Abstract
Intact tight junctional (TJ) proteins are required for tubular ion transport and waste excretion. Disruption of TJs may contribute to a decreased glomerular filtration rate in acute kidney injury (AKI) via tubular backleak. The effect of LPS-mediated AKI on murine TJs has not been studied extensively. We hypothesized LPS endotoxin administration to mice would disrupt tubular TJ proteins including zonula occludens-1 (ZO-1), occludin, and claudins. ZO-1 and occludin immunofluorescence 24 h post-LPS revealed a marked change in localization from the usual circumferential fencework pattern to one with substantial fragmentation. Renal ZO-1 expression was significantly reduced 24 h after LPS (decrease of 56.1 ± 7.4%, P < 0.001), with subsequent recovery. ZO-1 mRNA expression was increased 24 h post-LPS (4.34 ± 0.87-fold, P = 0.0019), suggesting disruption of ZO-1 protein is not mediated by transcriptional regulation, but rather by degradation or changes in translation. Similarly, claudin-4 protein expression was decreased despite elevated mRNA. LPS administration resulted in dephosphorylation of occludin and fragmented tubular redistribution. Protein expression of claudin-1, and -3 was increased after LPS. ZO-1, occludin, and claudin-1, -3, and -4 gene expression were increased 48 h after LPS, suggesting a renal response to strengthen TJs following injury. Interestingly, reduced mRNA expression was found only for claudin-8. This study provides further support that LPS-induced AKI is associated with structural injury and is not merely due to hemodynamic changes.
Keywords: claudin, LPS endotoxin, acute kidney injury, occludin, ZO-1
sepsis is a leading cause of mortality in the intensive care unit. In critically ill patients, sepsis is associated with kidney injury in 48% of cases, and the combination of both conditions portends a mortality rate near 60% (57). The proportion of severe sepsis cases, and mortality due to sepsis, continue to increase (16), reinforcing the importance of understanding underlying mechanisms of sepsis-induced acute kidney injury (AKI). We have previously described an animal model of sepsis which replicates the clinical features in humans, including AKI, following the administration of LPS endotoxin (12, 13, 24, 60). Mice display only subtle tubular injury after LPS injection, in contrast to the widespread tubular necrosis observed in ischemia-reperfusion models of AKI (44). These mild pathological manifestations in conjunction with weight loss and an elevated blood urea nitrogen (BUN)-to-creatinine ratio previously suggested that hemodynamic mechanisms such as hypotension may explain the decrease in glomerular filtration rate (GFR) seen in this model; however, tubular apoptosis and neutrophil infiltration are consistently observed (12).
Three major factors contribute to the profound decrease in clearance clinically associated with acute tubular necrosis: tubular backleak, cast obstruction, and tubuloglomerular feedback (37, 51, 54). The phenomenon of tubular backleak has been explored in humans following ischemic injury during kidney transplantation (31, 42). Tubular injury increases paracellular permeability, which is regulated by tight junction (TJ) proteins. Kwon et al. (31) showed increased fractional clearance of dextran and backleak of inulin following posttransplant ischemic AKI; these findings were associated with alterations in TJ protein distribution in proximal renal tubules. The TJ is composed of zonula occludens-1 (ZO-1), transmembrane proteins, and signaling molecules. ZO-1 is a 220-kDa intracellular scaffolding protein which links membrane-bound components of the TJs to the actin cytoskeleton (17, 58). ZO-1 interacts with signaling proteins such as Src as well as the TJ strand composed of claudins and TJ-associated marvel proteins: occludin, tricellulin, and marvelD3 (15, 19, 38, 39, 46). The expression of occludin and ZO-1 are higher in the distal nephron than the proximal nephron (21). While transepithelial electrical resistance (TEER) also increases in the distal nephron (14), a direct link between TEER and ZO-1 or occludin expression cannot be established and may depend significantly on claudins. Various members of the claudin family are expressed in different segments of the nephron, with some acting as pore-formers for specific ion passage, such as claudin-2, -10a, -10b, and -16, and others serving to generally tighten barrier function, such as claudin-3 in the distal convoluted tubule (4, 7, 29, 30).
Multiple in vivo and cell culture models have shown disruption of TJs in response to ischemic injury, free radical injury, or ATP depletion in various tissues. A mouse model of renal ischemia-reperfusion injury has revealed alterations in the TJ transcriptome (28). In rat and murine intestine, both ischemia-reperfusion and cecal ligation and puncture models have resulted in displacement of TJ proteins from their membrane-bound location (34, 35). In cultured renal epithelial cells, oxidative injury changed cellular distribution of TJ proteins and increased monolayer permeability (20, 40, 56). Similarly, ATP depletion in cells leads to dissociation of the TJ complex from its membrane anchorage (14, 23). Despite these findings, no publication to date has focused on protein and gene expression of TJ proteins in the kidney following LPS administration.
The aim of this study is to investigate the in vivo changes in epithelial TJ distribution, protein expression, and gene expression in the kidney following LPS exposure. A prior study of LPS-mediated AKI in the rat revealed increased tubular TJ permeability and ultrastructural abnormalities on electron microscopy but did not characterize individual TJ components (27). Here, we describe expression and localization of ZO-1, occludin, and claudin-1, -3, -4, and -8 in mice at various times following LPS exposure to provide a more integrated picture of renal structure following endotoxemia.
MATERIALS AND METHODS
Administration of LPS endotoxin to induce kidney injury in mice.
Thirty-nine male C57BL/6 mice were obtained commercially and studied at 9 wk of age (Charles River Laboratories, Boston, MA). At time 0, mice received a single intraperitoneal injection of either Escherichia coli LPS endotoxin (10 mg/kg in 0.1 ml 0.9% normal saline, Sigma, St. Louis, MO) or 0.9% normal saline. To alleviate a portion of volume depletion, mice were administered 0.25 ml sterile saline as a series of subcutaneous injections at time 0 relative to LPS injection and every 12 h thereafter. Blood was obtained for BUN and/or cytokine measurements at time 0, 2 h, and 6 h after LPS injection. Most mice were euthanized 24 h after LPS (n = 16) or saline (n = 9) injection, with harvest of blood and renal tissue. A subset of mice was euthanized at 6 (n = 4) and 48 h (n = 10). Two mice in the 48-h group died before tissue harvest; all mice survived at other time points. BUN concentrations were determined with a Beckman CX5CE autoanalyzer. Creatinine concentrations were determined by quantitative colorimetric determination (Stanbio, Elkhart, IN). This project was approved by the Institutional Animal Care and Use Committee of The University of Chicago.
Evaluation of reduced-dose LPS endotoxin in mice.
Ten male C57BL/6 mice were studied at 9 wk of age. At time 0, mice received a single intraperitoneal injection of either E. coli LPS endotoxin (0.5 mg/kg in 0.1 ml 0.9% normal saline, Sigma) or 0.9% normal saline. Mice were volume resuscitated as above and euthanized 24 h after LPS (n = 5) or saline injection (n = 5).
Cytokine measurement.
TNF-α levels were determined from sera obtained 2 h after LPS administration using a commercially available ELISA kit according to the manufacturer's instructions. (eBioscience Mouse TNF alpha ELISA Ready-SET-Go!, no. 88–7324).
Pathology.
