequences used for quantitative PCR in this study
| Target | Forward Primer | Reverse Primer |
|---|---|---|
| ActβA | ATCATCACCTTTGCCGAGTC | ACAGGTCACTGCCTTCCTTG |
| MSTN | TGGCCATGATCTTGCTGT | CCTTGACTTCTAAAAAGGGATTCA |
| GDF11 | ACCACCGAGACGGTCATAAG | TGCCTGGGTGTCCAAATGTAAC |
| BMP2 | TCTTCCGGGAACAGATACAGG | TCTCCTCTAAATGGGCCACTT |
| BMP4 | GAGGGATCTTTACCGGCTCC | GTTGAAGAGGAAACGAAAAGCAG |
| BMP7 | CGATACCACCATCGGGAGTTC | AAGGTCTCGTTGTCAAATCGC |
| TGFβ1 | GCTCCCACTCCCGTGGCTTCT | GCGCTTCCGTTTCACCAGCTC |
| TGFβ2 | AGCAAGCTGAAGCTCACCAGCC | CCTGCAGTAAGTCCCTGGTACTGTTG |
| TGFβ3 | AACTCCAGCTCTCTTCCCACTCCC | ACAGGTTCTGCCGGGCTCTG |
| FST | ACCTGAGAAAGGCCACCTG | ACTTTCCCTCATAGGCTAATCCA |
| FSTL3 | CAGGCCACTTGCTTCCTG | TGCAGCTCACACGAAGTTCT |
| Ins1 | CAGCAAGCAGGTCATTGTTTCAAC | TGGGTGGGTTTGGGCTCC |
| Ins2 | GAAGTGGAGGACCCACAAGT | AGTGCCAAGGTCTGAAGGTC |
| Glucagon | AGGGACCTTTACCAGTGATGT | AATGGCGACTTCTTCTGGGAA |
| Glut2 | CTGGAGCCCTCTTGATGGGA | CCAGTCCTGAAATTAGCCCACA |
| Irs2 | GTGGGTACATGCGAATGTGGT | GCGGGGCAAAGAGCTGTAG |
| Pdx1 | GAAATCCACCAAAGCTCACG | CGGGTTCCGCTGTGTAAG |
| MafA | CTCCAGAGCCAGGTGGAG | GTACAGGTCCCGCTCCTTG |
| MafB | GAGAGACGCCTACAAGGTCAACTG | GCTCAAGTCAAACAGGTCAAGGC |
| Arx | GGCCGGAGTGCAAGAGTAAAT | TGCATGGCTTTTTCCTGGTCA |
| HIf1a | TTCCCCTCTCCTGTAAGCAA | TCGACGTTCAGAACTCATCCT |
| PC1 | GGAGGCATAAGAATGCTGGATGGCA | TGCCAGGCCCCTCCACAGTTT |
| PC2 | CGGCGTCCAAGAGACGACGAC | CTGCCCACAAACCCCAGCTCC |
| Kcnj11(Kir6.2) | GGAGCCTGTACCGGGTTATT | AAACCAGTCCCGAGCTGAG |
| Abcc8 | TCTCCGGGGCCGTCTTCTGG | AGGCTACGGGGCCTCTGCTC |
| Gck | CTGGATGACAGAGCCAGGAT | CTCTGCCAGGATCTGCTCTAC |
| Sgk3 | AGGAGAGCTGCCCAAGTGTA | CTTCTGCCCACAGAGACCA |
| Ppy | GACTATGCGACACCTGAGCA | CCAGGAAGTCCACCTGTGTT |
| FSTL5 | CATTCCAGACCCTCAGCTTG | TGTGGACCTCACTGCCATT |
| Gremlin | GACCCACGGAAGTGACAGA | CCCTCAGCTGTTGGCAGTAG |
| Rpl19 | CCTGAAGGTCAAAGGGAATGTGTT | GCTTTCGTGCTTCCTTGGTCTTA |
Data analysis.
Body weight and fat mass comparisons in Fig. 1 were performed by linear regression with slopes compared by t-test using Prism software. Group mean determinations were compared by t-test when two groups were involved, whereas GTT and ITT results, islet area distribution analyses, and comparisons between more than two groups were determined by ANOVA with Tukey's post hoc test. Differences in islet response to treatments were tested by ANOVA with Tukey's post hoc test.
RESULTS
Growth and body composition.
Although the developmental alterations resulting from loss of activin B were described in the original report (29), growth and body composition were not explored. Regression analysis of body weights for nearly 200 males demonstrated that overall gain in body weight with age was significantly reduced in BBKO mice relative to WT littermates (Fig. 1A). Total fat mass, as determined by NMR, was also reduced in BBKO mice compared with age-matched WT mice, but the regression line slopes were not significantly different (Fig. 1B). However, when weight-matched mice were compared, the mean fat and lean masses were not significantly different (Fig. 1C), although BBKO males in this group were significantly older (Fig. 1D). Thus, in age-matched mice, body weight and fat mass were different, and this difference became significant after about 6 mo of age. To avoid bias in subsequent metabolic studies that included older mice, weight-matched WT and BBKO groups were compared.
