Abstract
Tuberous sclerosis complex (TSC), an inherited tumor predisposition syndrome associated with mutations in TSC1 or TSC2, affects ∼1 in 6,000 individuals. Eighty percent of TSC patients develop renal angiomyolipomas, and renal involvement is a major contributor to patient morbidity and mortality. Recent work has shown that mammalian target of rapamycin complex 1 (mTORC1) inhibition caused angiomyolipoma shrinkage but that this treatment may cause cytostatic not a cytotoxic effect. Endoplasmic reticulum (ER) stress can develop in TSC-associated cells due to mTORC1-driven protein translation. We hypothesized that renal angiomyolipoma cells experience ER stress that can be leveraged to result in targeted cytotoxicity. We used immortalized human angiomyolipoma cells stably transfected with empty vector or TSC2 (encoding tuberin). Using cell number quantification and cell death assays, we found that mTORC1 inhibition with RAD001 suppressed angiomyolipoma cell proliferation in a cytostatic manner. Angiomyolipoma cells exhibited enhanced sensitivity to proteasome inhibitor-induced ER stress compared with TSC2-rescued cells. After proteasome inhibition with MG-132, Western blot analyses showed greater induction of C/EBP-homologous protein (CHOP) and more poly (ADP-ribose) polymerase (PARP) and caspase-3 cleavage, supporting ER stress-induced apoptosis. Live cell numbers also were decreased and cell death increased by MG-132 in angiomyolipoma cells compared with TSC2 rescued. Intriguingly, while pretreatment of angiomyolipoma cells with RAD001 attenuated CHOP and BiP induction, apoptotic markers cleaved PARP and caspase-3 and eukaryotic translation initiation factor 2α phosphorylation were increased, along with evidence of increased autophagy. These results suggest that human angiomyolipoma cells are uniquely susceptible to agents that exacerbate ER stress and that additional synergy may be achievable with targeted combination therapy.
Keywords: angiomyolipoma, ER stress, mTOR, TSC
tuberous sclerosis complex (TSC), an inherited tumor predisposition syndrome, affects ∼1 out of 6,000 individuals. Eighty percent of TSC patients develop renal angiomyolipomata, which are emblematic of perivascular epithelioid cell tumors (or PEComas) consisting of smooth muscle, epithelioid, and adipocyte cells that are proliferative in nature, and whose progressive growth can lead to renal disease (36). Renal disease is the leading cause of death in the adult TSC patient (35). Recent clinical trials concluded that disruption of mammalian target of rapamycin complex 1 (mTORC1) caused a volumetric reduction in TSC patients′ angiomyolipomas, which was not sustained for many patients when therapy was discontinued (2). Thus targeted cytotoxic therapies must still be developed to more adequately care for the TSC renal patient.
TSC is caused by inactivating mutations in either TSC1 or TSC2, encoding hamartin and tuberin, respectively. These proteins form a heterodimer that regulates mTORC1. mTORC1, the rapamycin-sensitive complex composed of mTOR, regulatory associated protein of mTOR (raptor), mammalian LST8/G protein β-subunit-like protein, and PRAS40, functions as an integrator of internal and external environmental information including cellular energy level, nutrient availability, and redox status. mTORC1 integrates inputs from multiple upstream pathways to ultimately regulate cell growth, proliferation, motility, survival, transcription, and protein synthesis (13). In the TSC angiomyolipoma cell, the affected locus sustains a second somatic mutation causing constitutive activation of mTORC1, resulting in derangements in cell growth, including increased protein translation, which appear to contribute to renal tumorigenesis (14).
Mutations in the TSC genes impact the endoplasmic reticulum (ER). One function of the cellular ER is to regulate protein biosynthesis, folding, posttranslational modification, and trafficking. These ER functions are tightly regulated and exquisitely sensitive to stressors such as ischemia, glucose deprivation, oxidative stress, genetic mutation, and alteration of nutrient and energy homeostasis. One consequence of these types of insults is accumulation of misfolded proteins in the ER lumen, termed ER stress (reviewed in Ref. 18, 32). In reaction to this stress, the ER initiates an adaptive mechanism to compensate for aberrant protein accumulation, termed the unfolded protein response (UPR). Three main pathways initiate the compensatory mechanisms. The first pathway involves activation of the ER transmembrane protein inositol-requiring enzyme-1 (IRE-1) that occurs upon release from its normal binding partner from the ER luminal side, glucose-related protein-78 (GRP78 or BiP). Through its endoribonuclease activity, IRE-1 can splice various mRNAs such as X-box binding protein-1 thereby altering transcription of ER chaperones, such as BiP and ER-associated degradation proteins. A second pathway involves the ER transmembrane Ser/Thr protein kinase-like ER kinase (PERK), which phosphorylates eukaryotic initiation factor 2α (eIF2α) to halt translation. In the third pathway, activating transcription factor-6 (ATF6), an ER membrane-bound regulatory protein, is similarly activated by dissociation from BiP, and, following Golgi processing, translocates to the nucleus adjusting ER chaperone and ER-associated degradation component transcription. If persistent or severe ER stress overwhelms these compensatory mechanisms, apoptosis likewise can be facilitated by a number of pathways. PERK can induce expression of CCAAT/enhancer-binding protein homologous protein (CHOP), a proapoptotic transcription factor, partly through eIF2α signaling through ATF4. IRE-1 kinase activity can induce apoptosis via the c-jun NH2-terminal kinase (JNK) pathway. Caspase-mediated apoptosis can be initiated, in part through BAX/BAK mediated ER Ca2+ release, and can be identified by proteolytic cleavage of procaspase-3 and poly (ADP ribose) polymerase (PARP).
Recent murine findings highlight the possibility of a unique vulnerability of human TSC mutant cells to ER stress. TSC mutant cells exhibit constitutive mTORC1 activity and significantly upregulated protein translation. This translational burden can cause ER stress and can be exacerbated with thapsigargin, an ER Ca2+-ATPase inhibitor that alters ER Ca2+ and disturbs protein folding (27). Ozcan et al. (27) identified a ∼90% apoptotic index for Tsc2-null mouse embryonic fibroblasts (MEFs) treated with thapsigargin, which was absent in Tsc2-sufficient MEFs. Di Nardo et al. (9) obtained similar results in tuberin-deficient neuronal cells. Kang et al. (19) recently demonstrated that targeting the 26S proteasome with the inhibitor MG-132, and thereby exacerbating ER stress by inhibiting the protein degradation machinery, would also selectively eliminate Tsc1- or Tsc2-null MEFs. A limitation of such TSC genetic murine models is that they do not truly phenocopy human disease, raising the question whether human angiomyolipoma cells exhibit similar responses and survival to ER stress as their murine counterparts. We hypothesized that human TSC-associated human renal angiomyolipomas experienced ER stress due to constitutive mTORC1 driven protein translation and that angiomyolipoma cells would display increased sensitivity to ER stress exacerbation by proteasome inhibition.
METHODS
Cell culture.
The TRI102 human angiomyolipoma cell line was generated as described in by Hong et al. (17) by introduction of E6/E7 (pLXSN 16E6E7-neo; Ref. 11) and human telomerase (pLXSN hTERT-hyg) into a primary culture of TSC2 null human angiomyolipoma cells (621–101) isolated by Yu et al. (42). TRI103 was generated by stable transfection of TRI102 with wild-type TSC2 (pcDNA3.1 TSC2-zeo; Ref. 17). Both lines were obtained from the American Type Culture Collection. Cells were cultured in DMEM high glucose medium (GIBCO, Invitrogen) with l-glutamine, 10% FBS (unless otherwise noted), and 1% penicillin/ streptomycin at 37°C in 5% CO2.
Western blotting and antibodies.
Cells were lysed in RIPA buffer containing protease and phosphatase inhibitors, and protein concentration was determined using the BCA protein assay (Thermo Scientific). Equal amounts of protein were loaded into polyacrylamide gels, separated by SDS-PAGE, and transferred to PVDF membranes (Millipore). Membranes were blocked in 5% nonfat dry milk/TBS Tween (TBST) for 1 h at room temperature, washed with TBST, and subsequently incubated with primary antibodies in 5% milk/TBST overnight at 4°C. After being washed three times, membranes were incubated with mouse or rabbit horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) for 1 h at room temperature. Membranes were again washed three times, and signals were detected by chemiluminescence using ECL detection system (GE Healthcare). Primary antibodies were used against the following for Western blot analysis: tuberin, phospho-S6 (Ser235/236), ribosomal protein S6, IRE1α, CHOP, BIP, cleaved caspase-3 (Asp175), phospho-eIF2α (Ser51) and PARP (Cell Signaling Technologies), LC3 (MBL International), and GAPDH (Trevigen).
Crystal violet cell number quantification.
Then, 8 × 103 cells were seeded in 96-well plates and cultured for 24 h before drug treatment. At completion of treatment, wells were washed twice with PBS and fixed in 4% paraformaldehyde/PBS for 10 min at room temperature. Wells were washed twice with distilled water and incubated in 0.1% crystal violet/water for 30 min at room temperature. After again being washed three times, the stain was dissolved in 10% acetic acid and plates were agitated on shaker until solution was uniform in color. Absorbance was quantified at 540 nm. Background absorbance was measured in unstained cells from each group and subtracted from measurements. At least 8 wells of a 96-well plate were averaged for each treatment condition per experiment.
Propidium iodide uptake cell death assay.
