Abstract
Phosphatidylinositol phosphates (PIPs) are known to regulate epithelial sodium channels (ENaC). Lipid binding assays and coimmunoprecipitation showed that the amino-terminal domain of the β- and γ-subunits of Xenopus ENaC can directly bind to phosphatidylinositol 4,5-bisphosphate (PIP2), phosphatidylinositol 3,4,5-trisphosphate (PIP3), and phosphatidic acid (PA). Similar assays demonstrated various PIPs can bind strongly to a native myristoylated alanine-rich C-kinase substrate (MARCKS), but weakly or not at all to a mutant form of MARCKS. Confocal microscopy demonstrated colocalization between MARCKS and PIP2. Confocal microscopy also showed that MARCKS redistributes from the apical membrane to the cytoplasm after PMA-induced MARCKS phosphorylation or ionomycin-induced intracellular calcium increases. Fluorescence resonance energy transfer studies revealed ENaC and MARCKS in close proximity in 2F3 cells when PKC activity and intracellular calcium concentrations are low. Transepithelial current measurements from Xenopus 2F3 cells treated with PMA and single-channel patch-clamp studies of Xenopus 2F3 cells treated with a PKC inhibitor altered Xenopus ENaC activity, which suggest an essential role for MARCKS in the regulation of Xenopus ENaC activity.
Keywords: ENaC, MARCKS, phospholipids, PIP2, PKC
luminal sodium entry through amiloride-sensitive, highly selective sodium channels (ENaC) in the apical membrane of distal nephron, lung, and colon epithelial cells is the rate-limiting step for hormonally stimulated Na+ reabsorption. This implies that a thorough understanding of the mechanisms regulating these channels is fundamental to our understanding of total body sodium homeostasis and control of blood pressure. ENaC is formed by three distinct but structurally similar subunits (i.e., α, β, γ) (10). The topology of each subunit consists of a short amino terminus, two membrane-spanning domains that flank a large extracellular loop domain, and a short carboxy terminus (8).
Mutations in ENaC lead to inherited forms of hypertension and hypotension (43), while aberrant regulation of ENaC can lead to impaired alveolar fluid clearance and lung fluid balance (33). The regulation of ENaC by anionic phospholipids (APs) has been previously described (16, 25, 26, 31, 34–37, 46, 47). Ma et. al. (27) found that the regulation of ENaC by phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3) did not involve a change in surface expression of ENaC and occurred through a mechanism independent of ENaC trafficking. Zhang et al. (47) added that while PIP2 and PIP3 could stimulate ENaC activity, phosphatidylserine (PS) could maintain ENaC activity and phosphatidic acid (PA) could inhibit ENaC. Tong et al. (44) demonstrated that epidermal growth factor (EGF) can rapidly decrease ENaC activity by decreasing its open probability (Po) in a mechanism involving the depletion of PIP2. Our laboratory further demonstrated that ENaC is activated by a PIP-dependent mechanism after acute transforming growth factor-α or EGF treatment (23). Helms et al. (17) showed that PIP3 mediates aldosterone-induced ENaC activity. Several reports addressed the hypothesis that the positive charges within the amino terminus of ENaC interact with the anionic phospholipids of the inner leaflet of the plasma membrane to regulate ENaC activity (16, 25, 37, 47). Expression of truncated ENaC subunits suggested that the amino-terminal domain of ENaC β- and γ-subunits, but not the ENaC α-subunit, was necessary for PIP-induced ENaC activity.
Although there is accumulating evidence for the regulation of ENaC by PIPs, the molecular mechanism governing the interaction between ENaC and PIPs is obscure. ENaC is rare, as only one or two channels/μm2 are expressed at the apical membrane. PIPs (e.g., PIP2 and PIP3) are also rare, as they constitute fewer than 1 in 1,000 lipid molecules. Therefore, there must be an adaptor protein that could bind and sequester PIPs and then present them to ENaC. Ideally, such a protein should be abundantly available, capable of binding and sequestering PIPs, and able to quickly release the PIPs locally to ENaC to allow activation of ENaC. One such protein is myristoylated alanine-rich c-kinase substrate (MARCKS). MARCKS consists of a myristoylated amino-terminal domain and a basic effector domain, which contains sites for PKC phosphorylation, filamentous (F)-actin binding, and calcium/calmodulin binding (15). MARCKS reversibly associates with the plasma membrane through hydrophobic and electrostatic interactions of its myristoylation and basic effector domain, respectively (20). MARCKS cross-links actin, but binding is regulated by PKC phosphorylation and calmodulin binding (14, 15). PKC phosphorylates serine resides within the basic effector domain of MARCKS, leading to the translocation of MARCKS from the membrane to the cytoplasm.
In this paper, we investigated the role of MARCKS protein in the regulation of Xenopus ENaC by anionic phosphoinositides. We demonstrated a role for MARCKS in the PIP-dependent regulation of Xenopus ENaC, and we introduce various molecular tools including novel antibodies and recombinant fusion proteins that can be used to further investigate the molecular determinants governing this mechanism. MARCKS may allow regulation of Xenopus ENaC by PIPs, but MARCKS itself is subject to precise regulation at many different levels. It will be important to further investigate the temporal and spatial regulation of MARCKS at these levels to thoroughly understand the regulation of Xenopus ENaC by anionic phosphoinositides.