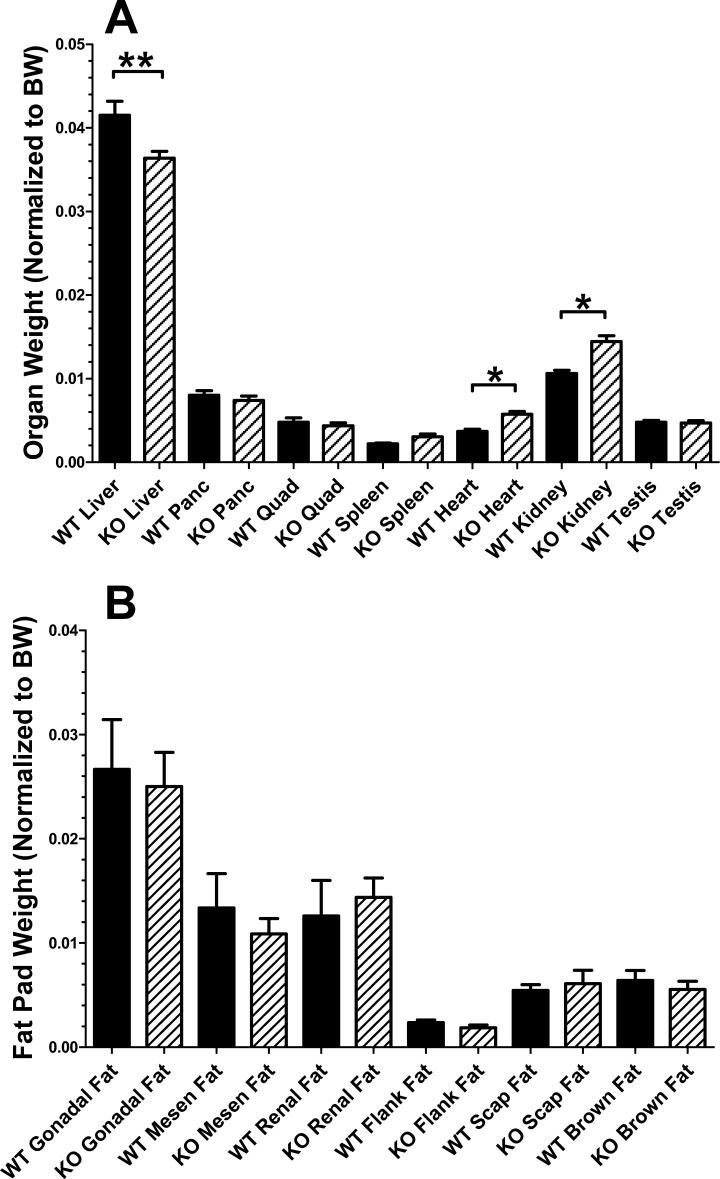
We also examined weights of individual organs to determine if this could account for the reduced body weight in BBKO mice. We found that liver weight, relative to body weight, was significantly reduced, but heart and kidney weights were significantly greater in BBKO males than in WT littermates (Fig. 2A), indicating that organ weight differences alone cannot account for the reduced body weight with age identified in BBKO males. When individual fat pads were removed and weighed, no significant differences were found (Fig. 2B), in agreement with the similar mean fat masses when WT and BBKO mice were weight matched (Fig. 1C).
Fig. 2.
Organ and fat pad weights for WT and BBKO mice. A: organ weights for body weight-matched WT and BBKO males. Liver weight was significantly reduced (P < 0.01), and both heart and kidney were significantly increased (P < 0.05) in BBKO mice. Thus, altered organ weights do not account for reduced body weight in BBKO mice. B: no differences in weight were detected in individual dissected fat pads from WT and BBKO mice; n = 5–21 animals/organ or fat pad, ranging from 7 to 14 mo of age.
Insulin and glucose homeostasis.
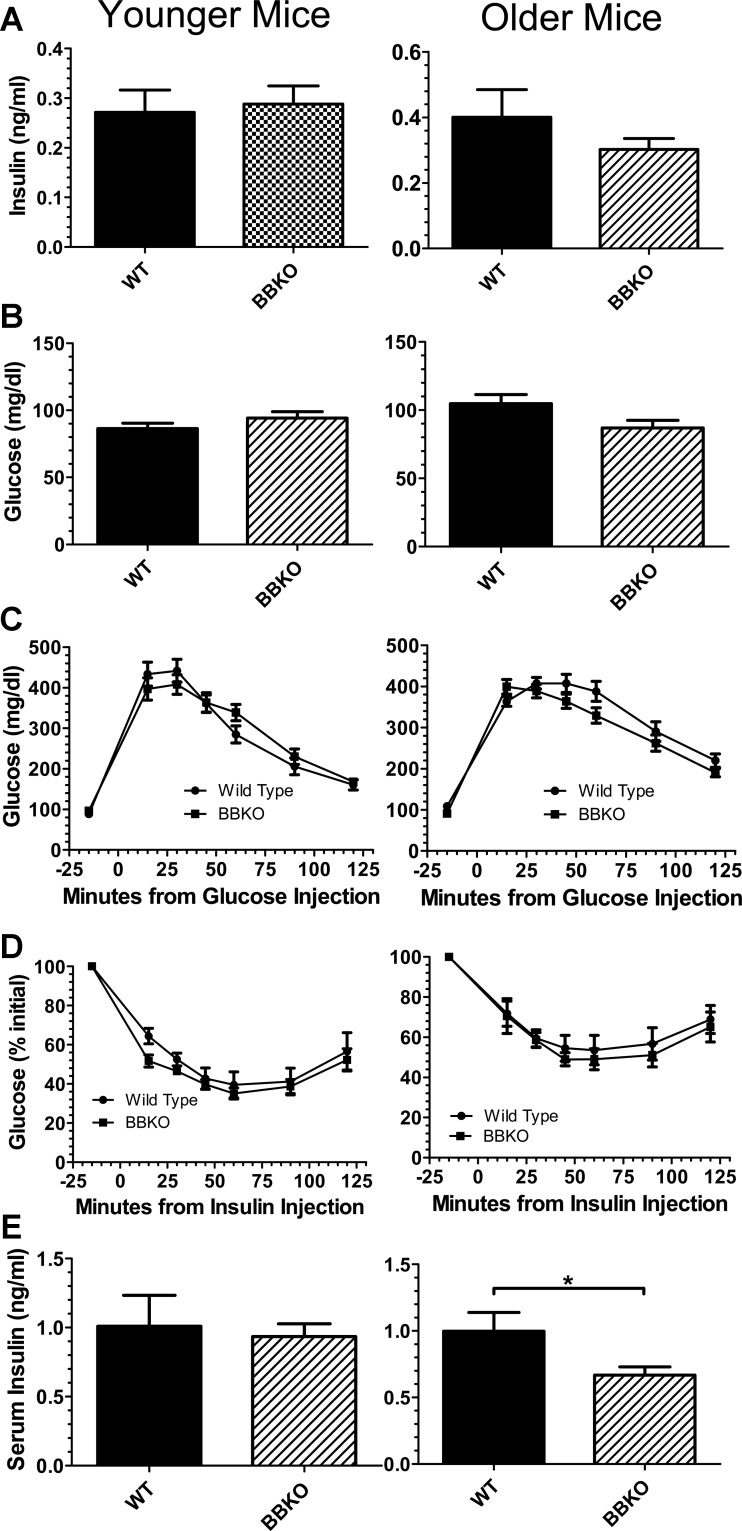
It was previously reported that ALK7-null mice were hyperinsulinemic from 2 wk of age and that the relative insulin resistance that was detectable at 2 mo of age was proposed to result from this elevation in serum insulin concentration (3). In addition, BBKO mice had insulin levels that were twice those of controls, although the body weights of these mice were not provided, consistent with the loss of activin B activity in ALK7-null mice causing hyperinsulinemia and subsequent insulin resistance (3). To assess the contribution of activin B to glucose homeostasis, we investigated insulin and glucose levels in BBKO mice compared with weight-matched WT mice. We detected no significant difference in fasting insulin levels for weight-matched groups of young or old mice (Fig. 3A). Although there were no differences in fasting glucose in younger or older animals, the BBKO mice were significantly older than WT controls in order to match body weights (Fig. 3B). Consistent with these observations, we found no differences in glucose tolerance (Fig. 3C) or insulin sensitivity (Fig. 3D) for younger or older weight-matched mice. However, serum insulin concentrations were