Cells were seeded in CulturPlate black opaque 96-well microplates (Perkin-Elmer). Treatments were administered as indicated in 190 μl total volume. At the time of assay, 10 ul of propidium iodide (100 ug/ml in PBS) was added to each well for a final concentration of 5 ug/ml, and cells were incubated for 30 min at 37°C. Propidium iodide is not membrane permeable and can only enter cells with compromised membranes. Once the dye is bound to nucleic acids, its fluorescence is enhanced 20- to 30-fold, the fluorescence excitation maximum is shifted ∼30–40 nm to the red and the fluorescence emission maximum is shifted ∼15 nm to the blue. Fluorescence intensity (535/617 excitation/emission) was measured using a Biotek Synergy H4 microplate reader. Background fluorescence was determined by measuring the propidium iodide signal in cell-free wells. At least 4 wells of a 96-well plate were averaged for each treatment condition per experiment. Parallel crystal violet assays were performed for visual inspection/confirmation.
Caspase-3 activity assay.
Caspase-3 activity was measured using the CaspACE colorimetric assay system (Promega) according to the manufacturer's specifications. Then, 3 × 105 cells/well were seeded in sixwell plates. At this initial density, cells were confluent at the time of assay. Following indicated treatments, cells were lysed and assayed, and A405 (corresponding to caspase-3 activity) was measured on a Biotek Synergy H4 microplate reader. For each treatment condition (including control), a parallel sample was also given the caspase inhibitor Z-VAD-FMK (50 μM). A405 values from Z-VAD-FMK-treated samples were subtracted from corresponding treatment samples, and the resulting values were then corrected for the amount of protein load (determined by BCA assay).
Immunofluorescence.
Cells on cover slips were fixed with 4% paraformaldehyde/PBS at room temperature, permeablized with 0.2% Triton X-100/PBS, washed extensively with PBS, and incubated overnight with primary antibodies [anti-CHOP, 1:1,500 (Cell Signaling Technologies) or anti-LC3, 1:200 (MBL International)] at 4°C. Alexa-conjugated secondary antibodies (1:500; Invitrogen) were applied for 1 h at room temperature. Coverslips were mounted on glass slides using ProLong Gold antifade mounting agent containing DAPI (Invitrogen). Images were obtained using a Zeiss Axiovert 400 microscope, and image analysis and pseudocoloring were performed with ImageJ (National Institutes of Health).
Electron microscopy.
Tissue culture samples were fixed in 3% buffered glutaraldehyde for 1 h, postfixed in 1% osmium tetroxide, dehydrated in graded alcohols, and embedded in LX-112 resin. The sections were cut with diamond knives on an ultramicrotome, stained with uranyl acetate and lead citrate, and examined in a Hitachi H-7600 electron microscope.
Construction of red fluorescence protein-ER plasmid and production of lentivirus.
The KDEL endoplasmic reticulum retention signal was added during PCR amplification of red fluorescence protein (RFP) using the forward primer TAGCTACCGGTTCTAGAGCCTCCTCCGAGGACGTCATC and the reverse primer AGCTACTCGAGTCACAGCTCGTCCTTCGAAGCTTGGGCGCCGGTGGA. This fragment was then digested with AgeI and XhoI and ligated into the pCSCGW lentiviral vector backbone (22). The ER targeting signal from calreticulin was then added to this newly created plasmid by digesting with AgeI and XbaI and ligating to the annealed oligonucleotides CCGGTATGCTGCTATCCGTGCCGTTGCTGCTCGGCCTCCTCGGCCTGGCCGTCGCCATCGATT and CTAGAATCGATGGCGACGGCCAGGCCGAGGAGGCCGAGCAGCAACGGCACGGATAGCAGCATA. The RFP-ER plasmid was cotransfected using calcium phosphate into 293T cells with pCMV-VSV-G (Addgene no. 8454), pRSV-REV (Addgene no. 12253), and pMDLg/pRRE (Addgene no. 12251). Two days posttransfection the viral supernatant was filtered using a 0.45 um syringe filter and stored at −80°C until needed.
Using RFP-ER to visualize ER vacuolization.
Then, 621–101 cells (42) were plated into 35-mm MatTek dishes into DMEM + 10%FBS containing 5 mg/ml polybrene and RFP-ER lentivirus. The following morning, media were removed and cells were treated with 0.1% DMSO or 20 nM rapamycin. Twenty-four hours later, cells were treated with 500 nM MG-132 or 0.1% DMSO in the presence or absence of 20 nM rapamycin for 8 h. Fluorescence images were acquired on a Zeiss AxioObserverZ1 microscope utilizing a c-apochromat ×40 water immersion objective.
Light microscopy and immunohistochemistry.
Following Cincinnati Children's Hospital Medical Center Institutional Review Board review and approval, representative sections from four cases of human renal angiomyolipoma, one case of Birt-Hogg-Dubé with renal cell carcinoma, and one case of normal kidney core needle biopsy were prepared from 10% formalin-fixed, paraffin-embedded tissue and stained with hematoxylin-eosin. Immunohistochemical stains were carried out on formalin-fixed, paraffin-embedded tissue using the avidin-biotin technique with diaminobenzidine as a chromogen on an automatic immunostainer (Benchmark XT; Ventana Medical Systems, Tucson, AZ). The following monoclonal antibodies were used: antibodies against BiP and phospho-eIF2α (purchased from Cell Signaling, Danvers, MA). In every case, formalin-fixed tissue was subjected to heat-induced antigen retrieval.
Statistical analysis.
Statistical significance was determined by repeated-measures ANOVA with Tukey-Kramer multiple comparison post hoc test using Prism software (Graphpad Software). All data are expressed as means ± SE.
RESULTS
TSC2 null human renal angiomyolipoma cells exhibit a cytostatic response to RAD001.
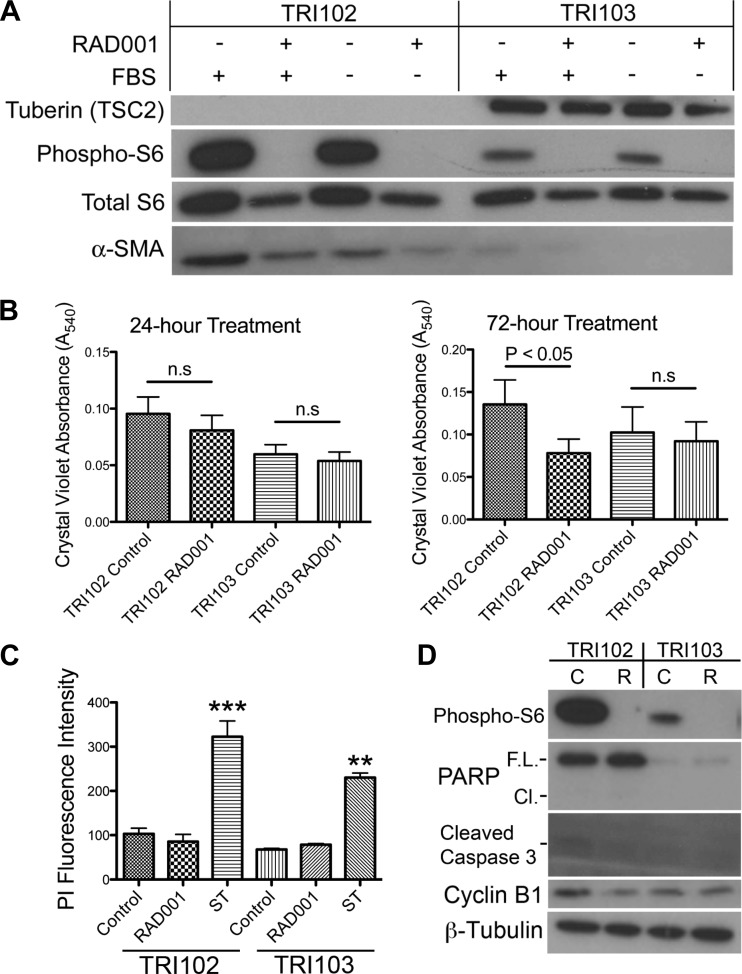
mTORC1 inhibition therapy caused angiomyolipoma shrinkage in patients that trended back toward baseline measurements when drug was discontinued, indicating a more cytostatic than cytotoxic effect (2, 7). To investigate the effect of mTORC1 inhibition on angiomyolipoma cells, tuberin, phospho-S6 ribosomal protein (phospho-S6) and total S6, and α-smooth muscle actin levels were measured by Western blot in TRI102 (TSC2−/− human renal angiomyolipoma) and TRI103 (TSC2-reexpressing human renal angiomyolipoma) cell lines in the presence or absence of FBS and the mTORC1 inhibitor RAD001 (20 nM, 24 h). The TRI102 angiomyolipoma line exhibited a robust P-S6 band that was lost with RAD001 treatment and greatly attenuated in the TSC2 reexpressing TRI103 line, indicating abnormally elevated mTORC1 activity in TRI102 cells that is rapamycin sensitive and corrected by tuberin reexpression (Fig. 1A). Also, TRI102 cells had an increased level of α-smooth muscle actin, a characteristic immunohistochemical finding of renal angiomyolipomas, which was slightly reduced in response to RAD001 (Fig. 1A). Cell number quantification was performed in the presence or absence of FBS and with and without RAD001 for 24 and 72 h. We found that, in the presence of FBS, RAD001 reduced the number of viable cells in both TRI102 and TRI103 at both 24 and 72 h (data not shown). This suggested a component of cell proliferation in both cell types that was driven by serum-induced mTORC1 activation and sensitive to mTORC1 inhibition. To isolate the tuberin-dependent, mTORC1-driven effect, we measured cell numbers in the absence of FBS. Under serum-free conditions, we found that RAD001 had no effect at 24 h but after 72 h significantly reduced the number of viable TRI102 cells, while TSC2 reexpressing TRI103 cells were not affected (Fig. 1B). The reduction in cell numbers after RAD001 treatment could either reflect a loss of cell viability or a reduction in cell proliferation. Therefore, cell death assays were utilized to determine whether RAD001 caused cell death. At the 72-h time point, RAD001 (20 nM) did not produce a significant increase in cell death measured by propidium iodide uptake (Fig. 1C). Staurosporine (250 nM), a potent inducer of apoptosis used here as a positive control, produced a threefold increase in propidium iodide fluorescence that was statistically significant in each cell type after only a 24-h treatment (Fig. 1C). Finally, levels of cyclin B1, which are increased during mitosis and decreased in cellular quiescence, decreased by 45% (measured by densitometric analysis, P < 0.05) in TRI102 cells after 24 h of RAD001, while TRI103 cells responded with a slight decrease that was not statistically significant (Fig. 1D). Cleaved caspase-3 or cleaved PARP was not detected by RAD001 treatment in either cell line (Fig. 1D). These findings support a cytostatic effect of RAD001, or an inhibition of proliferation, rather than induction of apoptosis or cell death.