MATERIALS AND METHODS
Cell culture.
2F3 cells, a clonal line derived from the Xenopus distal nephron epithelial cell line (A6), were maintained in DMEM/nutrient mix F-12 (Invitrogen, Carlsbad, CA) and NaHCO3, supplemented with 90 mM NaCl, 25 mM NaHCO3, 3.1 mM KCl, 0.8 mM CaCl2, 0.4 mM Na2HPO4, 0.3 mM NaH2PO4, 0.2 mM MgCl2, 0.3 mM MgSO4, 5% fetal bovine serum, 1.5 μM aldosterone, and 1% penicillin/streptomycin. Xenopus 2F3 cells were subcultured on glutaraldehyde-fixed, collagen-coated Millipore-CM filters (Millipore, Billerica, MA) attached to the bottom of Lucite rings for patch-clamp experiments or subcultured on 24-mm permeable inserts for all other experiments. Cells were allowed to form tight junctions and subcultured for 10 days before the experiments were performed. A549 cells, derived from human lung epithelial carcinoma cells, were cultured in F-12-K medium (Invitrogen) supplemented with 2 mm l-glutamine, 0.1% penicillin/streptomycin, and 10% fetal bovine serum. MpkCCDc14 cells, derived from the mouse kidney collecting duct, were incubated in a 1:1 mix of Dulbecco's modified Eagle's medium/Ham's F-12 medium (Invitrogen) supplemented with 50 nm dexamethasone, 1 nm triiodothyronine, 20 mm HEPES, 2 mm l-glutamine, 0.1% penicillin/streptomycin, and 2% fetal bovine serum.
SDS-PAGE and immunoblotting.
Cells or freshly isolated tissues (done in accordance with and after the approval of the Institutional Animal Care and Use Committee) were washed once with 1× PBS and then scraped into mammalian protein extraction reagent (MPER; Thermo Scientific, Rockford, IL) for cells or homogenized using an Omni TH homogenizer (Warrenton, VA) in tissue protein extraction reagent (TPER; Thermo Scientific) each containing protease and phosphatase inhibitors (Thermo Scientific). Cell lysates were passed through a 23-gauge needle and syringe five times before being incubated on ice for 1 h. Tissue lysates were centrifuged at 1,000 rpm at 4°C for 5 min to remove debris, and the supernatant was sonicated twice on ice at 10-s intervals. Protein concentration was calculated for cell and tissue lysates using a BCA protein assay (Thermo Scientific). One hundred micrograms of total protein prepared in Laemmli sample buffer (Bio-Rad, Hercules, CA) was loaded and resolved on 7.5% Tris·HCl polyacrylamide gels using the Criterion or Protean electrophoresis systems (Bio-Rad). The separated proteins were electrically transferred onto C-extra nitrocellulose membranes (GE Healthcare, Piscataway, NJ). The membranes were blocked in 5% wt/vol milk in 1× TBS (Bio-Rad) at room temperature for 1 h. The membranes were washed once with 1× TBS and then incubated with primary antibodies at a dilution of 1:1,000 in 5% wt/vol BSA in 1× TBS at 4°C for 8 h. The membranes were washed three times with 1× TBS at 5-min intervals before being incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody at a dilution of 1:3,000 in blocking solution. The membranes were incubated with SuperSignal Dura Chemiluminescent Substrate for 5 min before being developed using a Kodak Gel Logic 2200 Imager and Molecular Imaging software (Carestream Health, Rochester, NY).
Recombinant protein production.
Xenopus full-length α-, N-terminus α- (M2-V68), extracellular loop α- (S86-G529), C-terminus α- (H554-N643), N-terminus β- (M1-K51), C-terminus β- (D566-N647), and N-terminus γ-ENaC (M1-R49) coding sequences were subcloned into a pGEX expression vector. The constructs were transformed into competent bacterial cells, induced with IPTG for expression, and batch purified from inclusion bodies using glutathione-Sepharose 4B as previously described by Alli and Gower (4, 5).
Antibody production.
Polyclonal antibodies against the carboxy-terminal domain of Xenopus ENaC α- (ENaC 59) and β- (ENaC 60) subunits were generated after recombinant Xenopus glutathione-S-transferase (GST)-ENaC-α and -β fusion proteins were purified as described above, and then New Zealand White rabbits were immunized with 1–2 mg of the purified recombinant fusion protein per animal (Bio-Synthesis, Lewisville, TX). Animals were boosted/bled at 6, 8, and 10 wk thereafter before being exsanguinated. Each batch of serum was supplemented with sodium azide and evaluated for specificity and cross-reactivity using protein from the Wheat Germ In Vitro Translation System (Promega), Xenopus tissue lysates, and cellular lysates of various origins.
Transepithelial electrical measurements.
Transepithelial voltage and resistance were measured across confluent cell monolayers using an epithelial voltohmmeter (World Precision Instruments, Sarasota, FL). Transepithelial current was calculated based on Ohm's law and normalized to the surface area of the insert as described by the following equation: C = (V/R)l/4.67 μA·cm2, where C is current, V is voltage, and R is resistance. In some experiments, amiloride (10 μM) was added to the apical side of the inserts, and the difference in total current and amiloride-insensitive current was used to calculate amiloride-sensitive sodium current.
Patch-clamp, cell-attached, single-channel recordings.