significantly reduced at the 15-min time point of the GTT in older BBKO mice compared with WT controls, a difference not observed in younger mice (Fig. 3E). These results demonstrate that between weight-matched males there are no differences in glucose or insulin dynamics in BBKO males except for reduced insulin secretion after glucose challenge in older BBKO males.
Fig. 3.
Glucose homeostasis in weight-matched WT and BBKO mice. A: fasting insulin was not different in younger (3–5 mo; n = 8 WT and BBKO mice) or older (7–10 mo; n = 7, BBKO = 9 mice) WT and BBKO mice. B: fasting glucose was not different in weight-matched younger (3–5 mo; n = 12 WT and 15 BBKO males) or older (6–8.5 mo; n = 12 WT and 7–13 mo; n = 11 BBKO) mice. C: glucose tolerance test (GTT) was not different in younger (3–5 mo; n = 7 WT and 12 BBKO mice) or older (6–8.5 mo; n = 16 WT and 15 BBKO mice). ANOVA was used to analyze data for differences. D: insulin tolerance test (ITT) was not different in weight-matched younger (3.5–5 mo; n = 8 WT and 7 BBKO mice) or older (7–8 mo; n = 6 WT and 7 BBKO) mice. E: serum insulin at 15-min time point of GTT was not different for younger mice (4–5 mo; n = 6 WT and BBKO mice) but was significantly reduced in older (7–10 mo; n = 6 WT and BBKO mice) animals.
Islet morphometrics.
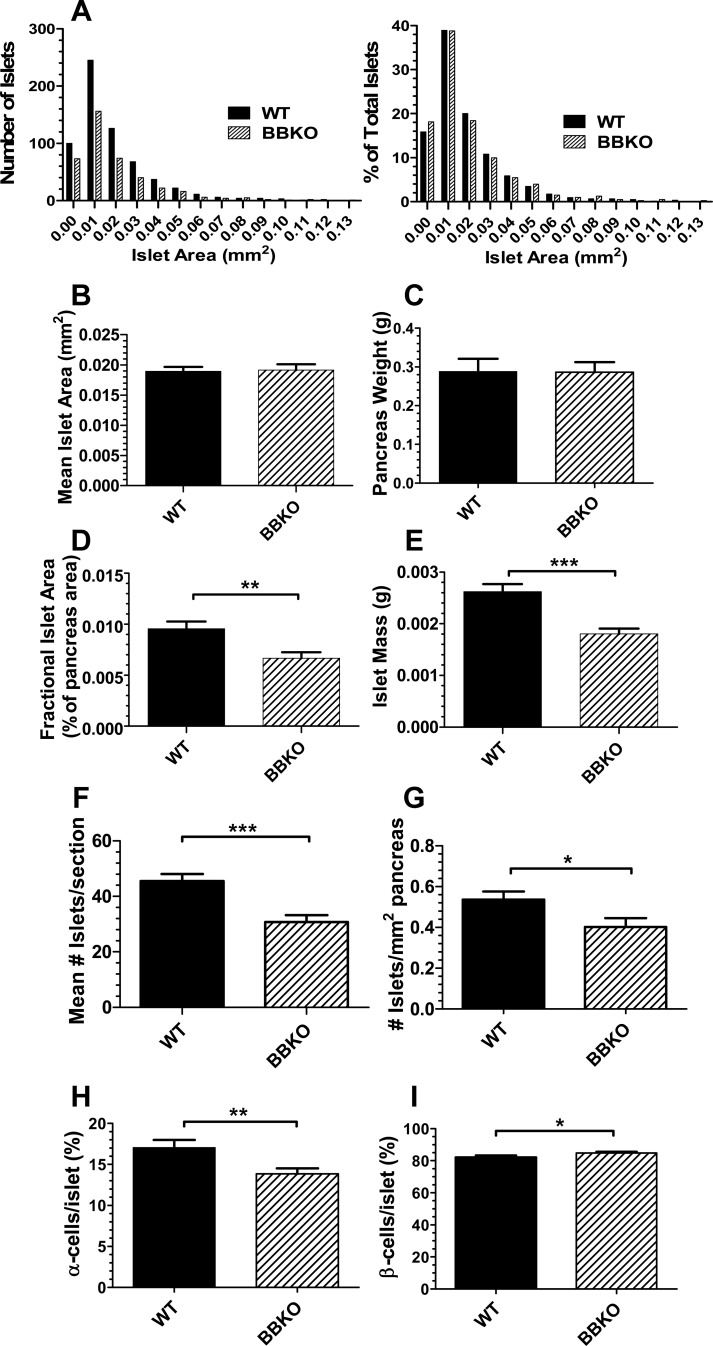
There were no significant differences in islet size between WT and BBKO mice as determined by analyzing absolute or relative islet area distribution (Fig. 4A) or mean area of all islets measured (Fig. 4B). There was also no difference in pancreas weight (Fig. 4C). However, the fractional islet area (Fig. 4D) and islet mass (Fig. 4E) were significantly reduced in BBKO mice compared with WT mice, suggesting that the total number of islets might be reduced in BBKO mice. This was indeed the case, as both mean number of islets per pancreas section (Fig. 4F) and number of islets per square millimeter of pancreas (Fig. 4G) were both significantly reduced in BBKO mice compared with WT controls. Interestingly, the proportion of α-cells/islet was significantly reduced in BBKO islets compared with WT islets (Fig. 4H), whereas β-cell proportion was significantly increased (Fig. 4I). These observations indicate that loss of activin B significantly impacts islet composition, islet mass, and islet number, but this difference is not sufficient to alter whole body glucose homeostasis.