For routine histological analysis, kidneys were sectioned coronally, fixed in 4% phosphate-buffered formalin, embedded in paraffin, and stained with periodic acid-Schiff base with hematoxylin counterstain. Histological sections for each animal were assigned a semiquantitative score for tubular injury as described by Nomura (43). A blinded observer assigned a score that ranged from 0 (no injury) to 3 (severe/widespread injury) for each of three variables: tubular dilatation/flattening, tubular casts, and tubular degeneration/vacuolization. For each animal, five cortical high-power fields (HPF) were examined at random. For each variable within each field, a score of 0 was assigned when <5% of the tubules were affected, a score of 1 when 5–33% were affected, a score of 2 when 34–66% were affected, and a score of 3 when >66% were affected. For immunohistochemistry, tissue was immediately frozen in OCT (Tissue-Tek, Torrence, Ca) compound at −80°C. Four-micrometer kidney cryostat sections were cut and stored at −80°C. A whole frozen kidney was concomitantly stored at −80°C for use in Western immunoblotting and real-time PCR.
Neutrophil staining.
Tissue immediately frozen in OCT compound at −80°C was cut into 4-μm kidney cryostat sections and fixed with ether/ethanol, incubated with 0.06% H2O2 for 30 min, and blocked with 0.3% BSA. Sections were stained for neutrophils by sequential incubation with rat anti-mouse neutrophil (mAb 7/4; Serotec, Raleigh, NC) serum at 1:60 dilution for 30 min followed by horseradish peroxidase-conjugated rabbit anti-rat IgG (Sigma) at 1:60 dilution for 30 min and diaminobenzidine reagent (Vector Labs, Burlingame, CA) for 5 min. A blinded observer counted the number of neutrophils per HPF and recorded the average of 10 fields for each tissue section.
SDS-PAGE and immunoblotting of frozen mouse kidney tissue.
A portion of frozen kidney was thawed, weighed, and homogenized at 4°C for 30 min in radio immunoprecipitation assay (RIPA; 500 μl) buffer with a mini-complete protease inhibitor (Roche Diagnostics, Indianapolis, IN). Samples were homogenized by mortar and pestle. The homogenized lysate was centrifuged at 5,000 g at 40°C for 5 min, and the supernatant was collected. Protein concentrations of each fraction were measured using the bicinchoninic acid (BCA) procedure (Pierce Chemical, Rockford, IL). Samples (30 μg protein) were electrophoresed through a 4–12% SDS-PAGE gel (Invitrogen Nu-PAGE) under reducing conditions. Proteins were transferred to an Immobilon-P nitrocellulose membrane (Millipore, Bedford, MA) and blocked overnight in PBS/5% BSA. Membranes were incubated overnight with polyclonal rabbit antibodies to ZO-1 (0.25 mg/ml, 1:200 dilution, Invitrogen), occludin (0.25 mg/ml, 1:200 dilution, Invitrogen), claudin-1 (0.25 mg/ml, 1:200 dilution, Invitrogen), claudin-3 (1 mg/ml, 1:500 dilution, Assay Biotech, Sunnyvale, CA), claudin-4 (0.267 mg/ml, 1:800 dilution, Protein Tech, Chicago, Il), and claudin-8 (0.5 mg/ml, 1:100 dilution, Invitrogen). Further 2-h incubations followed with either 680 nm goat anti-rabbit IgG (1:10,000 dilution, Li-Cor Biosciences, Lincoln, NE) or 800 nm donkey anti-mouse IgG (1:5,000 dilution, Li-Cor Biosciences). Activity was detected using an Odyssey infrared imager (ODY-1320, Li-Cor Biosciences) and Odyssey 2.1 software. A protein molecular size ladder control was run for each membrane with Precision Plus Protein (Bio-Rad, Hercules, CA). An actin control was performed for each membrane with mouse anti-actin antibody (1:2,000 dilution, Sigma). Band density was assessed with ImageJ software (v1.44p, National Institutes of Health, Bethesda, MD) and normalized to actin for each lane.
Immunofluorescence and laser-scanning confocal microscopy.
Frozen sections were air dried and fixed in 1:1 100% EtOH/ethyl ether for 10 min followed by 95% EtOH for 20 min. Sections were incubated overnight at humidified 4°C with primary ZO-1 (1:25 dilution), occludin (1:25 dilution), claudin-1 (1:25 dilution), claudin-3 (1:100 dilution), and claudin-8 (1:33 dilution) antibodies in sterile 0.3% BSA/PBS. Sections stained for claudin-4 were fixed in −20°C acetone for 20 min according to the supplier's instructions and incubated with primary claudin-4 antiserum (1:265 dilution) overnight. For localization studies, directly conjugated ZO-1 mouse monoclonal antibody-Alexa Fluor 488 (Invitrogen) was incubated with polyclonal goat antibody to megalin (0.2 mg/ml, Santa Cruz Biotechnology, Santa Cruz. CA) or polyclonal rabbit antibody to thiazide-sensitive sodium-chloride cotransporter (NCC; 1:1,500 dilution, custom manufacture by Pocono Farms). Subsequently, sections were incubated for 2 h with appropriate secondary antibodies at 1:100 dilution (including Alexa Fluor 647, Alexa Fluor 488, and rhodamine 594). Control slides with secondary antibody but without primary antibody were prepared to evaluate nonspecific staining and autofluorescence. Sections were washed and counterstained with 4′,6-diamidino-2-phenylindole (DAPI, 1:1,000 dilution, Invitrogen, Camarillo, CA) for 5 min. Slides were mounted with Fluorogel (Biomedia, Hatfield, PA). Localization of TJs was achieved using a Fluoview 200 laser-scanning confocal microscope equipped with a 647-nm argon laser at ×20 and ×60 magnification. Images were compiled by integration of images gathered at a Z-axis increment of 0.2 μm using the accompanying software. Alexa 647 fluorescence exposure was standardized at 800 ms, rhodamine exposure was 200 ms, Alexa 488 exposure was 100 ms, and DAPI 350 exposure was 5 ms.
Quantitative real-time PCR.
A portion of frozen kidney was placed in TRIzol reagent (GIBCO BRL, Grand Island, NY), from which total RNA was purified according to the manufacturer's instructions. RNA quantity and quality were determined using a Nanodrop ND-1000 UV-Vis spectrophotometer. To remove all traces of genomic DNA, samples were then treated with RNAse-free RQ1 DNAse (1 U/4 μg RNA, Promega, Madison, WI) in 10 μl reaction buffer (final concentration 40 mM Tris·HCl, 10 mM MgSO4, 1 mM CaCl2, pH 8.0) at 37°C for 30 min. This was followed by addition of 1 μl of 20 mM EGTA, pH 8.0, to stop the reaction, and incubation at 65°C for 10 min to inactivate the DNAse. cDNA was generated from RNA using random hexamers as primers with a SuperScript III first-strand synthesis kit (GIBCO BRL) according to the manufacturer's instructions and diluted fivefold before analysis. Real-time PCR was performed using the Applied Biosystems 7900 system and the SybrGreen intercalating dye method with HotStar DNA polymerase (Applied Biosystems, Foster City, CA). Each reaction was conducted in triplicate in a total volume of 20 μl with primers at 200 nM, 1 mM dNTPs, 3 mM MgCl2, and 4 μl of sample or standard cDNA. PCR was carried out with a hot start at 95°C (10 min) followed by 40 cycles at 95°C (15 s)/59°C (60 s). For each sample, the number of cycles required to generate a given threshold signal (Ct) was recorded. Using a standard curve generated from serial dilutions of kidney cDNA, the ratio of all gene expressions relative to 18S expression was calculated for each experimental animal and normalized relative to an average ratio from the control (no LPS) group. Qualitative PCR revealed products of each reaction as a single band when run on an agarose gel, confirming specific amplification. Primers were designed using Primer3 and Blast. Synthesis was performed by Invitrogen Custom Primers with sequences as follows: 18S forward primer 5-GTT GGT GGA GCG ATT TGT CT-3, 18S reverse primer 5GAA CGC CAC TTG TCC CTC TAT-3; occludin forward primer 5-TGG CTG CTG CTG ATG AAT A-3, occludin reverse primer 5-CAT CCT CTT GAT GTG CGA TAA T-3; ZO-1 forward primer 5-GAC CTT GAG CAG CCG TCA TA-3, ZO-1 reverse primer 5-CCG TAG GCG ATG GTC ATA GTT-3; claudin-1 forward primer 5-ACC GCT CAG GCC ATC TAC-3, claudin-1 reverse primer 5-CCA GCA GGA TGC CAA TTA C-3; claudin-3 forward primer 5-GAG ATG GGA GCT GGG TTG TA-3, claudin-3 reverse primer 5-GGA TCT TGG TGG GTG CAT AC-3; claudin-4 forward primer 5-GCT GGG AAG GGC AGT AGA G-3, claudin-4 reverse primer 5-GGG CGT AAT GGC AAG AGT AG-3; claudin-8 forward primer 5-GGC AAC CTA CGC TCT TCA AA-3, claudin-8 reverse primer 5-CAG GGA GTC GTA GAC CTT GC-3 (1); and macrophage inflammatory protein (MIP)-2 forward primer 5-CAC CAA CCA CCA GGC TAC A-3, MIP-2 reverse primer 5-GCC CTT GAG AGT GGC TAT GA-3.