Pipettes were pulled from filamented borosilicate glass capillaries (TW-150F, World Precision Instruments) using a two-stage puller (Narishige, Tokyo, Japan). The resistance of pipettes filled with patch solution (96 mM NaCl, 3.4 mM KCl, 0.8 mM MgCl2, 0.8 mM CaCl2, and 10 mM HEPES) was 5–10 MΩ. High-resistance seals (>20 GΩ) were formed after application of negative pressure on the patch membrane of confluent 2F3 cells subcultured on Lucite rings with the apical and basolateral membranes exposed to the patch solution. Single-channel current data were filtered at 1 kHz and recorded at 5 kHz using pClamp 9.2 software (Axon Instruments). The product of the number of channels times the open probability (NPo) was used as a measure of ENaC activity.
Lipid raft fractionation.
2F3 cells were lysed in ice-cold 1% Brij 96/TNEV buffer (10 mM Tris·HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 2 mM Na vanadate, and protease and phosphatase inhibitor cocktail) and passed five times through a 23-gauge needle with a syringe and then incubated on ice for 1 h. Lysates were centrifuged at 10,000 rpm for 5 min at 4°C. Five hundred microliters of the supernatant was mixed with an equal volume of 80% sucrose in TNE (without Brij 96) and then transferred to a 13 × 23-mm Beckman centrifuge tube. Eighteen hundred microliters of 35% sucrose in TNE were carefully layered on top of the mixture followed by 500 μl of 5% sucrose. The sucrose gradient was then centrifuged in a SW50.1 rotor (Beckman) at 34,000 rpm for 16 h at °4 C. Thirteen fractions of 250-μl volumes were carefully collected from the top to the bottom of the tube and then analyzed by Western blotting using Cav-1 and μ-2 antibodies as protein markers.
PIP strip overlay binding assay.
PIP strips (Molecular Probes) nitrocellulose membranes containing 15 different phospholipids each spotted at 20 pmol and a blank control were blocked with 3% fatty acid-free BST in TBS-T for 1 h at room temperature. The membrane was incubated with 1 μg/ml of the GST fusion protein in blocking buffer for 8 h at 4°C. The membranes were washed with TBS-T three times for 10-min intervals. The membranes were incubated with a peroxidase-conjugated anti-GST antibody for 4 h at 4°C before being subjected to chemiluminescent substrates for detection. All incubations were performed with gentle agitation, and membranes were not allowed to dry between steps.
Fluorescence resonance energy transfer.
Fluorescence resonance energy transfer (FRET) studies were performed as previously described by Karpova and McNally (18). Briefly, 2F3 cells cultured to 70% confluence on permeable inserts in 12-well plates were transiently cotransfected with ENaC fused to yellow fluorescent protein (YFP) with MARCKS fused to cyan fluorescent protein (CFP), CFP fused to YFP (19), unconjugated CFP, unconjugated YFP, and unconjugated CFP with unconjugated YFP in parallel experiments using the calcium phosphate method. After 48 h of being transfected, the cells were fixed for 20 min in 2% formaldehyde, washed in PBS, and the insert was mounted onto a microscope slide with a coverslip. FRET was determined by the acceptor photobleaching method.
Assessment of colocalization of MARCKS and PIP2 fluorescence.
We used the “Colocalization Finder” plugin (20a) for the image-analysis program “Image J” (41) to analyze our images for colocalization of the labels for MARCKS antibodies and for the PIP2 reporter. This plugin displays a correlation diagram as a scatterplot for two images (8 bits, same size). Drawing a rectangular selection on this diagram allows the user to identify pixels that contain both channel 1 (MARCKS fluorescence, red) and channel 2 (PIP2 fluorescence, green). These pixels will then be highlighted in white on separate images. We restricted the analysis to pixels having ratios of intensity values in the two channels >0.1, but then set the threshold above the background levels, which are relatively easy to identify in a scatterplot as the cluster of points near the origin.
Generation of mutant MARCKS fusion protein.
Spontaneous mutations occurred within the coding sequence of native MARCKS during PCR amplification. DNA sequencing and protein sequencing by mass spectrometry were performed to confirm the mutations.
Statistical analysis.
SigmaStat and SigmaPlot software (Jandel Scientific) were used to perform statistical analysis. Data are reported as means ± SE, and P < 0.05 was considered statistically significant.
RESULTS
Polyclonal antibodies made against the carboxy terminus of ENaC from recombinant fusion proteins.