Fig. 4.
Islet characteristics in WT and BBKO weight matched mice. A: absolute and relative distribution of islet areas measured in WT and BBKO pancreata (n = 5 mice/genotype; 3 sections analyzed/pancreas; 629 WT and 402 BBKO islets measured). ANOVA analysis detected no significant differences between WT and BBKO islet distribution. B: no difference in mean islet area was detected between WT and BBKO islets. C: no difference was observed in mean pancreas weight for mice used in islet analysis. D: islet area for each section was divided by total pancreas area on that section to calculate fractional islet area, which was significantly reduced in BBKO mice relative to weight-matched WT littermates. E: fractional islet area was multiplied by pancreas weight for each mouse to calculate islet mass, which was significantly reduced in BBKO mice relative to weight-matched WT littermates. F: mean number of islets/section of pancreas was significantly reduced in BBKO mice. G: number of islets/mm2 of pancreas was significantly reduced in BBKO islets. H: α- and β-cells were identified by double immunofluorescence and counted and percentage of α-cells was significantly reduced in BBKO islets vs. weight-matched WT islets. Results are expressed as %total cells in the islet identified by DAPI staining; n = 7 mice/genotype, 40 WT and 51 BBKO islets counted. I: proportion of β-cells was significantly increased in BBKO islets relative to islets from weight-matched WT littermates. Mice used for this study were weight-matched males 9 ± 3 mo of age.
Islet function.
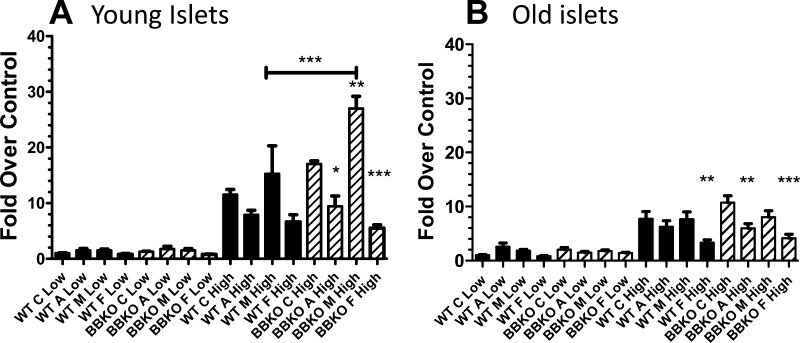
Islet function was determined for islets isolated from either younger or older WT or BBKO males. In addition, we assessed the effects of activin A, FST, and MSTN, since we had previously identified MSTN as the major TGFβ superfamily ligand in mouse islets (5). Insulin secretion was identical for young WT and BBKO islets in low glucose, and this was not altered by treatments (Fig. 5A). Elevated glucose increased insulin secretion equally in WT and BBKO islets (Fig. 5A). While activin and FST both tended to inhibit insulin secretion in WT islets exposed to high glucose, these effects were both greater and reached statistical significance in BBKO islets. Alternatively, MSTN stimulated insulin secretion in the presence of high glucose, and this stimulation reached significance in BBKO islets.
Fig. 5.