Fig. 1.
A: patient-derived AML cell line that had been transfected with vector alone (TRI102), or the vector containing the tuberous sclerosis complex 2 (TSC2) gene (TRI103) were cultured with and without 10% FBS, and treated with or without RAD001 (20 nM, 24 h). Lysates were prepared, and tuberin, total- and phospho-S6 ribosomal proteins, and α-smooth muscle actin (α-SMA) were detected by Western blot. B: cell numbers were quantified under serum free conditions by crystal violet DNA dye binding in TRI102 and TRI103 cells treated with or without RAD001 (20 nM) for 24 h (n = 6), or 72 h (n = 10). C: cell death was measured under serum free conditions by propidium iodide uptake assay in TRI102 and TRI103 cells treated with or without RAD001 (20 nM) for 72 h or stuarosporine (ST; 250 nM) for 24 h (n = 3; **P < 0.01; ***P < 0.001). D: Western blot of apoptosis and cell cycle markers in TRI102 and TRI103 cells treated without (“C”) or with (“R”) RAD001 (20 nM) for 24 h. Results are represent 3 separate experiments. PARP, poly (ADP-ribose) polymerase; F.L., full length; Cl., cleaved.
Enhanced ER stress response and cytotoxicity by proteasome inhibition in TSC2 null human renal angiomyolipoma cells.
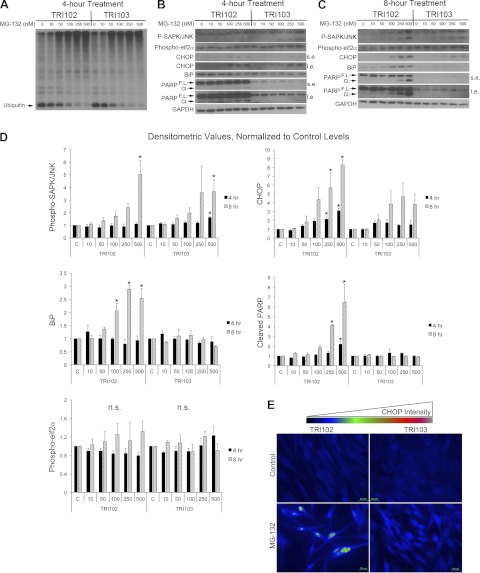
The effects of proteasome inhibition with MG-132 were evaluated by Western blot analysis. MG-132 treatment produced a dose-dependent decrease in monomeric, unlinked ubiquitin (8.5 kDa) and corresponding increase in higher molecular weight species representing ubiquitinated proteins to similar degree in both TRI102 and TRI103 cells, indicating inhibition of proteasome activity (Fig. 2A). The ER stress response to MG-132 was measured by Western blot analysis of phospho-SAPK/JNK, CHOP, BiP and PARP cleavage (Fig. 2, B–D). Dose-dependent increases in phospho-SAPK/JNK were measured in both cell types that reached statistical significance at 500 nM MG-132 after 8 h. CHOP levels were significantly increased by MG-132 in TRI102 cells. CHOP expression increased to a lesser degree in TRI103 cells, which did not reach statistical significance compared with control levels. BiP and PARP cleavage each increased significantly in the TSC2-deficient TRI102 cells, while levels in TRI103 cells were unaffected. Phospho-eIF2α trended toward in increase in TRI102 cells at the 8-h time point and were unaffected in TRI103 cells. By immunofluorescence microscopy, we found enhanced CHOP induction by MG-132 treatment in TRI102 compared with TRI103 cells and also observed that induced CHOP expression appeared to be localized to the nucleus (Fig. 2E). These findings suggest that TSC2-null renal angiomyolipoma cells are more sensitive than their TSC2-expressing counterparts with respect to an ER stress response to proteasome inhibition.
Fig. 2.
A: Western blot for ubiquitin in TRI102 and TRI 103 cells following 4-h treatment with MG-132 (doses ranging from 0 to 500 nM). B and C: Western blots for phospho-SAPK/JNK, phospho-eukaryotic initiation factor 2α (eIF2α), BiP, C/EBP-homologous protein (CHOP), BiP, full-length and cleaved PARP, and GAPDH in TRI102 and TRI103 cells following 4-h (B) and 8-h (C) treatment with MG-132 (doses ranging from 0 to 500 nM) in serum free conditions (“s.e.,” short exposure; “l.e.,” long exposure). D: graphs depicting densitometric analysis of phospho-SAPK/JNK, CHOP, phospho-eIF2α, BiP, and cleaved PARP levels normalized to untreated control values (n = 4). E: immunofluorescence images of TRI102 and TRI103 cells labeled with anti-CHOP 1° and Alexa 488-conjugated 2° antibodies with and without MG-132 treatment (500 nM, 8 h, serum free conditions). Image acquisition settings remained constant. Results represent 3 separate experiments.
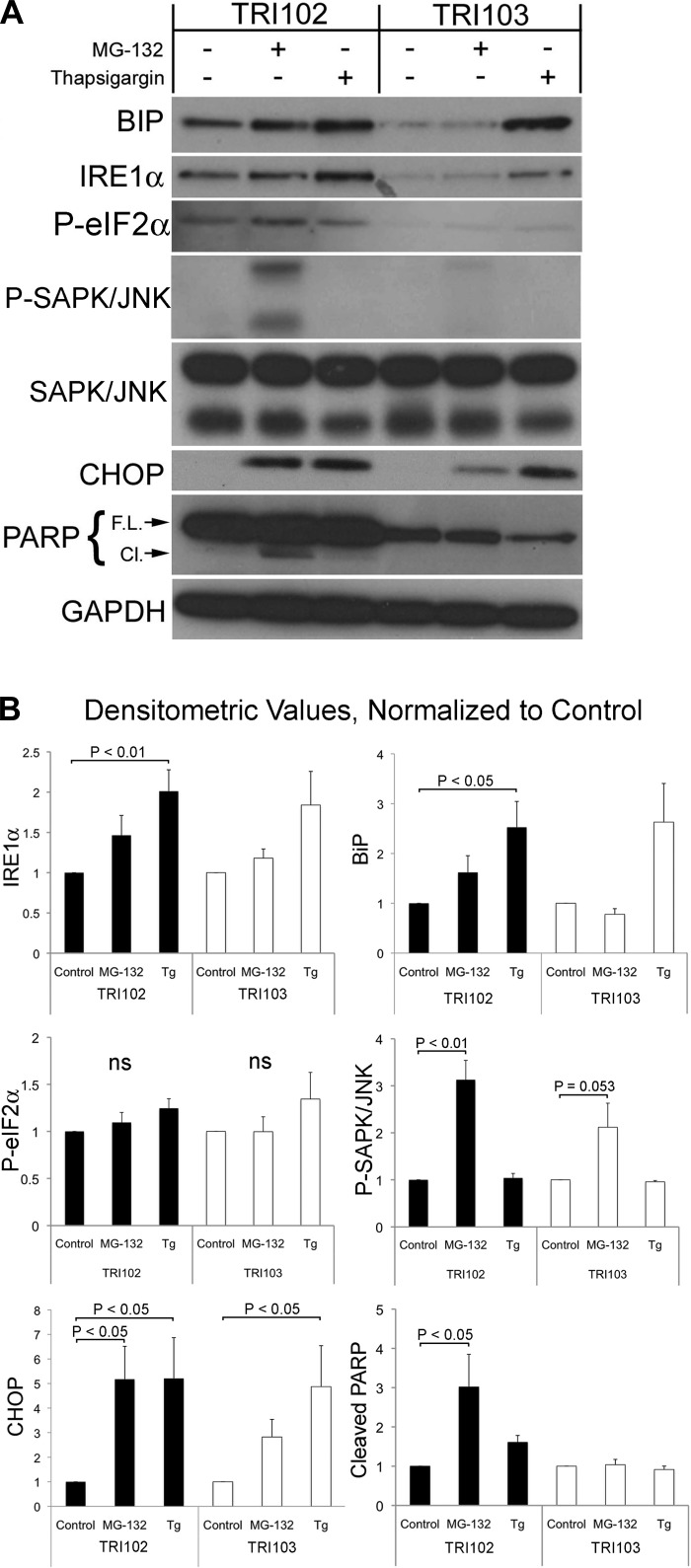
The specificity of response to different ER stress-inducing agents was evaluated by Western blot analysis of BiP, IRE1α, phospho-eIF2α, CHOP, and PARP cleavage in untreated cells, or following treatment with the proteasome inhibitor MG-132 (500 nM, 8 h), or the ER Ca2+-ATPase inhibitor thapsigargin (1 μM, 8 h). Both treatments are known to cause or exacerbate ER stress. Basal levels of BiP, IRE1α and phospho-eIF2α appeared elevated in TRI102 compared with TRI103 cells (Fig. 3A). Thapsigargin treatment significantly increased BiP, IRE1α, and CHOP expression in both cells types, while phospho-eIF2α appeared to modestly increase, but did not reach statistical significance. With MG-132 treatment, IRE1α, BiP and phospho-eIF2α appeared to be increased, although in these experiments did not reach statistical significance (Fig. 3, A and B). TRI102 cells displayed increased sensitivity to MG-132 as evidenced by a greater degree of CHOP induction and cleavage of PARP, indicating induction of ER stress-mediated apoptosis, which did not occur in TRI103 cells (Fig. 3, A and B). These findings suggest that the tuberin-null TRI102 cells have enhanced sensitivity to ER stress induced by proteasome inhibition compared with TRI103 cells but not to thapsigargin-induced ER stress.