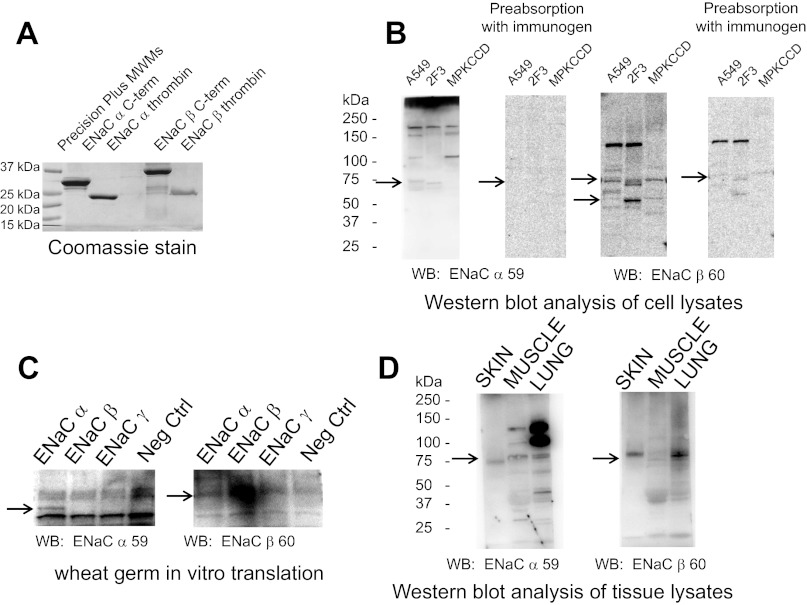
To investigate the underlying mechanism for the PIP-dependent regulation of ENaC, we made recombinant ENaC carboxy-terminal GST fusion proteins using a bacterial system. The ENaC fusion proteins were used as a renewable source of exogenous protein to perform overlay binding assays and served as immunogens to produce highly immunogenic polyclonal antibodies against the α- and β-subunits of ENaC. Several rabbits were immunized with the recombinant GST-ENaC fusion proteins that were ∼37 kDa in size (Fig. 1A), and each antibody was evaluated in various immunoassays for sensitivity and specificity. The optimal dilution of each antibody varied, and each antibody was found to cross-react with Xenopus, rat, and mouse. We did not check the efficacy against other species. However, we proceeded to fully characterize one antibody against the carboxy-terminal domain of α-ENaC and one antibody against the carboxy terminus of β-ENaC. Both the α- and β-ENaC antibodies produced multiple immunoreactive bands in Western blots when used at a dilution of up to 1:2,000 (Fig. 1B). Each of the immunoreactive bands was attenuated or absent in competition studies involving preabsorbing the antibody with the recombinant fusion protein used as the immunogen (Fig. 1B). To determine whether the antibodies were specific for each ENaC subunit, we performed wheat germ in vitro translation studies in which the antibodies were used to probe blots containing crude wheat germ extracts with or without each of the translated ENaC subunits. The α- and β-ENaC antibodies nonspecifically detected components of the wheat germ system but also specifically detected the corresponding ENaC subunits (Fig. 1C). Finally, we used the α- and β-ENaC antibodies to probe blots containing lysates from Xenopus skin, muscle, and lung. The antibodies confirmed that α- and β-ENaC subunits are abundantly present in the skin and lung (Fig. 1D).
Fig. 1.
Characterization of epithelial Na channel (ENaC) α- and β-polyclonal antibodies made from recombinant fusion proteins. A: Coomassie-stained gel showing Xenopus glutathione-S-transferase (GST)-ENaC α- and β-carboxy-terminal fusion proteins after being expressed in bacterial cells and purified over glutathione-Sepharose. The fusion proteins were used as immunogens for generating polyclonal antibodies. ENaC α-thrombin and ENaC β-thrombin refer to digestion of the recombinant fusion proteins with thrombin to remove the GST tag from the purified protein. B: Western blots showing immunoreactive bands corresponding to Xenopus ENaC α- and β-subunit proteins after protein lysates from Xenopus distal nephron 2F3 cells, human lung A549 cells, and mouse kidney collecting duct MpkCCDc14 cells were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked and probed for Xenopus ENaC α- and β-subunit proteins using bleeds corresponding to ENaC α- or β-antibodies. In parallel experiments, the antibodies were incubated with the immunogen before being used to probe for Xenopus ENaC α- or β-subunit proteins. C: Western blots showing immunoreactive bands corresponding to Xenopus ENaC α- or β-subunit proteins and demonstrating antibody specificity after probing of blots with ENaC α- or β-antibodies that contained Xenopus ENaC subunit proteins generated by the wheat germ system. D: Western blot analysis of blots showing immunoreactive bands corresponding to Xenopus ENaC α- and β-subunit proteins after protein lysates from various Xenopus tissues were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked and probed for Xenopus ENaC α- or β-subunit proteins using bleeds corresponding to ENaC α- or β-antibodies. Arrows in B–D indicate specific immunoreactive bands for ENaC α- and β-antibodies that are attenuated or absent after preincubation with the immunogen used to produce each antibody.
The amino termini of GST-ENaC-β and GST-ENaC-γ selectively bind phospholipids.