Function of WT and BBKO islets. Islets from young and older males were isolated and tested for glucose-stimulated insulin secretion (GSIS) in the presence or absence of activin A, myostatin (MSTN), or follistatin (FST). A: in islets from younger mice, no effect of activin, MSTN, or FST was detected in low glucose. In high glucose, activin A and FST significantly inhibited GSIS, and stimulation of GSIS by MSTN was significantly greater in BBKO islets than in WT. B: in islets from older mice, no effect of activin, MSTN, or FST was observed in low glucose, and in high glucose, activin A and FST both inhibited GSIS in BBKO islets but only FST inhibited GSIS in WT islets. No effect of MSTN was observed in older islets (n = 3 or 4 separate islet isolations and assessments for each group).
As with young islets, islets from older WT and BBKO mice secreted identical amounts of insulin in low glucose, and there was no effect of treatment (Fig. 5B). However, islets from older mice secreted less insulin in response to elevated glucose than younger islets. In addition, under high-glucose conditions, FST inhibited insulin secretion in both WT and BBKO islets, whereas activin A inhibited insulin secretion only from BBKO islets (Fig. 5B). Stimulation of insulin secretion by MSTN was not observed in older islets (Fig. 5B).
Islet gene expression.
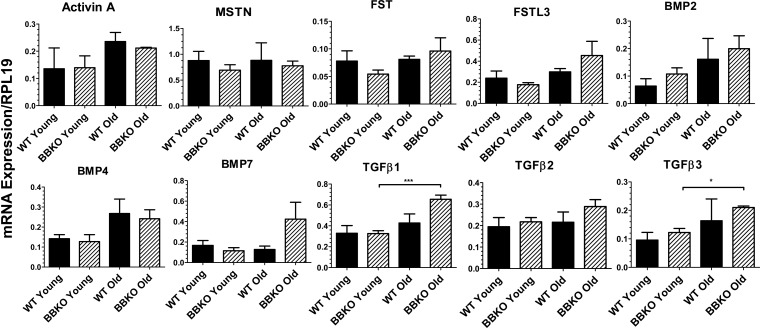
We first examined expression of the major TGFβ family ligands that we previously identified in C57Bl6 mouse islets (5). There were no significant differences in TGFβ family gene expression when comparing WT and BBKO islets (Fig. 6). However, expression levels of TGFβ1 and TGFβ3 were significantly higher in older BBKO islets than in younger ones (Fig. 6). These results indicate the absence of compensation for loss of activin B transcripts through amplification of another TGFβ superfamily growth factor.
Fig. 6.
mRNA expression of TGFβ family ligands and antagonists in young and old WT and BBKO islets. Islet mRNA expression was analyzed by qPCR. No differences between genotypes were observed, but TGFβ1 and -β3 were increased in older BBKO islets; n = 3 separate islet extracts for each group.
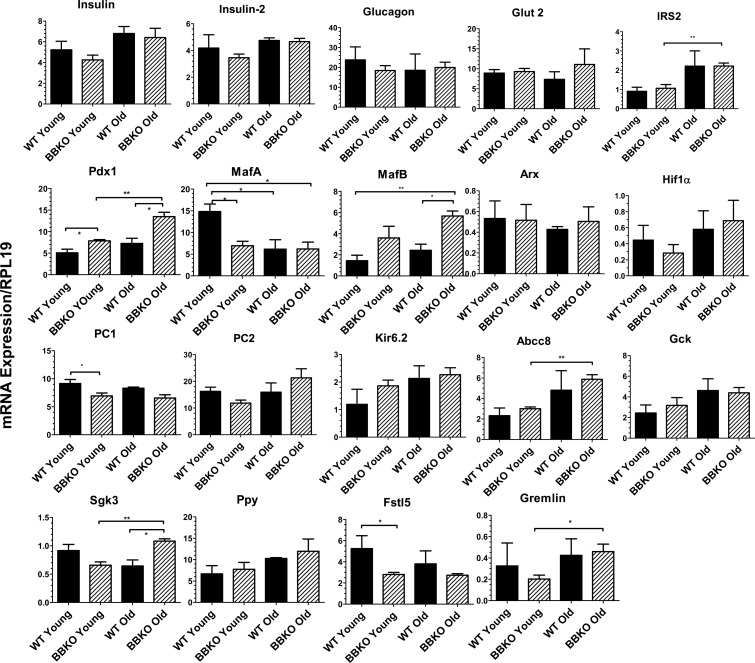
Among β-cell genes, Pdx1 mRNA was increased in BBKO relative to WT islets and in older BBKO islets compared with younger KO islets (Fig. 7). Interestingly, MafA expression was reduced in younger BBKO islets compared with WT but also in islets from older WT and KO mice. Like MafA, PC1 mRNA expression was reduced in BBKO relative to WT islets, but this was significant only in the younger islets. PC2 mRNA expression followed the same pattern in young mice, but the differences were not significant. IRS-2 mRNA expression was increased in islets from older BBKO mice compared with younger BBKO mice but were not different from WT mice, suggesting this difference did not result from the loss of activin B. Expression levels of Ins1, Ins2, Kir6.2, Hif1α, Glut2, and glucokinase were not altered by loss of activin B. Expression of Abcc8 was increased in older islets, and this difference was significant for BBKO islets.
Fig. 7.
mRNA expression of islet genes in young and old WT and BBKO islets. Expression of islet genes was analyzed by qPCR in young and old islets. β-Cell genes such as Pdx1 were increased in BBKO islets, as were some α-cell genes such as MafB; n = 3 separate isolations for each group.
MafB, an important α-cell gene in adult mice, was increased in BBKO islets relative to WT, but this difference was significant only in older islets (Fig. 7). Other α-cell genes, such as Arx and glucagon, were not different in BBKO islets. To explore possible causes for the reduced α-cell percentage in BBKO islets, we examined some genes recently identified as differentially expressed in immature relative to mature β-cells (23). We found that Sgk3, a gene that is expressed more highly in immature β-cells, was higher in older BBKO islets compared with both older WT islets and younger BBKO islets. Expression of Ppy followed this same trend, but the differences were not significant. On the other hand, Fstl5, another gene more highly expressed in immature β-cells, was expressed at lower levels in BBKO islets compared with WT. In addition, gremlin, a gene more highly expressed in mature β-cells (23), was increased in older BBKO islets relative to younger BBKO islets but was not different from WT islets at the same age.