Fig. 3.
A: Western blots for BiP, inositol-requiring enzyme-1α (IRE1α), phospho (P)-eIF2α, phospho-SAPK/JNK, SAPK/JNK, CHOP, full-length and cleaved PARP, and GAPDH in TRI102 and TRI103 cells given no treatment, 500 nM MG-132, or 1 μM thapsigargin (Tg) for 8 h in serum free conditions. B: graphs depicting densitometric analysis of BiP, IRE1α, phospho-eIF2α, phospho-SAPK/JNK, CHOP, and cleaved PARP levels normalized to untreated control values (n = 4).
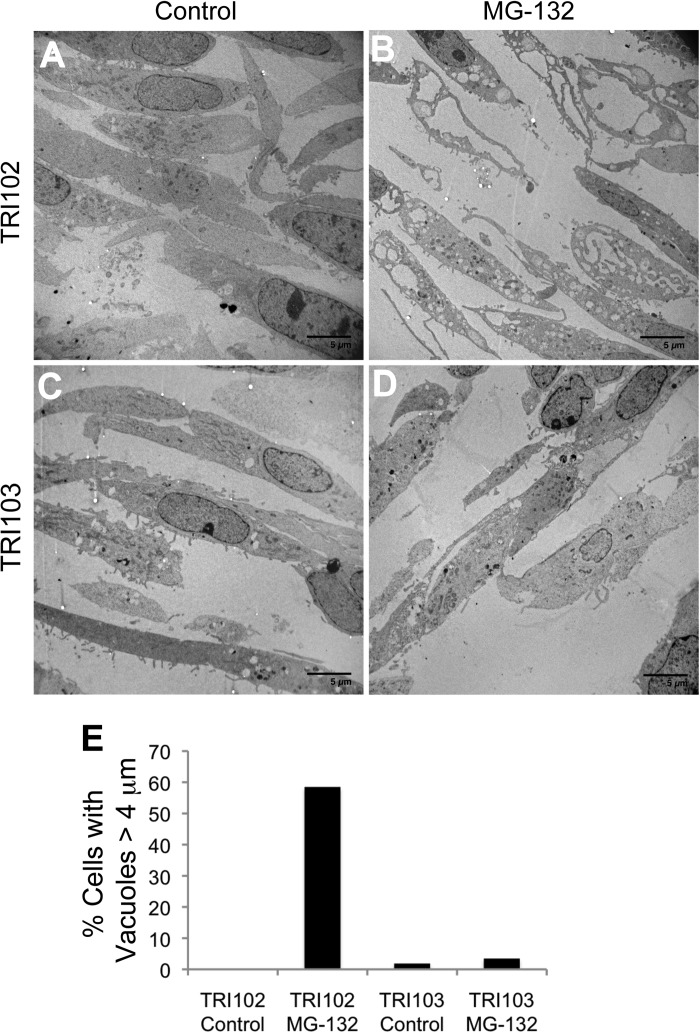
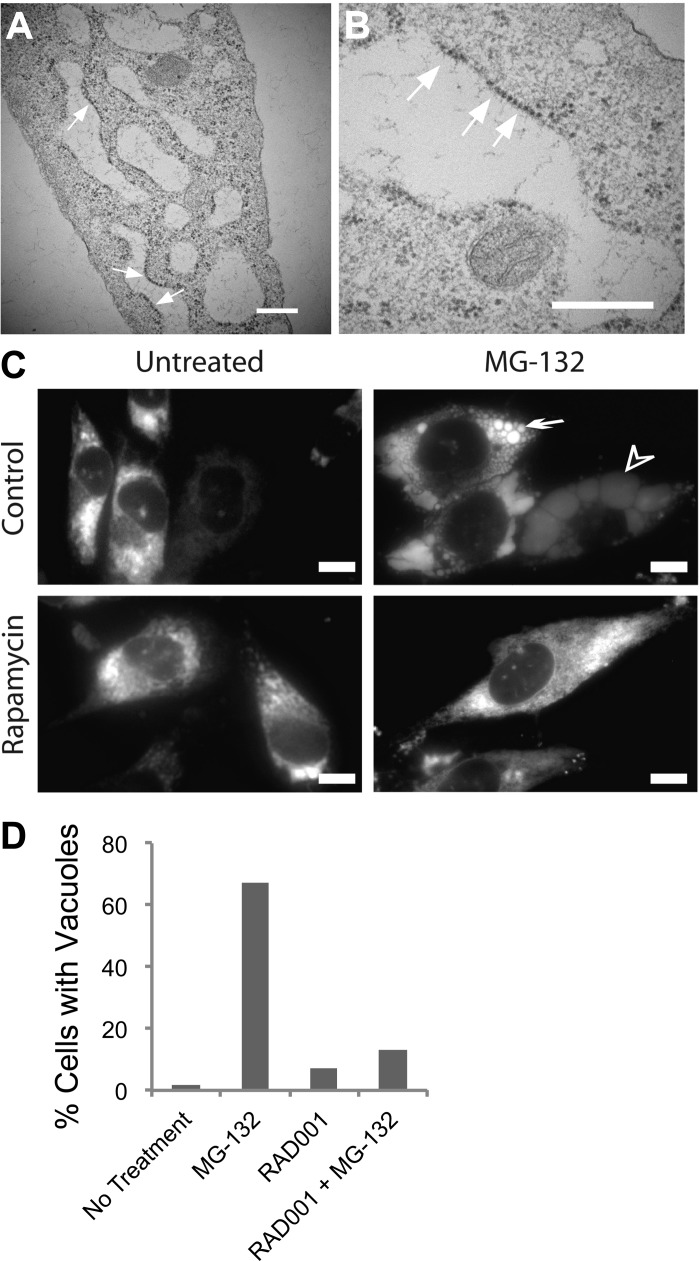
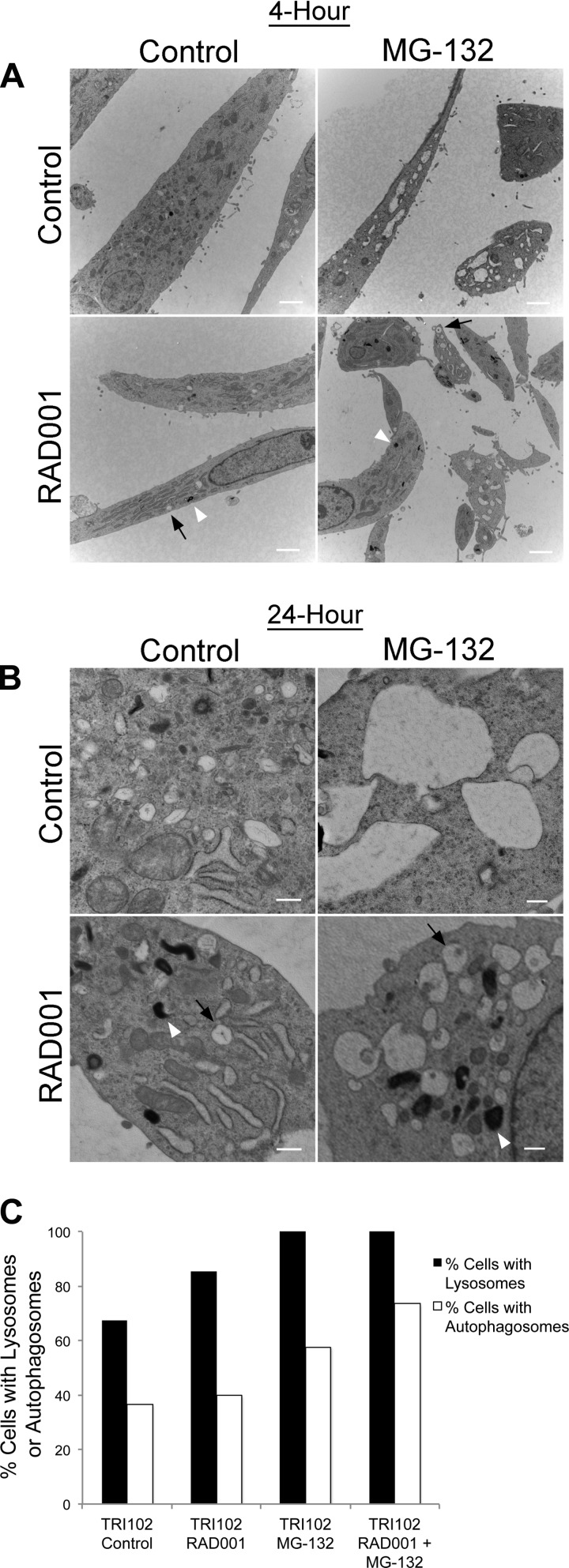
We observed the presence of numerous, large, irregularly shaped, longitudinal, and/or round vacuoles in TRI102 cells with MG-132 treatment that were absent in TRI103 cells (Fig. 4). Electron microscopy (EM) images demonstrate the large vacuoles observed only in MG-132-treated TRI102 cells are “studded” with ribosomes, indicating that these are of ER origin (Fig. 5, A and B). In 621–101 human renal angiomyolipoma cells expressing an ER-targeted RFP, a diffuse pattern of fluorescence consistent with ER staining was evident in untreated cells, while MG-132 induced numerous vacuoles that displayed accumulation of RFP, confirming their ER origin (Fig. 5C).
Fig. 4.
Electron micrographs of TRI102 (A and B) and TRI103 (C and D) cells untreated or treated with MG-132 (500 nM, 8 h). Note the pronounced vacuolization in MG-132 treated TRI102 cells (B) compared with other groups. E: graph depicting the percentage of cells containing vacuoles >4 μm in longest diameter. A minimum of 50 cells were counted for each group.