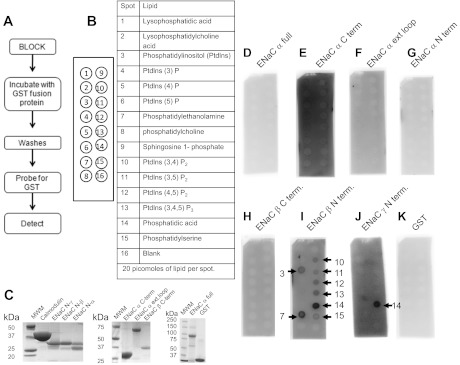
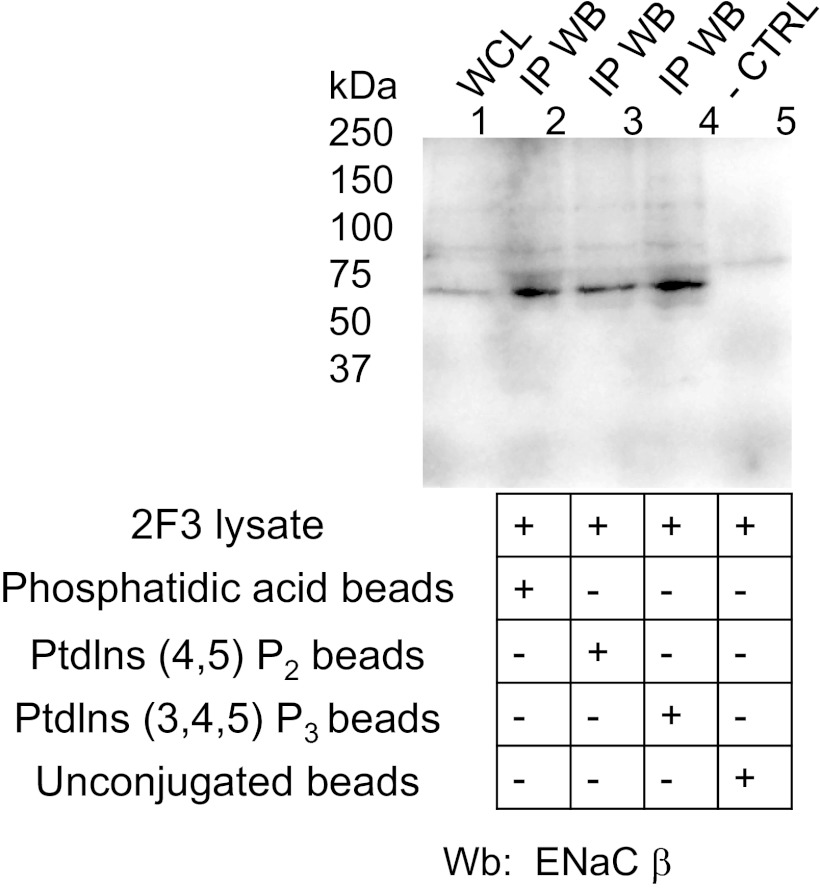
PIPs can directly bind ENaC in vitro as shown by transient transfection of various constructs and the use of specific antibodies (25). To identify the specific ENaC subunit and the specific ENaC domain that are involved in the binding, we created recombinant GST fusion proteins to specific domains of each of the three distinct ENaC subunits. The recombinant fusion proteins were used to perform PIP strip overlay binding assays. As shown in Fig. 2, A–G, the full-length, carboxy-terminal domain, extracellular loop domain, and amino-terminal domain of the ENaC α-subunit all failed to bind various phosphoinositides. Although the carboxy-terminal domain of the ENaC β-subunit failed to exhibit any appreciable binding, the amino-terminal domains of both the ENaC β- and γ-subunits selectively bound to various phosphoinositides (Fig. 2, H–J). In either case, there was no binding observed for GST alone or with the negative control well of the PIP strip assay (Fig. 2K). To confirm that PIP2, PIP3, and phosphatidic acid (PA) could directly bind to ENaC in vivo, we utilized beads conjugated to each of these phospholipids to perform pulldown studies. The proteins eluted from the phospholipid-conjugated beads after pulling down putative protein binding partners of the phospholipids were subject to SDS-PAGE and Western blot analysis. Probing the blots with an antibody against the ENaC β-subunit (Fig. 3) corroborated the findings from the PIP strip overlay binding assays.
Fig. 2.
Phosphatidylinositol phosphate (PIP) strip overlay binding assay using recombinant Xenopus GST-ENaC fusion proteins. Schematic of the general procedure (A) and format (B) of the PIP strip binding assay. C: Coomassie-stained gel showing the purity and size of each recombinant protein used. GST fusion proteins of full length Xenopus ENaC-α (D), Xenopus ENaC-α carboxy-terminal domain (E), Xenopus ENaC-α extracellular loop domain (F), Xenopus ENaC-α amino-terminal domain (G), and Xenopus ENaC-β carboxy-terminal domain (H) did not directly bind to any of the 15 different phospholipids or banks. GST fusion proteins of Xenopus ENaC-β amino-terminal domain (I) and Xenopus ENaC-γ amino-terminal domain (J) directly bound to various phospholipids, but with different affinities. GST alone did not bind with any appreciable affinity to any of the spotted phospholipids (K). The white dots present in various spots are due to burnout of the signal of the film.
Fig. 3.
Immunoprecipitated Western blot (IP WB) confirming endogenous Xenopus ENaC β-subunit associates with phosphatidic acid, phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3) in 2F3 cells. Cell lysate from Xenopus 2F3 cells was incubated in the presence of beads conjugated to either phosphatidic acid, PIP2, or PIP3. The complex of conjugated beads and interacting proteins were subject to a series of washes before being eluted in sample buffer. Eluted proteins were separated by SDS-PAGE and transferred onto nitrocellulose membranes before being blocked and probed for Xenopus ENaC β-subunit using ENaC-β 60 antibody. As a control, unconjugated beads were used to determine nonspecific binding. WCL represents whole cell lysate.