DISCUSSION
Although activins A and B have similar activities in vitro, their roles in vivo appear to be at least partially distinct. Loss of activin A was neonatally lethal (13), whereas loss of activin B was tolerated, although several developmental defects were reported (29). Further support for in vivo distinctions in activity was obtained when the sequence for the mature portion of the activin B peptide was inserted into the locus for activin βA, so that activin B was expressed everywhere that either activin A or B is normally expressed (Inhbabk/bk or BK mice) (4). Although this rescued the neonatal lethality of complete loss of activin A (13), BK mice were deficient in both growth and metabolism (4, 10). These studies indicate that while there is substantial overlap in function, activins A and B may have additional distinct roles in vivo involved in growth and metabolism. Activin B-specific roles could be achieved via signaling through the ALK7 type I receptor, which is not used by activin A but is also utilized by other TGFβ superfamily ligands such as GDF3 and nodal (16, 26). Based on analysis of ALK7-null mice, it was proposed that one such activin B-specific role was to oppose the islet enhancing activity of activin A (3).
We hypothesized that if this proposed role for activin B was critical, then activin B-null islets would have enhanced function in vitro and in vivo, which would result in improved glucose-stimulated insulin secretion, expanded islet size, increased islet mass, and enhanced glucose tolerance as was described in ALK7-null mice (3). However, we detected no differences in fasting insulin, fasting glucose, glucose tolerance, or insulin sensitivity in younger or older mice. While insulin levels following glucose challenge during the GTT were lower in older BBKO mice than in WT controls, given identical whole body insulin sensitivity, the physiological significance of this finding is unclear. Therefore, we conclude that activin B does not play a critical role in regulating islet function that is sufficient to alter whole body glucose tolerance or insulin sensitivity.
Growth curves demonstrated that BBKO males grew significantly more slowly that WT littermates, so that using age-matched WT controls would lead to significant differences in body weight between the groups at ages above 6 mo. This would introduce a second variable that could have substantial influence on glucose homeostasis and insulin sensitivity. We therefore used weight-matched WT controls for all metabolic experiments. For example, differences in fat mass between age-matched WT and BBKO mice were eliminated when weight-matched controls were utilized even though the BBKO mice were older.
To determine whether loss of activin B alters islet function itself, we examined the response to elevated glucose in young and older islets in the presence or absence of activin A, MSTN, or FST treatments. BBKO islets were more sensitive to activin A, MSTN, and FST treatment in terms of insulin secretion compared with WT islets. However, activin A inhibited insulin secretion in these studies, a finding that agrees with chronic activin treatment of mouse islets (22) but conflicts with studies in rat (24) and human islets (7), as well as with the proposed model in which activin B would inhibit β-cell-enhancing effects of activin A (3). Interestingly, FST also inhibited insulin secretion induced by elevated glucose, whereas MSTN, a TGFβ superfamily member closely related to activin and which uses similar signaling pathways (1, 12, 25), enhanced insulin secretion. Since FST is an activin/MSTN/GDF11 antagonist (17), the seemingly paradoxical observation that both activin and FST inhibit insulin secretion may derive from both molecules inhibiting MSTN stimulation of insulin secretion, with FST directly binding and neutralizing MSTN while activin displaces MSTN from a common type II receptor. Moreover, since MSTN reportedly utilizes ALK5 as its primary type I receptor whereas activins utilize ALK4 and -7 (25), differential downstream signaling by the distinct type I receptors could explain opposite actions for activin and MSTN in islets. Thus, the outcome of altering either antagonist (e.g., FSTL3) or ligand (activin A, activin B, MSTN, or GDF11) bioavailability may depend on the relative concentrations of these ligands, their locations relative to the antagonists, and the degree to which their actions can override those of the other ligands. Importantly, our group and others (5, 22) recently demonstrated that adult mouse islets produce activins A and B, MSTN, and GDF11, with MSTN being synthesized in greatest abundance. Moreover, immunohistochemical analyses demonstrated that both activin A and B proteins were preferentially localized to α-cells (5), whereas the source for MSTN and GDF11 remains unknown. In addition, microarray experiments identified FSTL3 in mRNA from β-cells and not α-cells (33). Taken together, these findings suggest that FSTL3 from β-cells may regulate activin action in α-cells as well as MSTN and GDF11 that could be produced in α- or β-cells. Thus, the overall effect of altering an individual TGFβ family ligand or inhibitor may depend on whether the action is autocrine or paracrine as well as the relative concentration of the various ligands and inhibitors. In vitro, however, loss of activin B appears to increase islet sensitivity to the stimulatory effects of MSTN as well as the inhibitory effects of activin A. In addition, the accentuated effects of FST in BBKO islets may indicate that the stimulatory effect of MSTN is stronger than the inhibitory effect of activin, or that the concentration of MSTN is greater, so that more MSTN than activin is neutralized (reducing insulin secretion) while less activin is inhibited (further reducing insulin secretion). These effects will have to be sorted out in an environment where endogenous ligands can be removed, such as under perifusion, which would also allow derepression from downregulation that continuous exposure to endogenous ligands might produce.