Fig. 5.
A and B: electron micrographs of MG-132-treated TRI102 cells demonstrating the presence of ribosomes on the edges of large vacuoles. White arrows indicate examples of ribosomes. White bars = 500 nm. C: fluorescence images of 621–101 human renal angiomyolipoma cells expressing an endoplasmic retituclum (ER)-localized RFP-containing an ER targeting and retention sequence. A diffuse pattern of fluorescence consistent with ER can be seen in untreated control (top left). Pronounced vacuolization is evident with MG-132 treatment (500 nM, 8 h; top right). Small, bright ER-derived vacuoles can clearly be seen (white arrow) and also very large vacuoles with a weaker, more diffused RFP signal due to increased size (arrowhead). Rapamycin suppressed vacuole formation by MG-132 (bottom right). White bars = 10 μm. D: graph depicting the percent of vacuolated TRI102 cells treated as follows in serum free conditions: no treatment, 500 nM MG-132 (8 h), 20 nM RAD001 (72 h), or RAD001 + MG-132 (as previous). Vacuolated cells were determined from immunofluorescence imaging of cells labeled with anti-LC3 (see Fig. 8). Importantly, LC3 did not appear to directly label the numerous large vacuoles, but vacuoles were clearly discernable inside cells in these images (see Fig. 8E).
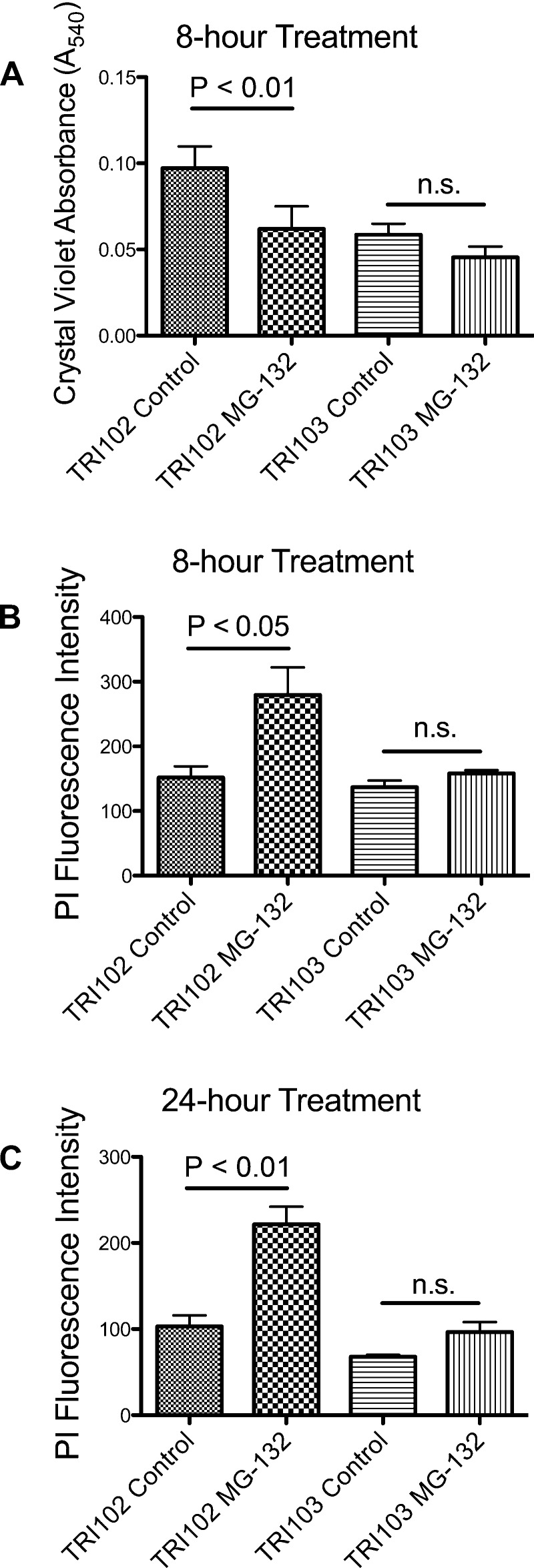
To understand if the ER stress leveraged a targeted cytotoxic effect, cell number quantification was performed with and without MG-132 (500 nM, 8 or 24 h) by crystal violet DNA dye binding assay and cell death was measured by propidium iodide uptake assay. Proteasome inhibition significantly reduced the number of viable cells in TRI102 but not TRI103 after 8 h (Fig. 6A). Propidium iodide uptake was significantly increased in TRI102 cells following MG-132 treatment, indicating cell death (Fig. 6, B and C). These findings are consistent with a cytotoxic effect. Such a cytotoxic response did not occur in TRI103 cells even after 24-h exposure to MG-132. These findings suggest tuberin deficiency, and mTORC1 activation may uniquely sensitize human renal angiomyolipoma cells to proteasome inhibition therapies that exacerbate ER stress toward cytotoxicity.
Fig. 6.
A: graph depicting cell number quantification measured by crystal violet DNA dye binding assay in TRI102 and TRI103 cells without and with 500 nM MG-132 treatment for 8 h in serum free conditions (n = 5). B and C: graph depicting cell death measured by propidium iodide (PI) uptake assay in TRI102 and TRI103 cells following 8-h (B) and 24-h (C) treatment with 500 nM MG-132 in serum free conditions (n = 3, each).
Effects of combined RAD001 and MG-132 treatment on TSC2 null human renal angiomyolipoma cells.
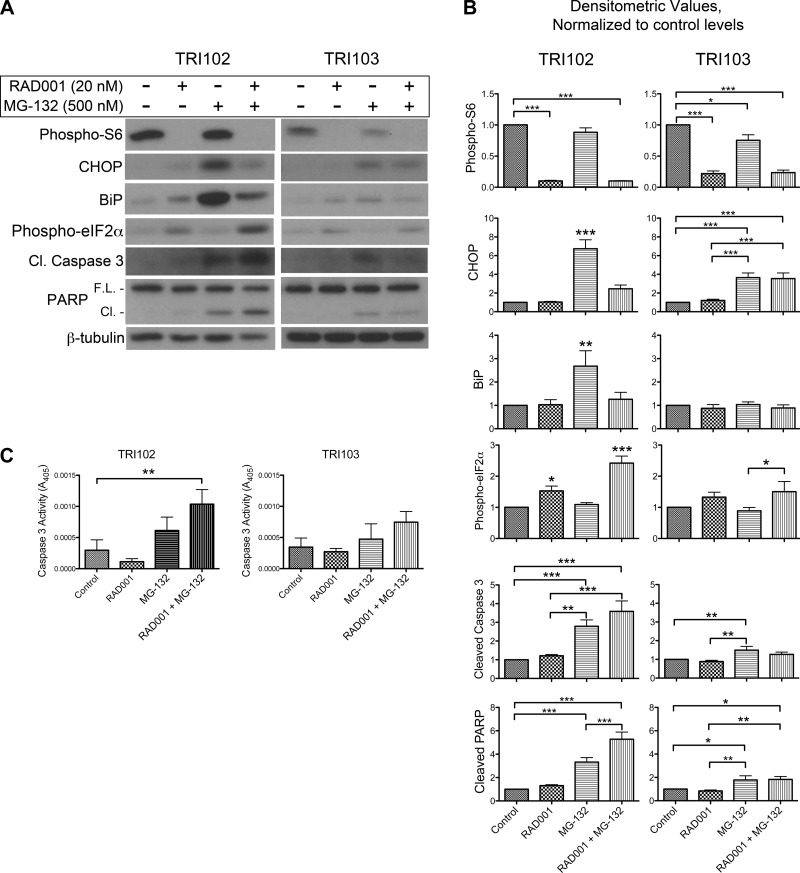
We hypothesized that pretreatment of TRI102 cells with the mTORC1 inhibitor RAD001 would relieve ER stress and thereby reduce sensitivity to the MG-132 treatment. TRI102 cells were pretreated with 20 nM RAD001 in serum free conditions for 64 h then MG-132 was added for 8 h, and whole cell lysates were obtained for Western blot analysis (Fig. 7, A and B). The 72-h time point for RAD001 exposure was chosen to ensure that levels of ER stress-adaptive proteins (such as BiP) would acclimatize to the pharmacologically reduced level of mTORC1 activity. As expected, after 72 h of RAD001 exposure, BiP and CHOP induction by MG-132 was attenuated. Also, the vacuolization induced by MG-132 in TRI102 and parental 621–101 cells was not present in RAD001 pretreated cells (Figs. 4, 5, C and D, 8, and 9). Surprisingly, MG-132-induced PARP and caspase-3 cleavage was increased in RAD001-pretreated groups compared with nonpretreated groups (Fig. 7, A and B). Also, phospho-eIF2α levels were higher in TRI102 cells that received both treatments. Assays measuring caspase-3 activity supported the enhanced apoptotic signaling in TRI102 cells given combined RAD001 pretreatment and MG-132 (Fig. 7C). Thus, while ER stress was attenuated, proapoptotic stimuli were increased in RAD001 pretreated TRI102 cells upon MG-132 administration.
Fig. 7.
A: Western blots for phospho-S6, CHOP, BiP, phospho-eIF2α, cleaved caspase-3, full-length and cleaved PARP, and β-tubulin in TRI102 cells given no treatment, or 500 nM MG-132 (8 h) with and without 20 nM RAD001 pretreatment (72 h; n = 7). B: graphs depicting densitometric analysis (values normalized to untreated control levels). *P < 0.05; **P < 0.01; ***P < 0.001. C: graphs depicting caspase-3 activity measured in TRI102 and TRI103 cells given no treatment, or 500 nM MG-132 (8 h) with and without 20 nM RAD001 pretreatment (72 h; n = 4). **P < 0.01.