Statistics.
Statistical analysis was performed using the SigmaStat 10.0 software package (Systat, San Jose, CA). Unless noted otherwise, data are given as means ± SE. Groups were compared by two-tailed t-test or ANOVA (Holm-Sidak) when more than two groups were compared. Bonferroni's correction for multiple comparisons was used when more than two groups were compared. Adjusted P values <0.05 were considered significant. Unadjusted P values are provided in the text with insignificant comparisons clearly denoted. Because expression data obtained by real-time PCR were not normally distributed, ANOVA was performed on log-transformed data.
RESULTS
ZO-1 expression and distribution are altered after LPS administration.
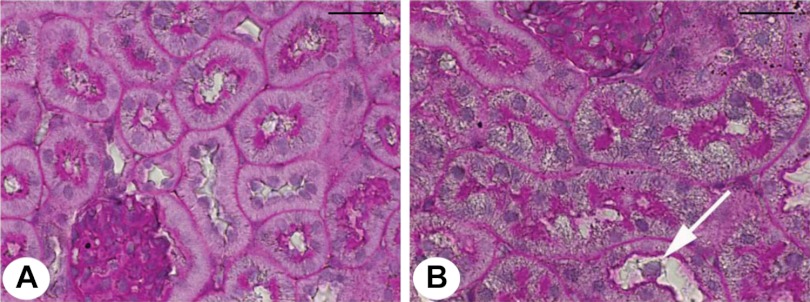
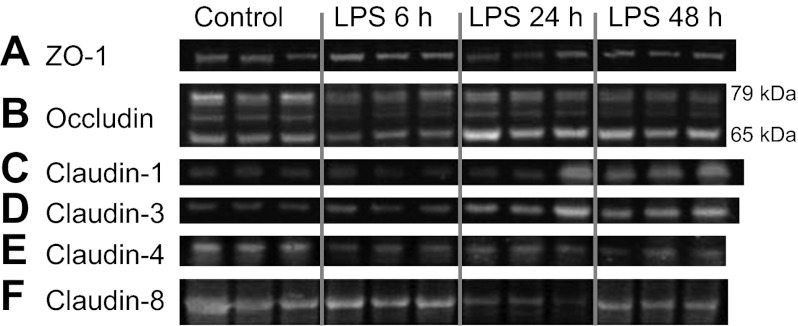
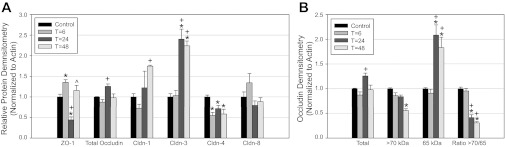
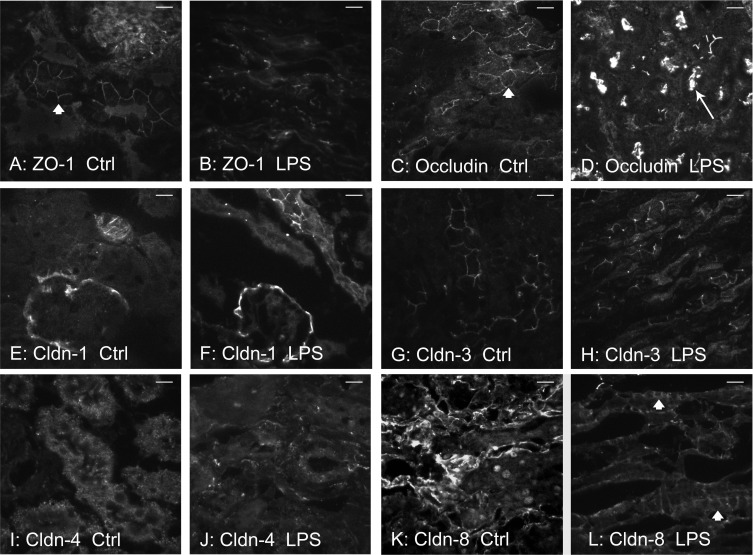
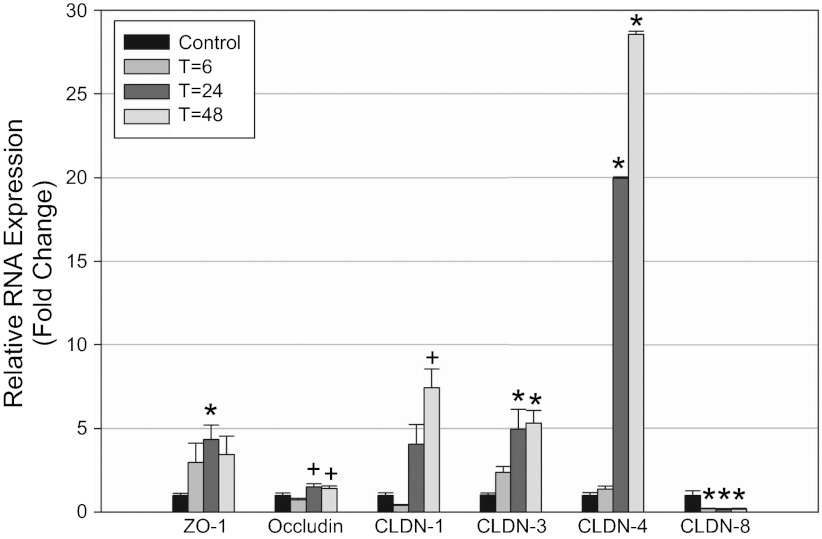
To determine changes in TJ protein and gene expression over time, mice were euthanized 6, 24, and 48 h after LPS injection. Laboratory values, animal weights, and changes in renal histology are provided in Table 1. LPS injection produced a notable rise in BUN by 6 h, which increased further by 24 h. Mice were administered a saline bolus at time 0 and every 12 h thereafter for the duration of the study to alleviate a portion of volume depletion. Light microscopy 24 h post-LPS revealed a subtle, patchy distribution of injury with intratubular vacuolization, and occasional areas of tubular dilatation and casts (Fig. 1). ZO-1 protein expression varied between time points (P < 0.001, Figs. 2A and 3A). Total ZO-1 expression seen by immunoblotting was increased at 6 h (35.5 ± 6.4%, P = 0.009). Compared with baseline, expression was significantly reduced 24 h after LPS (56.1 ± 7.4%, P = 0.00003), and subsequently recovered at 48 h. These findings are consistent with immunofluorescence staining of ZO-1 in frozen mouse kidney (Fig. 4). Not only was total expression decreased at 24 h, a marked change occurred in localization from the usual circumferential fencework pattern to a disorganized pattern with greater fragmentation (Fig. 4, A and B). ZO-1 mRNA expression also varied significantly between time points (P = 0.014, Fig. 5), but opposite in direction to changes in protein expression at 24 h. Relative to control, mRNA expression was increased at all time points, most significantly 24 h post-LPS (4.34 ± 0.87-fold, P = 0.0019). Together, these results suggest the disruption of ZO-1 seen 24 h post-LPS is not mediated by transcriptional regulation, but rather by protein degradation or changes in translational regulation.