Although there was no difference in mean islet size, morphometric analysis revealed that BBKO islets have a reduced proportion of α-cells/islet and a corresponding increase in β-cells/islet compared with WT islets. In addition, fractional islet area and islet mass were also reduced in BBKO mice, as was the number of islets per section and islet density per section. This decrease in islet number likely accounts, at least in part, for the reduced islet mass in BBKO mice. The cause of reduced α-cell proportion is unclear, but activin B could stimulate α-cell proliferation or promote survival, resulting in reduced α-cell number with loss of activin B in BBKO mice. Moreover, since ALK7-null mice had larger islets and increased islet mass, these results support the conclusion that the phenotypes observed in ALK7-KO mice are not due to loss of activin B signaling but perhaps to another ligand that uses ALK7, such as GDF3 (16).
We examined gene expression in young and old BBKO islets compared with WT controls to assess whether loss of activin B altered expression of important α- or β-cell genes or was compensated for by increased expression of TGFβ family genes. We found no evidence for compensatory increase in expression of any related TGFβ family member, suggesting that the phenotype of BBKO mice is related to reduced activin B activity. With respect to islet genes, important regulators of β-cell fate and function, like Pdx1, were increased in BBKO islets. However, other genes important for β-cell function, such as MafA and PC1, were reduced, and many genes, such as Ins1, Ins2, and Glut2, were unchanged. Moreover, MafB, an important regulator of α-cells in adult islets, was increased in BBKO islets, whereas Arx and glucagon expression levels were unchanged. The lack of clarity in gene expression changes may derive from divergent effects on α- and β-cells within the extracted islets.
Alternatively, it is also possible that loss of activin B resulted in a continuous process of enhanced β-cell neogenesis, resulting in a range of β-cell maturities within each islet with different mRNA expression patterns. Previous studies have demonstrated that MafA expression is restricted to mature β-cells, whereas MafB is expressed in insulin-positive cells during development that do not yet express MafA, suggesting that β-cells mature through a MafB+Ins+MafA− immature stage to a MafB−MafA+Ins+ mature stage (2, 15). Moreover, the MafB-to-MafA transition occurs in Pdx1-positive β-cells; that is, Pdx1 and MafB are coexpressed in maturing β-cells (15). We found that Pdx1 and MafB expressions were significantly elevated in BBKO islets, which might be expected from the presence of an expanded pool of immature β-cells in both young and old BBKO mice that are in the process of maturing. To explore this possibility further, we examined genes that were reported to be more highly expressed in immature than in more mature β-cells (23). We found increased expression of some genes that were elevated in immature β-cells (e.g., Sgk3) but not others (e.g., Fstl5) and even increased expression of some genes that were more highly expressed in mature β-cells (e.g., gremlin). Thus, although there is no clear genetic signature indicative of an altered β-cell maturation state in BBKO islets, it remains possible that formation of new β-cells is increased in BBKO mice but that their maturation or survival is suppressed by loss of activin B, leading to reduced islet mass. This possibility is being addressed through lineage tracing technology.
Our results demonstrate that loss of activin B does not alter whole body glucose homeostasis or insulin sensitivity in young or older mice, nor does it alter islet function relative to WT islets, findings that do not support the proposal that activin B acts as a negative regulator of activin A action (3). Moreover, the absence of altered glucose homeostasis in BBKO mice indicates that the hyperinsulinemic and glucose-intolerant phenotype of ALK7-null mice is not due to loss of activin B signaling alone, suggesting that other growth factors using ALK7 might have roles in modulating glucose control. However, activin B does have significant actions regulating the proportion of α- to β-cells within islets as well as islet mass and number in that they are all reduced in BBKO islets, suggesting that BBKO mice might be more susceptible to obesity-induced hyperglycemia. Our results, therefore, suggest that enhancing activin B bioactivity within islets may provide a method for increasing islet mass that could be pursued for potential therapeutic effectiveness for diabetes.
GRANTS
This work was supported by: R01 DK-075058 and 3R01 DK-75058–3S1 RA from the National Institute of Diabetes and Digestive and Kidney Diseases (A. Schneyer) and R01 HD-032067 (M. M. Matzuk) from the National Institute of Child Health and Human Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.B., M.L.B., M.M.M., and A.S. conception and design of research; L.B., M.L.B., N.U., M.M., and A.S. performed experiments; L.B., M.L.B., N.U., M.M., and A.S. analyzed data; L.B., M.L.B., N.U., M.M., M.M.M., and A.S. interpreted results of experiments; L.B., M.L.B., M.M., and A.S. prepared figures; L.B., M.M., and A.S. drafted manuscript; L.B., M.L.B., N.U., M.M., M.M.M., and A.S. edited and revised manuscript; L.B., M.L.B., N.U., M.M., M.M.M., and A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We are indebted to Brooke Bentley for efficient and expert preparation of tissues for histological examination.