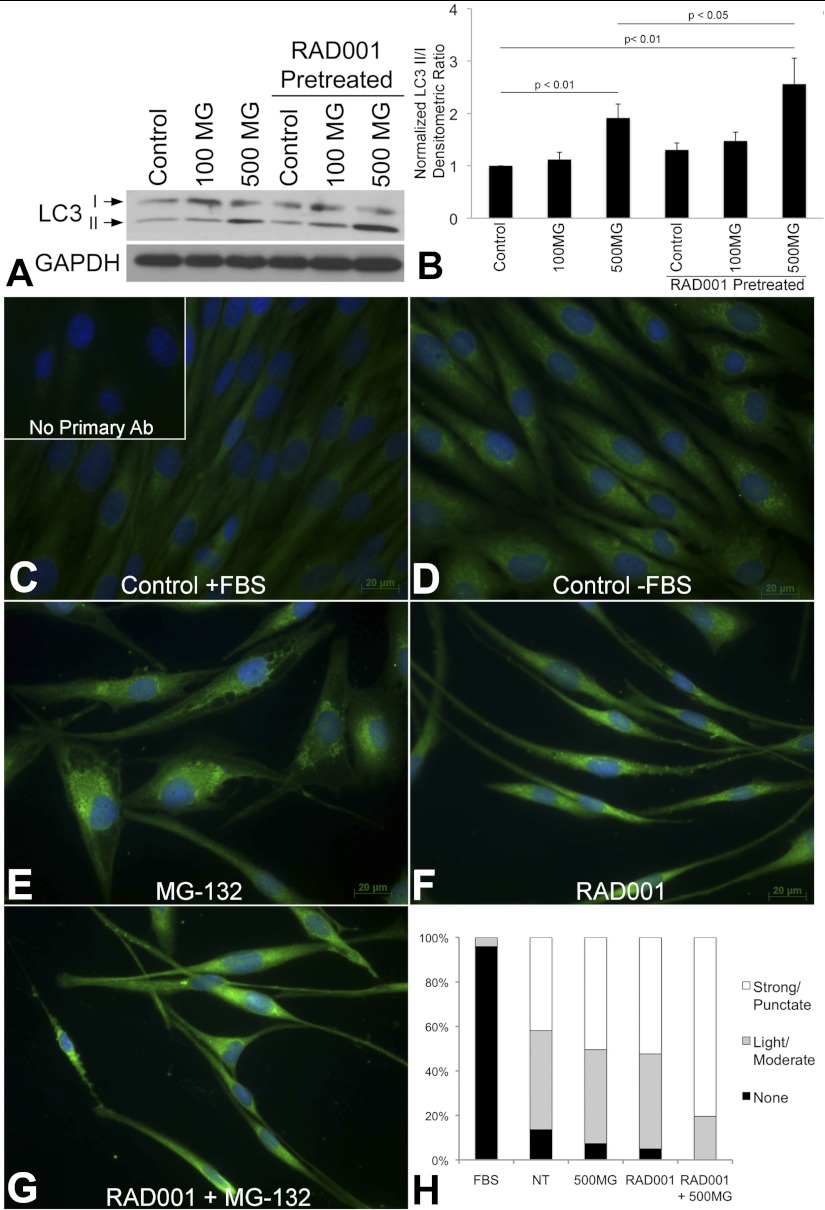
Fig. 8.
A: Western blot of LC3 I and II in TRI102 cells given no treatment, 100 nM or 500 nM MG-132 (8 h) with and without 20 nM RAD001 pretreatment (72 h) in serum free conditions (n = 7). B: graph depicting densitometric analysis. C–G: immunofluorescence images of TRI102 cells stained for LC3. C: control with FBS; inset: no primary antibody negative control. D: control, serum free for 72 h. E: 500 nM MG-132 for 8 h, serum free 72 h. F: 20 nM RAD001 pretreatment for 72 h, serum free 72 h. G: 20 nM RAD001 pretreatment for 72 h, 500 nM MG-132 for final 8 h, serum free 72 h. H: graph depicting percentage of TRI102 cells with LC3 staining patterns of “None,” “Light/Moderate,” or “Strong/Punctate” given the aforementioned treatments; 175 to 260 individual cells in randomly selected fields from 6 separate experiments were evaluated separately by 2 investigators (B. J. Siroky, unblinded and J. J. Bissler, blinded) with a correlation coefficient of 0.992 between data sets.
Fig. 9.
Electron micrographs of TRI102 cells treated with RAD001 (20 nM) and/or MG-132 (500 nM) for 4 h (A) or 24 h (B). A: images are lower magnification (white bars = 2 μm) and demonstrate vacuole formation with MG-132 treatment that is attenuated with RAD001 pretreatment. Electron dense lysosomes (white arrowheads) and autophagosomes surrounding partially digested cellular material (black arrows) are present in RAD001-treated cells, but appear more abundant in RAD001/MG-132 treated groups. B: images are higher magnification (white bars = 500 nm) and demonstrate similar findings. C: graph depicting the percentage of TRI102 cells expressing lysosomes and autophagosomes after 24 h of each indicated treatment. For each group, ≥50 cells were examined from 3 different experiments.
Since mTORC1 inhibition can induce cellular autophagy (26), we hypothesized that an autophagic impetus might contribute to the increased apoptosis caused by the combined treatment. We measured the conversion of LC3 I to II (a lipidation/proteolytic modification that accompanies autophagosome formation) by Western blot and observed LC3 staining by immunofluorescence under the aforementioned treatment conditions. We found that LC3 conversion, measured as the densitometric ratio of LC3 II to I (25), was increased in TRI102 cells given the combined treatment compared with MG-132 alone (Fig. 8, A and B). Also, immunofluorescence imaging supported this finding in that cells from the combined treatment group had more frequent punctate LC3 staining (indicating autophagosomes) compared with MG-132 alone (Fig. 8, C–H). Adding support to the induction of autophagy in this case, EM images revealed the presence of electron dense lysosomes and double membrane-bound autophagosomes in TRI102 cells that were treated with MG-132, RAD001 or the combination (Fig. 9, A and B). These were more abundant with the combined treatment than with either MG-132 or RAD001 alone (Fig. 9C). Notably, autophagosomes were clearly evident in RAD001-treated TRI102 cells, indicating cellular autophagy, but LC3 conversion was not increased compared with control levels on Western blot at the 72-h time point (Fig. 8, A and B). The absence of observable LC3 conversion is likely because autophagic flux returned to baseline levels after the 72-h duration of RAD001 exposure in this experiment.
Evidence of ER stress observed in human renal angiomyolipoma tissue.
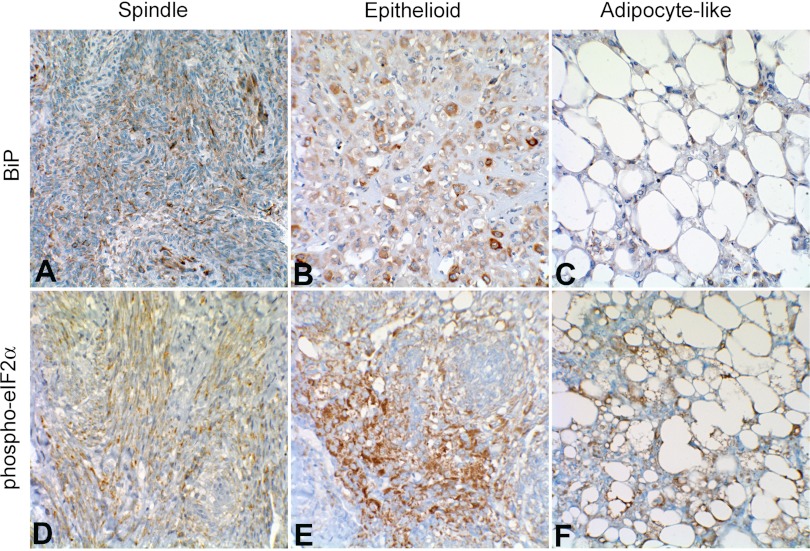
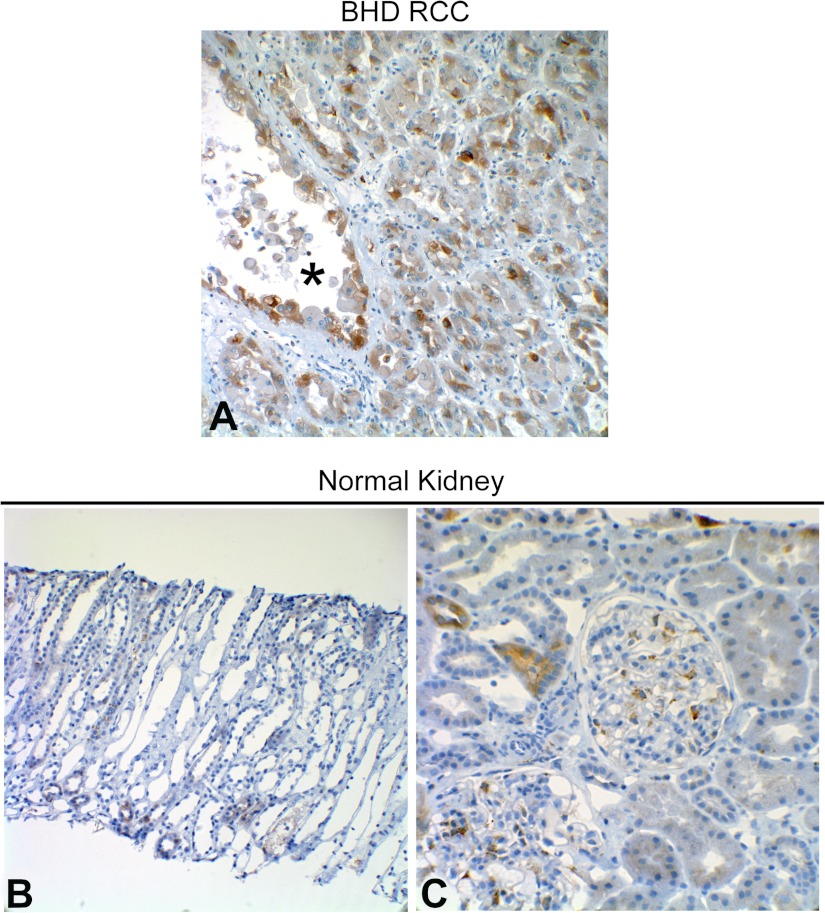
To determine whether ER stress is evident in renal lesions from TSC patients, renal angiomyolipoma tissue was probed for BiP and phospho-eIF2α. Moderate to strong BiP positivity was detected in angiomyolipoma tissue from three of the four TSC patients that were investigated (Table 1). Specifically, BiP positivity was observed in all cellular components (spindle, adipocyte-like, and epithelioid) of angiomyolipomas (Fig. 10, A–C) and also in spindle cells interspersed in vascular walls inside angiomyolipomas. BiP positivity has been identified in renal cell carcinomas (RCC; Ref. 10). Therefore, as a positive control, we observed a RCC and RCC cyst from a patient with Birt-Hogg-Dubé syndrome and found that each stained positive for BiP (Fig. 11A). The BHD mutation in Birt-Hogg-Dubé syndrome leads to folliculin inactivation and thus to mTOR activation (5), which could lead to ER stress and may explain the BiP positivity in this case. As a comparative negative control, we examined normal kidney tissue that had been obtained by biopsy because of a history of macroscopic hematuria (Fig. 11, B and C). A very small number of tubules from this tissue were mildly BiP positive, and there was occasional BiP positivity in mesangial cells in the glomeruli, but, by and large, all cell types in this tissue were negative. Each of the four TSC patient samples also stained positively for phospho-eIF2α (Table 1 and Fig. 10, D–F).