Table 1.
Experimental data in control mice and animals given LPS
| Control Mice (n = 9) | LPS-Injected Mice (n = 30) | P Value | |
|---|---|---|---|
| Weight loss at 24 h, % | 4.40 (0.70) | 8.86 (0.50) | P ≤ 0.01 |
| BUN at time 0, mg/dl | 27.7 (1.94) | 26.6 (0.89) | P = 0.57 |
| BUN at 6 h, mg/dl | 23.9 (1.03) | 38.6 (1.18) | P ≤ 0.01 |
| BUN at 24 h, mg/dl | 22.2 (0.70) | 102.2 (5.81) | P ≤ 0.01 |
| BUN at 48 h, mg/dl | N/A | 110.9 | N/A |
| Creatinine at 24 h, mg/dl | 0.50 (0.04) | 1.23 (0.10) | P ≤ 0.01 |
| TNF-α at 2 h, pg/ml | 1.9 (1.2) | 1,665.5 (179.7) | P ≤ 0.01 |
| PAS microscopy score | |||
| Vacuolization (0–3) | 0.47 (0.24) | 2.77 (0.11) | P ≤ 0.01 |
| Tubule dilatation (0–3) | 0.96 (0.25) | 0.95 (0.18) | P = 0.98 |
| Cast formation (0–3) | 0.31 (0.11) | 0.81 (0.19) | P = 0.07 |
| Neutrophil infiltration, PMN/hpf | 3.00 (0.92) | 18.56 (1.73) | P ≤ 0.01 |
Values are means (SE). BUN, blood urea nitrogen; PAS, periodic acid-Schiff; PMN, polymorophonuclear neutrophils; hpf, high-power field. Mice receiving LPS had statistically greater weight loss, rise in BUN and creatinine, serum TNF-α, neutrophil infiltration, and light microscopy changes.
Fig. 1.
Effect of LPS on light microscopic pathology. Periodic acid-Schiff-stained light microscopy of mouse kidney tissue is shown. A: normal mouse kidney. B: mouse kidney 24 h post-LPS. Widespread vacuolization and occasional denuded cells (arrow) are seen in tubules. Magnification: ×60. Bar = 20 μm.
Fig. 2.
Effect of LPS on renal tight junction (TJ) protein expression. Mice were injected with LPS (10 mg/kg) and euthanized 6, 24, and 48 h after injection. Representative immunoblots are shown. Each lane represents a different mouse. A: zonula occludens-1 (ZO-1) expression is decreased 24 h following LPS administration. B: occludin is heavily phosphorylated, revealing multiple bands from 79 to 65 kDa. Total occludin expression is unchanged; however, a shift from higher molecular weight bands to the lower molecular weight 65-kDa band occurs following LPS administration. C: claudin-1 expression increases over time after LPS administration. D: claudin-3 expression increases over time after LPS administration. E: claudin-4 expression decreases at all time points after LPS administration. F: claudin-8 expression was not significantly changed at any time point after LPS administration.
Fig. 3.
TJ protein densitometry. Protein levels of ZO-1, occludin, and claudin-1, -3, -4, and -8 were determined at each time point. Band densities are expressed as a ratio to actin. Protein expression in control mice was normalized to a value of 1. A: total protein expression in whole mouse kidney for all 6 TJ proteins. B: occludin densitometry. Total occludin did not differ significantly between baseline and post-LPS mice. Highly phosphorylated occludin band (those >70 kDa in size) intensity decreased while the lower molecular weight 65-kDa occludin increased. The ratio of bands >70 kDa to the 65-kDa band decreased 24–48 h (T) after LPS administration. *Significant comparison with baseline with adjusted P < 0.05. +Significant comparisons to T = 6 with P < 0.05. ^Significant comparisons to T = 24 with P < 0.05.
Fig. 4.
TJ staining in mouse kidney. Frozen sections of kidney tissue were stained via immunofluorescence for TJ proteins in control mice and mice 24 h post-LPS. A: ZO-1 control stain. Tubules are shown with circumferential “fencework” cellular staining (arrowhead). B: ZO-1 24 h post-LPS. Tubules lack this usual circumferential pattern. Instead, there were significant fragmentation and nonlinear, diffuse staining of ZO-1. C: occludin control stain. Circumferential cellular staining is denoted by the arrowhead. D: occludin 24 h post-LPS. Tubules reveal decreased circumferential staining, with increased apical and intratubular distribution (arrow). E: claudin-1 control stain. Bowman's capsule and occasional tubular staining are observed. F: claudin-1 staining 24 h post-LPS. No discernible changes were noted compared with control. G: claudin-3 control stain. Circumferential tubular staining is shown. H: claudin-3 staining 24 h post-LPS. Tubules revealed increased total staining after LPS, but much of the staining was less specific to the cellular membrane, giving a more diffuse, cytoplasmic pattern. I: claudin-4 control stain. Staining appears to be mainly cytoplasmic, which may represent fixation artifact. J: claudin-4 staining 24 h post-LPS. Intensity of staining is decreased. Circumferential tubular cell staining showed occasional areas of fragmentation; circumferential staining was not appreciated. K: claudin-8 control stain. Bright linear tubular staining is noted with some circumferential staining. Occasional artifact nuclear staining was noted as well. L: claudin-8 staining 24 h post-LPS. Circumferential cellular staining is noted (arrowheads). Magnification: ×60. Bar = 20 μm.
Fig. 5.
Effect of LPS on renal TJ mRNA expression. mRNA expression by real-time PCR is provided as a ratio to 18S. RNA expression in control mice was normalized to 1. ZO-1 mRNA expression was increased 4.3 ± 0.9-fold at 24 h vs. baseline. Occludin mRNA expression was increased 1.5 ± 0.2-fold at 24 h vs. 6 h. Claudin-1 mRNA expression was increased 7.4 ± 1.1-fold at 48 h. Claudin-3 mRNA expression was increased 5.3 ± 0.8-fold at 48 h vs. baseline. Claudin-4 mRNA expression was increased 28.6 ± 9.9-fold at 48 h. Claudin-8 mRNA expression was decreased 5.7 ± 0.3-fold. *Significant comparison with control with adjusted P < 0.05. +Significant comparisons to T = 6 with P < 0.05; n = 4 for all groups except 24-h group, where n = 7.
Occludin expression and distribution are altered following LPS administration.
Occludin protein and RNA expression were determined in mice 6, 24, and 48 h after LPS exposure. The posttranscriptional modification of the occludin protein is complex, with several splice variants and limited proteolysis by matrix metalloproteinases (11). Additionally, occludin is posttranslationally modified with heavy serine and threonine phosphorylation, typically yielding a spectrum of bands between 65 and 85 kDa (23, 48). Sakakibara (48) showed that treatment of protein with a phosphatase led to convergence of bands toward 65 kDa. Increased phosphorylation of occludin is associated with higher transepithelial resistance and more complete localization to the TJ (34, 47, 48, 52). Protein expression is shown for whole kidney total occludin, as well as the ratio of higher molecular weight occludin bands (at or above 70 kDa) to lower molecular weight occludin bands (at 65 kDa).