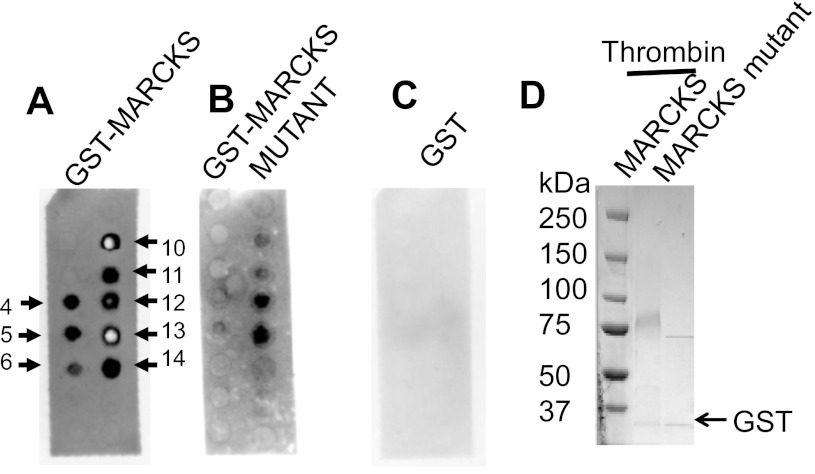
MARCKS and MARCKS mutant proteins differentially bind phospholipids in vitro.
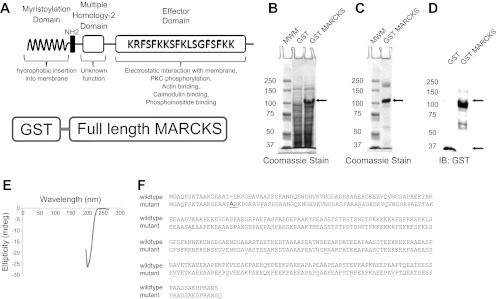
MARCKS is an interesting protein because of its unique biochemical and biophysical properties. Theoretically, MARCKS is a 32-kDa protein but migrates close to 75 kDa in SDS-PAGE gels. Native MARCKS adopts an unfolded conformation but binds to various molecules and proteins. Its function is dependent on its cellular localization and its association with other proteins. In particular, one domain of MARCKS, the so-called effector domain, is critical for the binding of PIPs and contains the PKC phosphorylation site. We evaluated whether the binding of MARCKS to various phospholipids was affected by mutations in this effector domain. Recombinant native MARCKS (Fig. 4, A–E) and mutant MARCKS GST (Fig. 4F) fusion proteins were expressed and purified from bacterial cells. As shown in Fig. 4E, the absence of two negative bands of similar magnitude at ∼208 and 222 nm and the presence of a positive band near 190 nm suggest the protein lacks an all-α helical conformation. Also, the absence of a negative band between 210 and 220 nm and a positive band in the 195- to 200-nm region suggest the protein lacks an all-β sheet conformation. Instead, the strong negative band near 200 nm is characteristic of a disorderly, or random coil protein. The mutant MARCKS construct presented here is novel and was created after directly subcloning a sequence of MARCKS containing spontaneous mutations into the pGEX expression vector. PIP strip overlay binding assays indicated the binding of various phospholipids was significantly attenuated or absent for the mutant MARCKS compared with the native MARCKS protein (Fig. 5).
Fig. 4.
Characterization of GST mutant and native myristoylated alanine-rich C-kinase substrate (MARCKS). A: schematic of the GST-MARCKS fusion protein (bottom) and the anatomy of native MARCKS protein (top). The myristoylation domain of MARCKS and the basic effector domain of MARCKS both contribute to the reversible association of MARCKS with the membrane. Binding of the basic effector domain with actin or calcium-calmodulin or posttranslational modification (phosphorylation) of the basic effector domain results in dissociation of MARCKS from the membrane. B: Coomassie-stained gel showing a predominant 100-kDa band after expression of GST-MARCKS with IPTG induction. C: Coomassie-stained gel showing purification of the 100-kDa band after batch purification over Sepharose and eluting with reduced glutathione. D: Western blot analysis of GST-MARCKS confirming a 100-kDa immunoreactive band after probing with a peroxidase-conjugated antibody against GST. E: far UV circular dichroism analysis showing GST-MARCKS is not dominated by secondary structural elements such as α-helixes and β-sheets. F: amino acid sequence alignment comparing native MARCKS with the mutant MARCKS protein.
Fig. 5.
Molecular analysis of the association between MARCKS and PIPS. Overlay binding assay showing purified GST-MARCKS (A) and a purified GST-MARCKS mutant (B) fusion protein, but not GST (C) each bind directly to various phospholipids in vitro, but with different affinities. The format for the PIP strip assays is the same as presented in Fig. 2. The sequences for the native MARCKS and mutant MARCKS constructs used to perform the PIP strip assays are given in the previous figure. The white dots present in various spots are due to burnout of the signal of the film.