Table 1.
Summary of immunohistochemical findings from renal angiomyolipoma tissue from four TSC patients
| BiP |
Phospho-eIF2α |
|||||
|---|---|---|---|---|---|---|
| TSC Renal Angiomyolipoma Tissue Sample | Spindle | Epithelioid | Adipocyte | Spindle | Epithelioid | Adipocyte |
| 1 | + | + | + | + | + | + |
| 2 | + | −/+* | − | + | + | + |
| 3 | + | + | + | − | + | + |
| 4 | − | −/+* | − | + | + | + |
TSC, tuberous sclerosis complex; BiP, binding protein; eIF2α, eukaryotic initiation factor-2α.
Epithelioid cells from this patient sample displayed weak, interspersed BiP positivity.
Fig. 10.
Immunohistochemical analysis of BiP (A–C) and phospho-eIF2α (D–F) in de-identified human renal angiomyolipoma tissue from two TSC patients. A: spindle cells displaying strong interspersed BiP staining. B: epithelioid cells with weakly BiP-positive and scattered strongly BiP-positive large cell populations. C: adipocyte-like cells with positive BiP staining in cytoplasm and weakly positive epithelioid cells interspersed. D: spindle cells with strong phospho-eIF2α staining. E: epithelioid cells displaying strong phospho-eIF2α staining. F: adipocyte-like cells with strong cytoplasmic phospho-eIF2α staining.
Fig. 11.
A: immunohistochemical analysis of BiP expression in renal cell carcinomas (RCC) and RCC cyst (lumen marked with *) tissue from a patient with Birt-Hogg-Dubé syndrome. Lower magnification (B) and higher magnification (C) immunohistochemical images of normal renal tissue (obtained via core needle biopsy) stained for BiP.
Human renal angiomyolipoma tissue displays cells with expanded rough ER.
It has been previously demonstrated that rough ER expands as an adaptation to ER stress (33). We investigated whether rough ER was expanded in angiomyolipoma cells from human TSC patients. EM images demonstrate a pronounced expansion of rough ER in smooth muscle, epithelioid, and adipocyte cells from TSC patient-derived angiomyolipoma tissues (Fig. 12, B–D), compared with cells from normal renal tissue (Fig. 12A).
Fig. 12.
Electron microscopy demonstrating expanded endoplasmic reticulum in angiomyolipoma cells from different TSC patients compared with non-TSC associated kidney tissue. Arrows denote endoplasmic reticulum. A: normal renal tubule cell. B: angiomyolipoma spindle-morphology cell with significantly expanded endoplasmic reticulum. C: expanded endoplasmic reticulum in epithelioid cell angiomyolipoma. D: expanded endoplasmic reticulum in epithelioid cell with lipid droplets. White bar = 500 nm.
DISCUSSION
The current treatment options for renal angiomyolipoma remain limited to excisional or embolitic procedures. Recent clinical research offers evidence that mTORC1 inhibition may be cytostatic and thus a means to control this renal manifestation (2). The cytostatic effects of mTORC1 inhibition were emphasized in the present studies by the viable/dead cell assay results and the failure of PARP or caspase-3 cleavage with RAD001 treatment demonstrated in the angiomyolipoma derived cell lines. Such cytostatic effects may also be at play in the recent clinical trials with renal cystic disease (34, 39), although cyst size is a function not only of cystic epithelial cell number but also secretion of fluid into the cysts. Although cytostatic therapies represent a significant step forward, there is still a very large need for cytotoxic therapies to assuage the renal manifestations of TSC.
An important finding from this study is that TSC patient-derived human renal angiomyolipoma cells experience ER stress. This is supported by observation of elevated BiP, IRE1α, and phospho-eIF2α in TRI102 compared with TRI103 cells. This is in general agreement with other recent cell culture studies (19, 27) conducted using Tsc1-null and/or Tsc2-null MEFs in which BiP, CHOP, and spliced X-box binding protein-1 mRNAs were elevated, and phospho-PERK and phospho-eIF2α protein levels were increased, and in rat hippocampal neurons in which Tsc2-knockdown increased CHOP, ATF4, and GRP78/BiP mRNA levels (9). The present findings extend the hypothesis, that constitutive mTORC1 activity causes ER stress, into a cell type relevant to human TSC renal disease, the renal angiomyolipoma cell. The immunohistochemical findings of BiP and phospho-eIF2α positivity from TSC patient-derived angiomyolipoma tissue coupled with the pronounced ER expansion observed by EM strongly support the contention that TSC renal angiomyolipomas experience ER stress. This evidence provides solid rationale for testing whether TSC renal angiomyolipoma cells would be sensitized and susceptible to further exacerbation of ER stress toward a cytotoxic endpoint.
We also demonstrate that TSC2-deficient human renal angiomyolipoma cells have increased sensitivity, and in fact cytotoxicity, to exacerbation of ER stress by proteasome inhibition compared with TSC2 reexpressing counterparts. The shorter time course required to observe a decreased number of viable cells (8 h, compared with 72 h with mTORC1 inhibition) and increase in cell death measured by propidium iodide uptake, coupled with the increased proapoptotic signaling (caspase-3 and PARP cleavage) indicate that MG-132 does indeed produce a cytotoxic response in TRI102 cells. Notably, the differential sensitivity between TRI102 and TRI103 cells with respect to BiP, IRE1α, CHOP, and cleaved PARP response to MG-132 was not evident in response to thapsigargin. This is important because the mechanism by which ER stress is induced/exacerbated by MG-132 is the accumulation of unfolded/misfolded protein due to inhibition of the proteasomal degradative process (20). It follows that TRI102 cells would be more sensitive to this specific type of challenge given their translational drive due to constitutive mTORC1 activation. In contrast, both cell types appear equally sensitive to thapsigargin, which drives ER stress by disrupting ER Ca2+ homeostasis and thus folding capacity for existing protein, but without further increasing accumulation of misfolded protein. We submit that TRI102 cells undergo a compensatory upregulation of UPR proteins (as evidenced by BiP and IRE1α steady state levels) that facilitates the equivalent responses by TRI102 and TRI103 cells to thapsigargin-induced stress (due to ER calcium dysregulation) but that TRI102 cells are nearer to the threshold capacity to compensate for an additional increase/accumulation of the total misfolded protein load as occurs upon proteasome inhibition. Furthermore, as noted by Kang et al. (19) in Tsc-mutant MEFs, the renal angiomyolipoma cells may lack the ability to properly downregulate protein translation as part of the UPR, thereby contributing to the proposed sensitization. In fact, we observed a decrease in phospho-S6 levels (Fig. 7, A and B) in tuberin-expressing TRI103 cells after MG-132 treatment, which was not evident in the TSC2-null TRI102 line. We did not observe a truncated ER stress response to MG-132 in our TSC2-null cell line as was reported by Kang et al. in TSC-null MEFs. Here we actually observe an enhanced response to proteasome inhibition in TSC2-null compared with reexpressing cells. This discrepancy may be explained by the fact that in our studies we used a 20-fold lower concentration of MG-132 and we are measuring an enhanced sensitivity to a lower dose rather than a truncated response to a higher one.
Additional evidence of the increased sensitivity of TRI102 over TRI103 cells to proteasome inhibition was the finding of ER vacuolization in MG-132-treated TRI102 cells. The ER vacuolization observed in these cells from this study is consistent with observations of vacuolization in COS-7 and MCF-7 cells induced by proteasome inhibition (bortezomib) combined with HSP90 inhibition (geldanamycin; Ref. 23). The effect of geldanamycin in cells from that study was to destabilize HSP90-chaperoned “client” proteins, thereby decreasing cellular protein folding capacity such that subsequent proteasome inhibition caused accumulation of misfolded proteins that overwhelmed that ER secretory pathway, causing the ER distension, and ultimately sensitizing the COS-7 or MCF-7 cells to cytotoxicity by ER stress exacerbation (24). We posit that the constitutive mTORC1 activation in angiomyolipoma cells produces a similar sensitization. Thus the present findings further legitimize the concept that TSC-affected human angiomyolipoma cells can be leveraged by proteasome inhibitor-mediated ER stress exacerbation due to their mutant gain-of-function phenotype.