Compared with baseline, total occludin protein expression was not significantly changed at any time point after LPS injection. A modest nonsignificant increase in total protein (25.7 ± 5.9%) was noted 24 h post-LPS, although this was significantly increased vs. the 6-h densitometry (P = 0.0028) (Figs. 2B and 3B). In contrast, high molecular weight occludin bands (>70 kDa) were significantly reduced at 48 h (44.7 ± 3.9%, P = 0.000049). Furthermore, the 65-kDa band of occludin revealed significantly increased density 24 and 48 h post-LPS (108.3 ± 20.8% increase, P = 0.0011 and 82.9 ± 21.2% increase, P = 0.0052, respectively). The ratio of the heavier occludin bands to the 65-kDa band was significantly decreased at both 24 and 48 h after LPS vs. controls.
Notably, these changes in occludin posttranslational isoforms correlate with occludin redistribution. Occludin staining was changed following LPS administration in a pattern similar to ZO-1 (Fig. 4, C and D). Analogous to ZO-1, occludin failed to localize in its usual circumferential, fence-like pattern 24 h after LPS administration. Occludin staining became fragmented, disrupted, and in selected areas densely localized to the apical surface of tubules. A significant difference in mRNA expression was noted between time points (P = 0.0060, Fig. 5); however, the effect size was small. Compared with control, expression of occludin mRNA 24 h after LPS administration was increased (P = 0.037), but did not reach significance after adjustment for multiple comparisons. Pairwise comparisons between 6 h and either 24 or 48 h were significant (P = 0.0012, P = 0.0068, respectively); however, the change in mRNA expression was less than one-fold different. Thus transcriptional regulation appears to play a modest role, if any, in changes in occludin protein expression after LPS-induced AKI.
Claudin-1 and -3 protein and gene expression are increased following LPS administration.
Claudin-1 and -3 protein and gene expression were determined in mice 6, 24, and 48 h after LPS exposure. For claudin-1, protein levels were decreased at 6 h (nonsignificant) and subsequently increased 24 and 48 h after LPS exposure (Figs. 2C and 3A). Claudin-1 protein was significantly increased between 6 and 48 h (142.1 ± 10.1% increase, P = 0.009). Protein expression between baseline and 48 h was also elevated (74.8 ± 2.3%, P = 0.01, nonsignificant when adjusted for multiple comparisons). For claudin-3, total protein expression was unchanged on immunoblotting at 6 h (Figs. 2D and 3A), but increased markedly at 24 and 48 h post-LPS vs. baseline (240 ± 24.4% increase, P = 0.00020, and 224 ± 11.7% increase, P = 0.00046, respectively). However, despite this increased protein expression on immunoblotting, changes in claudin-1 and -3 seen on immunofluorescence were subtle. Claudin-1 immunofluorescence was not significantly altered between control and 24 h (Fig. 4, E and F). As seen in prior studies, staining was observed predominantly in Bowman's capsule with occasional tubular staining; its distribution did not change after LPS administration (7, 29). The claudin-3 immunofluorescence pattern 24 h after LPS exposure revealed increased total staining of tubules, but the staining was less specific to the cellular membrane. The pattern was more diffuse and intracellular (Fig. 4, G and H), as has been noted in other tissues (34). mRNA expression of claudin-1 and -3 followed a similar trend to protein expression. Claudin-1 expression increased at 24 and 48 h (7.41 ± 1.13-fold increase at 48 h, P = 0.0064, Fig. 5). Similarly, mRNA expression of claudin-3 was significantly increased at 24 and 48 h (5.30 ± 1.34-fold increase at 48 h, P = 0.0014, Fig. 5).
Claudin-4 and -8 expression are altered following LPS administration.
We focused on claudin-4 and -8 as relevant TJ proteins to study because of their expression in the distal tubule, including the collecting duct, where backleak through injured TJs may have a significant effect upon clearance. In contrast to claudins -1 and -3, total claudin-4 protein expression was decreased on Western blotting at all time points relative to control (Figs. 2E and 3A), including 48 h (41.9% ± 22.3% decrease, P = 0.0092). Immunofluorescence 24 h after LPS injection revealed reduced intensity and some fragmentation of claudin-4 (Fig. 4, I and J). Surprisingly, despite this clear decrease in claudin-4 protein expression, claudin-4 mRNA expression was markedly increased at 24 and 48 h after LPS (28.56 ± 9.88-fold increase at 48 h, P = 0.00088, Fig. 5). Claudin-8 protein expression showed a trend toward a decrease 24 h post-LPS, although this did not reach statistical significance compared with baseline. Immunofluorescence also suggested a decrease in claudin-8; however, there was clearly preservation at the cell membrane in many areas (Fig. 4, K and L). Unlike all other claudins studied, mRNA expression of claudin-8 after LPS injection was significantly decreased at all time points (5.68 ± 0.31-fold decrease at 48 h, P = 0.00143, Fig. 5).
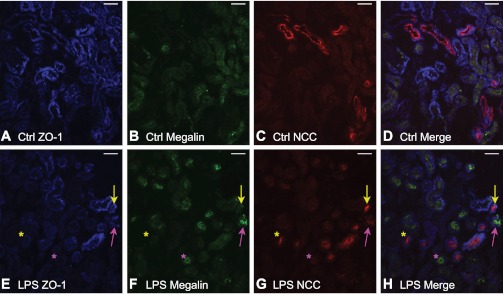
Reduced ZO-1 expression after LPS is found in both proximal and distal cortical tubular segments.
Frozen sections from control mice and from mice 24 h after LPS were stained with tubular markers of proximal and distal nephron segments. Antibodies to megalin and NCC labeled the proximal and distal convoluted tubule, respectively. Colocalization of ZO-1 with tubular markers was observed in the proximal tubule and distal convoluted tubule (Fig. 6, A–D), consistent with its known distribution throughout the nephron. A directly conjugated ZO-1 antibody was used for localization and had reduced intensity of staining compared with its unconjugated rabbit counterpart (used elsewhere in this study). Nonetheless, significant changes in immunofluorescence were observed in mice following LPS-mediated AKI (Fig. 6, E–H). Following LPS, a ZO-1 decrease was found sporadically in both the proximal and distal cortical tubular segments. No pattern suggesting greater disruption in a particular segment of the nephron could be reliably discerned. Areas of preserved tubular ZO-1 staining were found in proximal and distal convoluted tubular sections following LPS administration, similar to the classic patchy pattern described in acute tubular necrosis.
Fig. 6.
Localization of ZO-1 after LPS administration. Frozen sections of control mice and mice 24 h post-LPS were stained with tubular markers of nephron segments. A: control section labeled with ZO-1 (blue). ZO-1 demonstrates expression in most tubules. B: control section labeled with megalin (green, proximal tubule). C: control section labeled with sodium-chloride cotransporter (NCC; red, distal convoluted tubule). D: merged image of A–C indicating ZO-1 is found distributed in both proximal and distal tubules. E: section 24 h post-LPS labeled with ZO-1 (blue). There is patchy, irregularly decreased ZO-1 expression after LPS administration. F: LPS section labeled with megalin (green, proximal tubule). G: LPS section labeled with NCC (red, distal convoluted tubule). H: Merged image of E–G indicating ZO-1 is found distributed in both proximal and distal tubules. Note that ZO-1 expression in proximal tubules may be either decreased (purple asterisk) or preserved (purple arrow) after LPS. ZO-1 expression in distal tubules may be either decreased (yellow asterisk) or preserved (yellow arrow). Magnification: ×20. Bar = 60 μm.