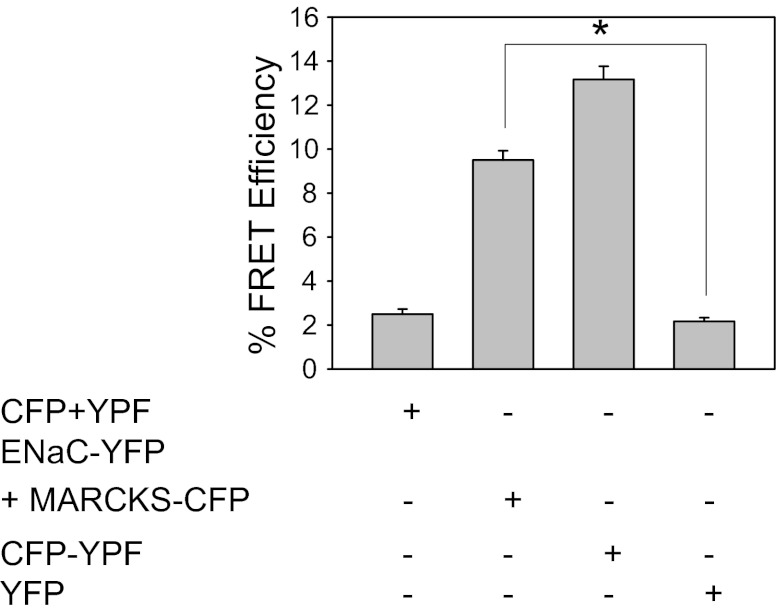
ENaC-YFP and MARCKS-CFP are close enough to allow FRET.
FRET decreases as the fourth power of the distance between the resonant molecules. Therefore, any significant energy transfer implies that the resonant molecules must be physically very close together. To analyze FRET between ENaC and MARCKS in vitro, we applied an established FRET method, acceptor photobleaching, as originally described by Karpova and McNally (18). This approach involves transient cotransfection of one protein of interest fused to YFP and the other protein of interest fused to CFP. As controls, CFP fused to YFP is transfected and serves as a positive control to establish the maximum detectable level of FRET under our imaging conditions. Unconjugated CFP, unconjugated YFP, and unconjugated CFP mixed with unconjugated YFP are also transfected separately in parallel experiments as negative controls. As shown in Fig. 6, the maximum FRET efficiency observed for the FRET pair of CFP directly fused to YFP was ∼13%. There was a statistically significant difference (*P < 0.05) in the FRET efficiency between the FRET pair of ENaC-YFP coexpressed with MARCKS-CFP (9.5 ± 0.4280) and the negative control YFP (2.1670 ± 0.1670) (Fig. 6). The distance between the donor and acceptor pair to achieve FRET was calculated to be 6.5 nm.
Fig. 6.
Statistical analysis of Fluorescence resonance energy transfer (FRET) efficiency as determined by acceptor photobleaching in Xenopus 2F3 cells. Xenopus 2F3 cells were transfected with the indicated constructs, fixed, and mounted on microscope coverslips for imaging. FRET determination is shown for images from 6 independent cells. YFP, yellow fluorescent protein; CYF, cyan fluorescent protein. Values are means ± SE (*P < 0.05).
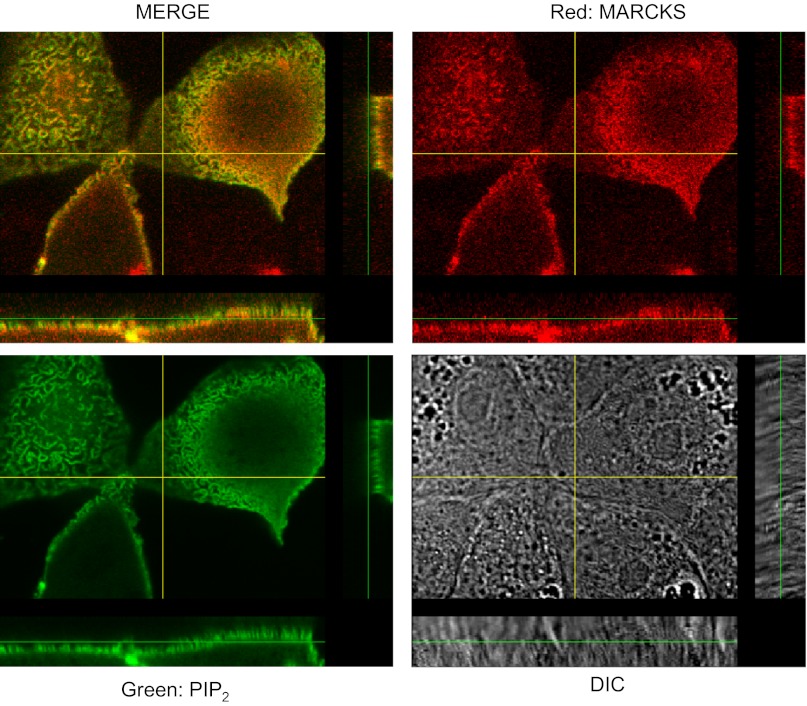
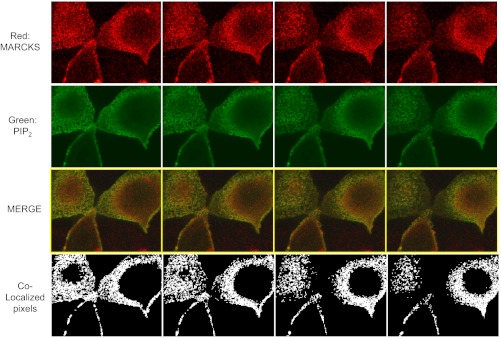
MARCKS colocalizes with PIP2 at the apical membrane of Xenopus 2F3 cells.