REFERENCES
- 1. Andersson O, Reissmann E, Ibanez CF. Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis. EMBO Rep 7: 831–837, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes 59: 2530–2539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertolino P, Holmberg R, Reissmann E, Andersson O, Berggren PO, Ibanez CF. Activin B receptor ALK7 is a negative regulator of pancreatic +/- cell function. Proc Natl Acad Sci USA 105: 7246–7251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown CW, Houston-Hawkins DE, Woodruff TK, Matzuk MM. Insertion of Inhbb into the Inhba locus rescues the Inhba-null phenotype and reveals new activin functions. Nat Genet 25: 453–457, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Brown ML, Kimura F, Bonomi LM, Ungerleider NA, Schneyer AL. Differential synthesis and action of TGFss superfamily ligands in mouse and rat islets. Islets 3: 367–375, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brun T, Franklin I, St-Onge L, Biason-Lauber A, Schoenle EJ, Wollheim CB, Gauthier BR. The diabetes-linked transcription factor PAX4 promotes (beta)-cell proliferation and survival in rat and human islets. J Cell Biol 167: 1123–1135, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Florio P, Luisi S, Marchetti P, Lupi R, Cobellis L, Falaschi C, Sugino H, Navalesi R, Genazzani AR, Petraglia F. Activin A stimulates insulin secretion in cultured human pancreatic islets. J Endocrinol Invest 23: 231–234, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Kim SK, Hebrok M, Li E, Oh SP, Schrewe H, Harmon EB, Lee JS, Melton DA. Activin receptor patterning of foregut organogenesis. Genes Dev 14: 1866–1871, 2000 [PMC free article] [PubMed] [Google Scholar]
- 9. Kimura F, Sidis Y, Bonomi L, Xia Y, Schneyer A. The follistatin-288 isoform alone is sufficient for survival but not for normal fertility in mice. Endocrinology 151: 1310–1319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li L, Shen JJ, Bournat JC, Huang L, Chattopadhyay A, Li Z, Shaw C, Graham BH, Brown CW. Activin signaling: effects on body composition and mitochondrial energy metabolism. Endocrinology 150: 3521–3529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin HM, Lee JH, Yadav H, Kamaraju AK, Liu E, Zhigang D, Vieira A, Kim SJ, Collins H, Matschinsky F, Harlan DM, Roberts AB, Rane SG. Transforming growth factor-beta/Smad3 signaling regulates insulin gene transcription and pancreatic islet beta-cell function. J Biol Chem 284: 12246–12257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Massague J. TGFbeta in cancer. Cell 134: 215–230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matzuk MM, Kumar TR, Vassilli A, Bickenbach RR, Roop DR, Jaenisch R, Bradley A. Functional analysis of activins during mammalian development. Nature 374: 354–356, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Mukherjee A, Sidis Y, Mahan A, Raher MJ, Xia Y, Rosen ED, Bloch KD, Thomas MK, Schneyer AL. FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci USA 104: 1348–1353, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishimura W, Kondo T, Salameh T, El KI, Dodge R, Bonner-Weir S, Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol 293: 526–539, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romano V, Raimondo D, Calvanese L, D'Auria G, Tramontano A, Falcigno L. Toward a better understanding of the interaction between TGF-beta family members and their ALK receptors. J Mol Model 2012. February 22 [Epub ahead of print] PMID: 22354277 [DOI] [PubMed] [Google Scholar]
- 17. Schneyer AL, Sidis Y, Gulati A, Sun JL, Keutmann H, Krasney PA. Differential antagonism of activin, myostatin and growth and differentiation factor 11 by wild-type and mutant follistatin. Endocrinology 149: 4589–4595, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Shiozaki S, Tajima T, Zhang YQ, Furukawa M, Nakazato Y, Kojima I. Impaired differentiation of endocrine and exocrine cells of the pancreas in transgenic mouse expressing the truncated type II activin receptor. Biochim Biophys Acta 1450: 1–11, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M, Schneyer A. Biological activity of follistatin isoforms and follistatin like-3 are dependent on differential cell surface binding and specificity for activin, myostatin and BMP's. Endocrinology 147: 3586–3597, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Smart NG, Apelqvist AA, Gu X, Harmon EB, Topper JN, MacDonald RJ, Kim SK. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS Biol 4: e39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Szabat M, Johnson JD, Piret JM. Reciprocal modulation of adult beta cell maturity by activin A and follistatin. Diabetologia 53: 1680–1689, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Szabat M, Pourghaderi P, Soukhatcheva G, Verchere CB, Warnock GL, Piret JM, Johnson JD. Kinetics and genomic profiling of adult human and mouse beta-cell maturation. Islets 3: 175–187, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Totsuka Y, Tabuchi M, Kojima I, Shibai H, Ogata E. A novel action of activin A: stimulation of insulin secretion in rat pancreatic islets. Biochem Biophys Res Commun 156: 335–339, 1988 [DOI] [PubMed] [Google Scholar]
- 25. Tsuchida K, Nakatani M, Uezumi A, Murakami T, Cui X. Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr J 55: 11–21, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Tsuchida K, Nakatani M, Yamakawa N, Hashimoto O, Hasegawa Y, Sugino H. Activin isoforms signal through type I receptor serine/threonine kinase ALK7. Mol Cell Endocrinol 220: 59–65, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Ueda Y. Activin A increases Pax4 gene expression in pancreatic beta cell lines. FEBS Lett 480: 101–105, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Vale W, Rivier C, Hsueh A, Campen C, Meunier H, Bicsak T, Vaughan J, Corrigan A, Bardin W, Sawchenko P, Spiess J, Rivier J. Chemical and biological characterization of the inhibin family of protein hormones. Rec Prog Horm Res 44: 1–34, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Vassalli A, Matzuk MM, Gardner HA, Lee KF, Jaenisch R. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev 8: 414–427, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med (Maywood) 227: 724–752, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Xia Y, Schneyer AL. The biology of activin: recent advances in structure, regulation and function. J Endocr 202: 1–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamaoka T, Idehara C, Yano M, Matsushita T, Yamada T, Ii S, Moritani M, Hata Ji Sugino H, Noji S, Itakura M. Hypoplasia of pancreatic islets in transgenic mice expressing activin receptor mutants. J Clin Invest 102: 294–301, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang YH, Szabat M, Bragagnini C, Kott K, Helgason CD, Hoffman BG, Johnson JD. Paracrine signalling loops in adult human and mouse pancreatic islets: netrins modulate beta cell apoptosis signalling via dependence receptors. Diabetologia 54: 828–842, 2011 [DOI] [PubMed] [Google Scholar]