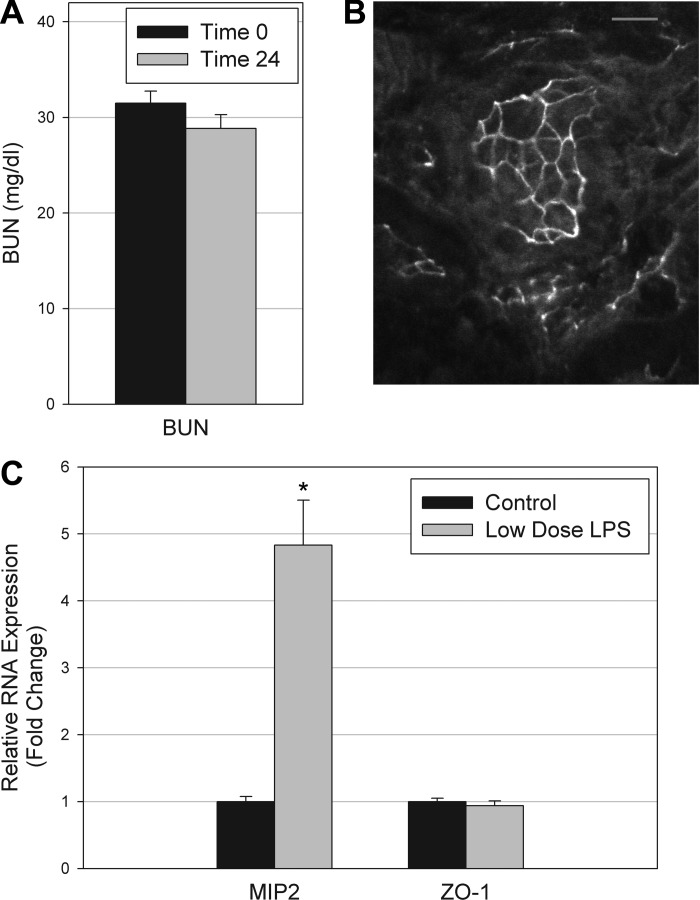
ZO-1 expression is unaltered by low-dose LPS administration.
To separate the effects of initial LPS signaling from severe end organ injury such as AKI, mice were given low-dose LPS (0.5 mg/kg), and compared with saline-injected controls. At the low dose, no mice developed renal failure at 24 h (Fig. 7A). Immunofluorescence staining for ZO-1 revealed intact circumferential staining in tubules 24 h after reduced-dose LPS administration (Fig. 7B). In contrast to higher-dose LPS, ZO-1 mRNA expression was unchanged 24 h after the reduced dose LPS (Fig. 7C). In contrast, expression of the chemokine MIP-2 was measured as a positive control because it is produced in the kidney and is strongly upregulated in conditions of endotoxemic or ischemic AKI (55). MIP-2 was found to be significantly elevated at the low dose of LPS (4.83 ± 0.68-fold increase, P = 0.001).
Fig. 7.
Administration of lower dose LPS. A: no statistical difference in blood urea nitrogen (BUN) was observed between control mice and mice receiving 0.5 mg/kg LPS. B: intact circumferential staining of ZO-1 was found in many tubules following low-dose LPS. C: real-time PCR revealed elevated gene expression of chemokine macrophage inflammatory protein (MIP)-2. In contrast, ZO-1 gene expression was unaltered by low-dose LPS.
DISCUSSION
Injury-induced TJ disruption has been well studied in cell and animal models. Ultrastructural abnormalities in pathology on electron microscopy have previously been documented in a rat model of LPS-mediated AKI (27); however, changes in gene and protein expression of individual TJ components were not described. In this study, we are the first to report and characterize alterations in individual TJ proteins ZO-1, occludin, and claudin-1, -3, -4, and -8 in mice during LPS-mediated AKI. A summary of our findings is provided in Table 2. The significance of this work rests in its specificity to the in vivo kidney, possible relevance to tubular backleak, and simultaneous determination of multiple TJ components over time. Furthermore, this study provides insights into the localization pattern of injury. These data suggest that AKI in the setting of endotoxemia is not just hemodynamic in nature but is associated with alterations in TJ protein and gene expression within the nephron.
Table 2.
Summary of protein, mRNA, and immunofluorescence results in kidneys
| Tight Junction Component | mRNA | Protein | Immunofluorescence |
|---|---|---|---|
| ZO-1 | Increased | Decreased 24 h post-LPS with recovery at 48 h | Fragmentation and reduced localization to membrane |
| Occludin | Mild increase | Shift to lower molecular weight | Fragmentation and reduced localization to membrane |
| Claudin-1 | Increased | Increased | No change |
| Claudin-3 | Increased | Increased | Modest increase in nonmembrane staining |
| Claudin-4 | Increased | Decreased | Reduced intensity with mild fragmentation |
| Claudin-8 | Decreased | No significant change | Preserved localization to cell membrane |
ZO-1, zonula occludens-1.
ZO-1 has been described as the organizer of the TJ because it anchors various components such as occludin and the claudins to the actin cytoskeleton (15, 58). Thus it is highly significant that ZO-1 was so strongly disrupted in LPS-induced AKI and may explain the failure of claudin-1, -3, and -4 to increase at the TJ despite an upregulation in transcription for each of them. A major finding in our study was the marked decrease in ZO-1 protein expression and localization in the kidney 24 h following LPS exposure. This finding is consistent with those of other cell culture and animal studies. A decrease in colonic ZO-1 protein expression has been demonstrated in a model of murine liver failure induced by d-galactosamine and LPS (53). Protein expression of ZO-1 has been demonstrated to move from the native, functional perimembrane fraction to the soluble, cytoplasmic fraction of the cell in a murine intestinal ischemia-reperfusion model (35). Neither of these studies addressed expression within the kidney. The location of ZO-1 was recently found to be decoupled from TJs within both the proximal convoluted tubule and the collecting duct in rat renal ischemia-reperfusion injury (19); however, total expression of ZO-1 protein assessed by immunoblotting was not discussed. Interestingly, decreased ZO-1 expression in the kidney has been demonstrated following chronic cyclosporine exposure (32), in the absence of overt renal disease. Decreased ZO-1 protein expression has also been demonstrated in cultured intestinal epithelial cells following a variety of insults such as cytokines and LPS (8, 10).
After an initial small but significant increase in ZO-1 protein expression at 6 h, ZO-1 expression was markedly decreased at 24 h after LPS, with subsequent recovery. This decrease in protein expression could not be explained by a drop in ZO-1 transcription, which was significantly changed in the opposite direction. Furthermore, the tissue distribution of ZO-1 visualized by immunofluorescence was highly fragmented at 24 h post-LPS. Its distribution became disorganized and was no longer consistently localized to the cell surface membrane of tubular cells. Thus the decrease in ZO-1 found at 24 h appears secondary to some combination of posttranslational regulation or degradation. In contrast to the normal circumferential location of ZO-1 at baseline, this change in location of ZO-1 could possibly lead to impairment of overall TJ structure and function in LPS-induced AKI. Colocalization staining revealed that these changes in ZO-1 expression were present irregularly, sometimes disrupted and sometimes preserved within different segments within the nephron, similar to the classic description of acute tubular necrosis as a patchy process. Given prior work suggesting that the endothelium is a target of cytokine-induced injury in the setting of sepsis (5, 59–61), we suggest that uneven perfusion from microvascular dysfunction could account for the patchy distribution of changes in tubular ZO-1 expression (60, 61). At 48 h, cellular repair processes may be underway with continued elevation of mRNA expression and restitution of protein expression.