Based on our observation of increased sensitivity of TRI102 over TRI103 cells to ER stress exacerbation by proteasome inhibition with MG-132, we hypothesized that mTORC1 inhibition with RAD001 would alleviate ER stress in TRI102 cells and attenuate the apoptotic response to MG-132. While RAD001 consistently reduced basal levels of MG-132-induced BiP and CHOP expression, surprisingly, cleavage of caspase-3 and PARP were increased, indicating a greater proapoptotic signal. This finding is consistent with that of Kang et al. (19) who found that rapamycin treatment also slightly enhanced active caspase-3 appearance in response to tunicamycin or thapsigargin. The finding that LC3 conversion/autophagosome formation appears to be stimulated to a greater degree by the RAD001/MG-132 combination in TRI102 angiomyolipoma cells suggests autophagy induction as a potential mechanism. Although not completely understood, crosstalk exists between autophagy and apoptotic signaling (21). The enhanced phosphorylation of eIF2α in TRI102 cells given the combination treatment could provide a mechanistic link, as persistent eIF2α phosphorylation can lead to apoptosis and also promote autophagy (30, 31, 41). Another possibility is that the enhanced autophagic response contributes directly to increased apoptotic signaling, but autophageal, or type II, cell death may also be the result. The relationship between mTORC1 and autophagy has been widely studied. Recently, Qin et al. (29) demonstrated that drug-induced ER stress reduces mTORC1 activity and subsequently activates autophagy. This group also reported that autophagy induction was attenuated in Tsc2−/− MEFs with acute ER stress stimuli. Our findings with respect to autophagy are consistent with those studies, and here we add that mTORC1 inhibition with RAD001 in TSC2-deficient angiomyolipoma cells potentiates autophagy upon acute ER stress induction. This important finding suggests that combination therapies of mTORC1 inhibition and ER stress induction may provide targeted cytotoxicity in TSC renal angiomyolipomas and autophagy may play a role in this response. We are actively working to uncover the additional molecular links in this phenomenon. A recent study by Parkhitko et al. (28) described a protective effect of autophagy in TSC2-null tumor cells treated with rapamycin and that chloroquine, which can inhibit autophagy by preventing lysosomal acidification and thus autophagolysosome formation, was synergistic with rapamycin toward cytotoxicity. Taking into account the present studies, autophagy may have both protective and cytotoxic roles in TSC tumor cells depending on the degree or context of activation. In fact, this idea of roles for autophagy in both cell death and survival has been proposed and is currently a topic of great interest and debate (8, 31).
The observation of BiP and phospho-eIF2α positivity in human tissues, coupled with the findings in human cell culture, suggest that TSC patient renal angiomyolipomas both experience ER stress due to aberrant mTORC1 activation and could be susceptible, in a targeted fashion, to therapies that exacerbate ER stress, such as proteasome inhibition (1). For example, the proteasome inhibitor bortezomib is an FDA-approved drug available for multiple myeloma that targets this tumor's exuberant translation and resultant UPR (4, 6, 12). Proteasome inhibitors also are effective against prostate cancer and head and neck squamous cell carcinomas (15, 16, 37, 38, 40). Regrettably, tumors are not universally susceptible to a single therapeutic approach. Fortunately, the additional therapeutic avenue involving perturbations in the ER stress and autophagy pathways likely offers an alternative approach for TSC-related renal disease.
GRANTS
This work was supported by the Tuberous Sclerosis Alliance Rothberg Courage Award (to J. J. Bissler) and National Institute of Environmental Health Sciences Grant 2T32-ES-7250-21A1 (to B. J. Siroky).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.J.S., J.T.B., A.R.H., B.P.D., L.A.Q., and J.J.B. conception and design of research; B.J.S., H.Y., J.T.B., L.L., A.R.H., B.P.D., and L.A.Q. performed experiments; B.J.S., H.Y., J.T.B., A.R.H., L.A.Q., and J.J.B. analyzed data; B.J.S., H.Y., J.T.B., L.A.Q., and J.J.B. interpreted results of experiments; B.J.S., J.T.B., and L.A.Q. prepared figures; B.J.S., J.T.B., and L.A.Q. drafted manuscript; B.J.S., J.T.B., L.A.Q., and J.J.B. edited and revised manuscript; B.J.S. and J.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nancy Kleene who assisted in the proofreading and critical review of the manuscript.
REFERENCES
- 1. Babcock JT, Quilliam LA. Rheb/mTOR activation and regulation in cancer: novel treatment strategies beyond rapamycin. Curr Drug Targets. [DOI] [PubMed] [Google Scholar]
- 2. Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, Salisbury S, Franz DN. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358: 140–151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Catley L, Weisberg E, Kiziltepe T, Tai YT, Hideshima T, Neri P, Tassone P, Atadja P, Chauhan D, Munshi NC, Anderson KC. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood 108: 3441–3449, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cocciolone RA, Crotty KA, Andrews L, Haass NK, Moloney FJ. Multiple desmoplastic melanomas in Birt-Hogg-Dube syndrome and a proposed signaling link between folliculin, the mTOR pathway, and melanoma susceptibility. Arch Dermatol 146: 1316–1318 [DOI] [PubMed] [Google Scholar]
- 6. Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ, Davies FE. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood 110: 2641–2649, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Davies DM, Johnson SR, Tattersfield AE, Kingswood JC, Cox JA, McCartney DL, Doyle T, Elmslie F, Saggar A, de Vries PJ, Sampson JR. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med 358: 200–203, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ 19: 87–95, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Nardo A, Kramvis I, Cho N, Sadowski A, Meikle L, Kwiatkowski DJ, Sahin M. Tuberous sclerosis complex activity is required to control neuronal stress responses in an mTOR-dependent manner. J Neurosci 29: 5926–5937, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fu W, Wu X, Li J, Mo Z, Yang Z, Huang W, Ding Q. Upregulation of GRP78 in renal cell carcinoma and its significance. Urology 75: 603–607 [DOI] [PubMed] [Google Scholar]
- 11. Furukawa T, Duguid WP, Rosenberg L, Viallet J, Galloway DA, Tsao MS. Long-term culture and immortalization of epithelial cells from normal adult human pancreatic ducts transfected by the E6E7 gene of human papilloma virus 16. Am J Pathol 148: 1763–1770, 1996 [PMC free article] [PubMed] [Google Scholar]
- 12. Gass JN, Gunn KE, Sriburi R, Brewer JW. Stressed-out B cells? Plasma-cell differentiation and the unfolded protein response. Trends Immunol 25: 17–24, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Henske EP, Neumann HP, Scheithauer BW, Herbst EW, Short MP, Kwiatkowski DJ. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer 13: 295–298, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, Schlossman R, Podar K, Munshi NC, Mitsiades N, Anderson KC. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood 101: 1530–1534, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res 61: 3071–3076, 2001 [PubMed] [Google Scholar]
- 17. Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell 30: 701–711, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Inagi R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp Nephrol 112: e1–9, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Kang YJ, Lu MK, Guan KL. The TSC1 and TSC2 tumor suppressors are required for proper ER stress response and protect cells from ER stress-induced apoptosis. Cell Death Differ 18: 133–144, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol 8: 397–403, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest 115: 2679–2688, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y, Hangoc G, Campbell TB, Goodman M, Tao W, Pollok K, Srour EF, Broxmeyer HE. Identification of parameters required for efficient lentiviral vector transduction and engraftment of human cord blood CD34(+) NOD/SCID-repopulating cells. Exp Hematol 36: 947–956, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, Gius D, Neckers L. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther 3: 551–566, 2004 [PubMed] [Google Scholar]
- 24. Mimnaugh EG, Xu W, Vos M, Yuan X, Neckers L. Endoplasmic reticulum vacuolization and valosin-containing protein relocalization result from simultaneous hsp90 inhibition by geldanamycin and proteasome inhibition by velcade. Mol Cancer Res 4: 667–681, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy 3: 542–545, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem 273: 3963–3966, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 29: 541–551, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parkhitko A, Myachina F, Morrison TA, Hindi KM, Auricchio N, Karbowniczek M, Wu JJ, Finkel T, Kwiatkowski DJ, Yu JJ, Henske EP. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proc Natl Acad Sci USA 108: 12455–12460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 6: 239–247 [DOI] [PubMed] [Google Scholar]
- 30. Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci 32: 469–476, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Schleicher SM, Moretti L, Varki V, Lu B. Progress in the unraveling of the endoplasmic reticulum stress/autophagy pathway and cancer: implications for future therapeutic approaches. Drug Resist Updat 13: 79–86, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Schuck S, Prinz WA, Thorn KS, Voss C, Walter P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol 187: 525–536, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wuthrich RP. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829 [DOI] [PubMed] [Google Scholar]
- 35. Shepherd CW, Gomez MR, Lie JT, Crowson CS. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 66: 792–796, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Siroky BJ, Yin H, Bissler JJ. Clinical and molecular insights into tuberous sclerosis complex renal disease. Pediatr Nephrol 26: 839–852, 2011 [DOI] [PubMed] [Google Scholar]
- 37. Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl Bancroft C, Sausville E, Adams J, Elliott P, Van Waes C. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res 7: 1419–1428, 2001 [PubMed] [Google Scholar]
- 38. Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell 1: 493–503, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N, Arns W, Pavenstadt H, Gaedeke J, Buchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840 [DOI] [PubMed] [Google Scholar]
- 40. Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med 5: 412–417, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34: 7–11, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Yu J, Astrinidis A, Howard S, Henske EP. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am J Physiol Lung Cell Mol Physiol 286: L694–L700, 2004 [DOI] [PubMed] [Google Scholar]