Occludin protein expression was also significantly altered in LPS-induced AKI. Extensive phosphorylation of occludin has been associated with higher transepithelial resistance and a greater degree of localization to the TJ. This study is the first to suggest such findings in vivo in the kidney. Following LPS administration, the ratio of heavier occludin bands (>70 kDa) relative to the 65-kDa band was markedly decreased. (34, 47, 48, 52). In cultured epithelial cells, ATP depletion has been shown to result in decreased levels of more highly phosphorylated isoforms of occludin (23). We speculate that this decrease in occludin size was due to dephosphorylation, as a result of ATP depletion within tubular cells (26) in the setting of local hypoxia and disturbances in the renal microcirculation during endotoxemia. Total occludin expression changed minimally. Immunofluorescence 24 h post-LPS revealed changes in distribution, with fractures in the usual circumferential cell staining and more luminal distribution. We postulate that as kidney injury develops and persists, occludin is increasingly found in its dephosphorylated, non-membrane-bound form, as has been suggested in prior studies in cultured epithelia (9, 23, 47). This pattern of minimally changed total occludin expression but with less localization to the cell membrane was similar to those reported in ischemia-reperfusion (19). mRNA expression of occludin was significantly increased after LPS exposure. While this was consistent with the increased mRNA expression also seen for ZO-1 and several claudins, the small magnitude of change suggests that phosphorylation or other posttranslational processes may be more important to occludin regulation. It is unclear why ZO-1 protein expression began to recover 48 h after LPS exposure, while changes in occludin molecular weight became more pronounced.
Claudin-1 and claudin-3 were studied because of their contributions to barrier function in several sections of the nephron, including the distal tubule (6, 41), where backleak of highly concentrated luminal toxins could reduce clearance. Protein and mRNA expression of claudin-1 and -3 were both increased following LPS-induced AKI. While these findings are novel in this LPS-induced AKI model, similar results have been reported in other tissues and models. Claudin-1 and -3 mRNA expression was increased 4.8- and 4.5-fold, respectively, in a murine kidney model of ischemia-reperfusion (28), similar to the magnitudes of increase we observed. The increased mRNA expression of claudin-1, -3, ZO-1, and occludin may represent an attempted repair response by kidney cells following injury. It has been suggested that increases in claudin-2 protein expression may serve as a compensatory response to disruption of other TJ proteins in intestinal epithelia injury (45, 49), so it is plausible that we are seeing an analogous adaptive phenomenon here with claudin-1 and -3. However, immunofluorescence 24 h post-LPS revealed no change in claudin-1, which was mainly within Bowman's capsule, and only a modest increase in total claudin-3 staining, without a clear increase in localization to the TJ. A similar finding was also noted in a murine sepsis model, where claudin-3 labeling was present diffusely within intestinal epithelial cells and no longer localized at the lateral cell boundaries (34). This may relate to changes in translational regulation or degradation as suggested by the more marked changes we observed for other TJ components, like ZO-1 and occludin. It is unclear why claudin-1 and -3 protein expression were greatly increased, unlike ZO-1 and occludin. TJ components undergo rapid and continuous remodeling in steady state (50), but claudin exchange with its intracellular pool is less rapid than for either ZO-1 or occludin (50). We speculate that this may account, in part, for the relative preservation of claudin-1 and -3 protein after LPS exposure.
Claudin-4 and -8 hold important distinctions from the other claudins studied. First, their distribution is more specific to the distal convoluted tubule and collecting duct (6, 22, 30, 36). Although both -4 and -8 are implicated in barrier function (4), claudin-4 has also been found to participate in paracellular anion transport in the distal nephron (25, 33). Little is understood about renal claudin expression in the setting of kidney injury. In this study, we are the first to describe that protein and mRNA expression of claudin-4 and -8 were altered following LPS-induced AKI. Claudin-4 mRNA expression markedly increased while protein expression decreased. In contrast, CLDN8 was the only gene in this study with reduced mRNA expression, although protein expression was not significantly altered. Hou et al. (25) described that in mouse collecting duct cells, claudin-8 was required for claudin-4 membrane localization, and these proteins were found to coprecipitate. This may explain why we found decreased claudin-4 protein expression despite a strong increase in claudin-4 mRNA. Cell models have been used in an effort to understand the regulatory factors of claudin-4 and -8. Claudin-4 expression increases in Madin-Darby canine kidney cells in response to epidermal growth factor (18). Additionally, aldosterone modulates claudin-4 phosphorylation without changing protein expression or localization (32). Further mechanistic investigations are needed to understand these gene and protein changes observed in LPS-induced AKI.
Endotoxemia is well known to cause hypotension, as is clearly seen in septic shock in humans. One question that had previously persisted with the LPS-induced AKI model was whether the effect on clearance was predominantly hemodynamic, with lower pressure inside the glomerulus driving less filtration. Indeed, the histological tubular injury seen in the kidney on light microscopy with LPS is modest. However, the data we report here show that there are impressive derangements to TJ proteins in the renal tubule. These changes were more extensive than a mere reduction in TJ protein and gene expression; the altered distribution and fragmentation imply changes in posttranslational modification or active protein degradation. Lower dose LPS did not induce renal failure in mice, nor did it disrupt circumferential ZO-1 immunofluorescence staining or alter ZO-1 gene expression. Nevertheless, this lower dose of LPS was sufficient to significantly increase the renal-derived chemokine MIP-2 (55). Taken together, this implies that ZO-1 disruption is a consequence of severe end-organ injury and is less likely to be a direct effect of LPS on the tubular cell. While prior work has clearly established that inflammatory signaling through TNF receptor 1 is crucial for AKI (12, 13), expression of TNF receptor 1 in the kidney is mainly endothelial, not tubular (2, 3). Thus a direct role for TNF-α in causing ZO-1 disruption within the tubule is also unlikely. However, TNF-α can trigger apoptosis and injury in cultured renal endothelial cells. Thus we propose that inflammatory pathways initiated in the renal microcirculation, in conjunction with global hemodynamic effects, are responsible for subsequent tubular hypoxia and disruption in tubular TJs. The patchy, uneven distribution of TJ changes in different nephron segments is suggestive of the patchiness seen in the microvascular dysfunction of sepsis. Current cell culture studies are underway to determine whether the mechanism of ZO-1 disruption is secondary to hypoxia vs. signaling through LPS and/or cytokines; preliminary results (data not shown) reveal that chemical hypoxia in tubular cells is capable of disrupting ZO-1. The extent to which these disruptions in TJs contribute to the decrease in clearance and dysfunctional electrolyte handling is presently unclear but is a worthy target of inquiry until the pathogenesis of septic AKI is better understood.
GRANTS
This work was supported by National Institutes of Health Grants R01DK080863, T32DK007510, T32GM007019, and K08DK081728.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.T.E., B.K.H., B.K., F.G.T., and P.N.C. provided conception and design of research; M.T.E., B.K.H., C.X., and P.N.C. performed experiments; M.T.E., B.K.H., C.X., B.K., and P.N.C. analyzed data; M.T.E., B.K.H., C.X., B.K., F.G.T., and P.N.C. interpreted results of experiments; M.T.E., C.X., and P.N.C. prepared figures; M.T.E. and P.N.C. drafted manuscript; M.T.E., F.G.T., and P.N.C. edited and revised manuscript; M.T.E., B.K., F.G.T., and P.N.C. approved final version of manuscript.
REFERENCES
- 1. Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 287: F305–F318, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Al-Lamki RS, Wang J, Skepper JN, Thiru S, Pober JS, Bradley JR. Expression of tumor necrosis factor receptors in normal kidney and rejecting renal transplants. Lab Invest 81: 1503–1515, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, Min W, Pober JS, Bradley JR. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J 19: 1637–1645, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Amasheh S, Fromm M, Gunzel D. Claudins of intestine and nephron—a correlation of molecular tight junction structure and barrier function. Acta Physiol (Oxf) 201: 133–140, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Amon M, Menger MD, Vollmar B. Heme oxygenase and nitric oxide synthase mediate cooling-associated protection against TNF-alpha-induced microcirculatory dysfunction and apoptotic cell death. FASEB J 17: 175–185, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Balkovetz DF. Claudins at the gate: determinants of renal epithelial tight junction paracellular permeability. Am J Physiol Renal Physiol 290: F572–F579, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Balkovetz DF. Tight junction claudins and the kidney in sickness and in health. Biochim Biophys Acta 1788: 858–863, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Chin AC, Flynn AN, Fedwick JP, Buret AG. The role of caspase-3 in lipopolysaccharide-mediated disruption of intestinal epithelial tight junctions. Can J Physiol Pharmacol 84: 1043–1050, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Clarke H, Soler AP, Mullin JM. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J Cell Sci 113: 3187–3196, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Costantini TW, Deree J, Loomis W, Putnam JG, Choi S, Baird A, Eliceiri BP, Bansal V, Coimbra R. Phosphodiesterase inhibition attenuates alterations to the tight junction proteins occludin and ZO-1 in immunostimulated Caco-2 intestinal monolayers. Life Sci 84: 18–22, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Cummins PM. Occludin: one protein, many forms. Mol Cell Biol 32: 242–250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol 168: 5817–5823, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Cunningham PN, Wang Y, Guo R, He G, Quigg RJ. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. J Immunol 172: 2629–2635, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol Renal Physiol 274: F1–F9, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Denker BM, Sabath E. The biology of epithelial cell tight junctions in the kidney. J Am Soc Nephrol 22: 622–625, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 35: 1244–1250, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273: 29745–29753, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Flores-Benitez D, Ruiz-Cabrera A, Flores-Maldonado C, Shoshani L, Cereijido M, Contreras RG. Control of tight junctional sealing: role of epidermal growth factor. Am J Physiol Renal Physiol 292: F828–F836, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141: 1539–1550, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez JE, DiGeronimo RJ, Arthur DE, King JM. Remodeling of the tight junction during recovery from exposure to hydrogen peroxide in kidney epithelial cells. Free Radic Biol Med 47: 1561–1569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, Valencia L, Muriel P, Ponce L, Reyes JL. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int 57: 2386–2402, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez-Mariscal L, Namorado Mdel C, Martin D, Sierra G, Reyes JL. The tight junction proteins claudin-7 and -8 display a different subcellular localization at Henle's loops and collecting ducts of rabbit kidney. Nephrol Dial Transplant 21: 2391–2398, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Gopalakrishnan S, Raman N, Atkinson SJ, Marrs JA. Rho GTPase signaling regulates tight junction assembly and protects tight junctions during ATP depletion. Am J Physiol Cell Physiol 275: C798–C809, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Guo R, Wang Y, Minto AW, Quigg RJ, Cunningham PN. Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol 15: 3093–3102, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci USA 107: 18010–18015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johannes T, Mik EG, Ince C. Nonresuscitated endotoxemia induces microcirculatory hypoxic areas in the renal cortex in the rat. Shock 31: 97–103, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Kang YH, Falk MC, Bentley TB, Lee CH. Distribution and role of lipopolysaccharide in the pathogenesis of acute renal proximal tubule injury. Shock 4: 441–449, 1995 [PubMed] [Google Scholar]
- 28. Kieran NE, Doran PP, Connolly SB, Greenan MC, Higgins DF, Leonard M, Godson C, Taylor CT, Henger A, Kretzler M, Burne MJ, Rabb H, Brady HR. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int 64: 480–492, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Kirk A, Campbell S, Bass P, Mason J, Collins J. Differential expression of claudin tight junction proteins in the human cortical nephron. Nephrol Dial Transplant 25: 2107–2119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Kwon O, Nelson WJ, Sibley R, Huie P, Scandling JD, Dafoe D, Alfrey E, Myers BD. Backleak, tight junctions, and cell- cell adhesion in postischemic injury to the renal allograft. J Clin Invest 101: 2054–2064, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee CH, Kim S, Kang CM, Kim WY, Kim J, Kim GH. Altered expression of tight junction proteins in cyclosporine nephrotoxicity. Am J Nephrol 33: 7–16, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Le Moellic C, Boulkroun S, Gonzalez-Nunez D, Dublineau I, Cluzeaud F, Fay M, Blot-Chabaud M, Farman N. Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. Am J Physiol Cell Physiol 289: C1513–C1521, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Li Q, Zhang Q, Wang C, Liu X, Li N, Li J. Disruption of tight junctions during polymicrobial sepsis in vivo. J Pathol 218: 210–221, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Li Q, Zhang Q, Wang C, Liu X, Qu L, Gu L, Li N, Li J. Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats. J Cell Mol Med 13: 4061–4076, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li WY, Huey CL, Yu AS. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol 286: F1063–F1071, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Lieberthal W. Biology of acute renal failure: therapeutic implications. Kidney Int 52: 1102–1115, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci 113: 2363–2374, 2000 [DOI] [PubMed] [Google Scholar]
- 39. McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci 109: 2287–2298, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Meyer TN, Schwesinger C, Ye J, Denker BM, Nigam SK. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J Biol Chem 276: 22048–22055, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Milatz S, Krug SM, Rosenthal R, Gunzel D, Muller D, Schulzke JD, Amasheh S, Fromm M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta 1798: 2048–2057, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Myers BD, Moran SM. Hemodynamically mediated acute renal failure. N Engl J Med 314: 97–105, 1986 [DOI] [PubMed] [Google Scholar]
- 43. Nomura A, Nishikawa K, Yuzawa Y, Okada H, Okada N, Morgan BP, Piddlesden SJ, Nadai M, Hasegawa T, Matsuo S. Tubulointerstitial injury induced in rats by a monoclonal antibody that inhibits function of a membrane inhibitor of complement. J Clin Invest 96: 2348–2356, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park P, Haas M, Cunningham PN, Alexander JJ, Bao L, Guthridge JM, Kraus DM, Holers VM, Quigg RJ. Inhibiting the complement system does not reduce injury in renal ischemia reperfusion. J Am Soc Nephrol 12: 1383–1390, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85: 1139–1162, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell 21: 1200–1213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368: 471–481, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol 137: 1393–1401, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 73: 283–309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol 181: 683–695, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sheridan AM, Bonventre JV. Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr Opin Nephrol Hypertens 9: 427–434, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Sheth P, Delos Santos N, Seth A, LaRusso NF, Rao RK. Lipopolysaccharide disrupts tight junctions in cholangiocyte monolayers by a c-Src-, TLR4-, and LBP-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 293: G308–G318, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Song HL, Lv S, Liu P. The roles of tumor necrosis factor-alpha in colon tight junction protein expression and intestinal mucosa structure in a mouse model of acute liver failure. BMC Gastroenterol 9: 70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 55. Thurman JM, Lenderink AM, Royer PA, Coleman KE, Zhou J, Lambris JD, Nemenoff RA, Quigg RJ, Holers VM. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol 178: 1819–1828, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol Renal Physiol 276: F737–F750, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell 20: 3930–3940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, Mayeux PR. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol 292: F261–F268, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Wu X, Guo R, Chen P, Wang Q, Cunningham PN. TNF induces caspase-dependent inflammation in renal endothelial cells through a Rho- and myosin light chain kinase-dependent mechanism. Am J Physiol Renal Physiol 297: F316–F326, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Wu X, Guo R, Wang Y, Cunningham PN. The role of ICAM-1 in endotoxin-induced acute renal failure. Am J Physiol Renal Physiol 293: F1262–F1271, 2007 [DOI] [PubMed] [Google Scholar]