The PIP strip overlay binding assays using recombinant MARCKS GST fusion proteins demonstrate direct binding between various phospholipids and MARCKS in vitro. To determine whether MARCKS associates with the apical membrane where ENaC is present, the basolateral membrane, or both membranes, we performed confocal microscopy studies. As a reporter for PIP2 levels, 2F3 cells were transfected with the GFP-tagged pleckstrin homology (PH) domain of PLC-δ1 (GFP-PH). As shown in Figs. 7 and 8, MARCKS colocalizes with PIP2 at the apical membrane of 2F3 cells.
Fig. 7.
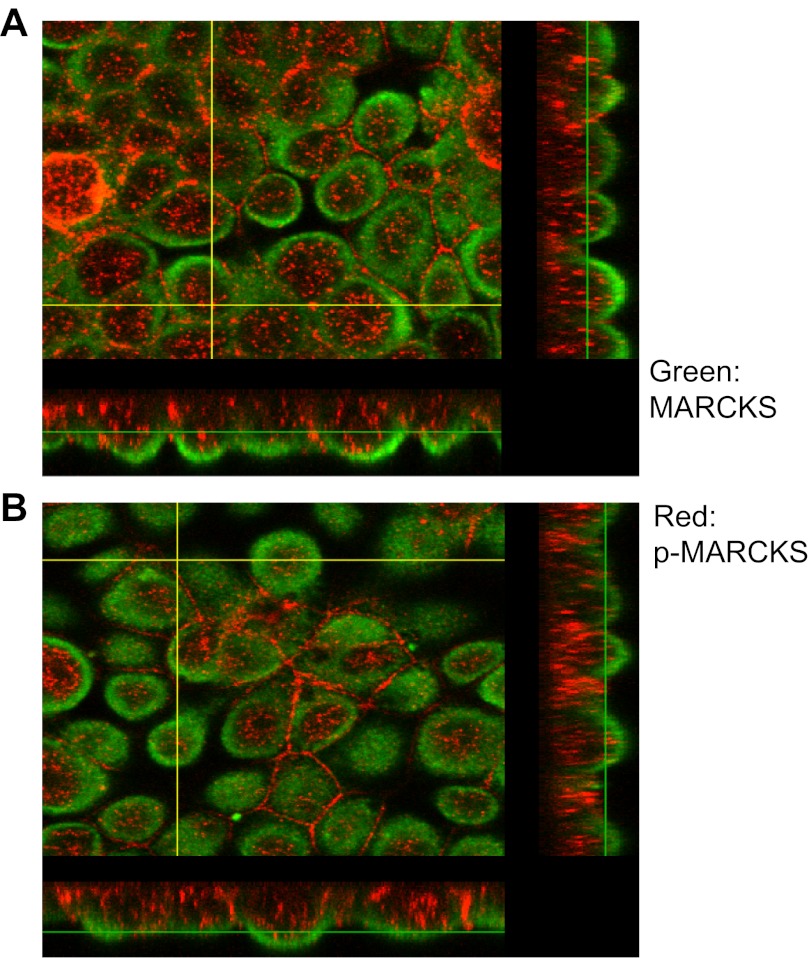
Localization of MARCKS and PIP2. 2F3 cells were transfected with the PIP2 reporter GFP-PLC-δ1 PH domain, fixed in paraformaldehyde, and stained with primary antibodies against MARCKS protein (host: mouse). Following treatment with a fluorescent secondary antibody, cells were examined using confocal microscopy using an Olympus Fluoview 1000 confocal microscope. The 4 panels show an X-Y optical slice near the apical membrane showing PIP2 (green), MARCKS (red), the merged image, and a differential interference white light image. Also, z-axis images are shown adjacent to the X-Y images. The overlap of red and green is best seen in the z-axis images.
Fig. 8.
Colocalization of MARCKS and PIP2. 2F3 cells were transfected with the PIP2 reporter GFP-PLC-δ1 PH domain, fixed in paraformaldehyde, and stained with primary antibodies against MARCKS protein (host: mouse). Following treatment with a fluorescent secondary antibody, cells were examined using confocal microscopy using an Olympus Fluoview 1000 confocal microscope. Sequential optical slice images at 0.25-μm intervals starting from the apical membrane were analyzed for colocalization of PIP2 (green) and MARCKS (red) pixels using a quantitative algorithm (Colocalization Finder plugin in the Image J program; see materials and methods). The top 4 slices (closest to the apical membrane) are shown in the montage. The bottom panel indicates colocalization (in white). Only the pixels that have an intensity value >20% of the maximum value for the green and 10% for the red component are used to generate the colocalized point shown in white (so only pixels with substantial green and red intensity are colocalized). The other areas of the figures represent a traditional merge of the green and red channels.
MARCKS translocates from the apical membrane to the cytoplasm after elevation of intracellular calcium concentration or after PKC stimulation.
The ability of MARCKS to bind and sequester PIPs as a source of PIPs for ENaC regulation depends on the presence of MARCKS at the apical membrane. The binding of a calcium calmodulin complex to a site within the basic effector domain of MARCKS or phosphorylation of serine residues by PKC within the basic effector domain of MARCKS leads to the translocation of MARCKS from the membrane to the cytoplasm. The regulation of MARCKS in Xenopus 2F3 cells has not been investigated; therefore, we investigated whether MARCKS could be regulated by PKC and calmodulin as it is in other cell types. As shown in Fig. 9, A and B, the unphosphorylated form of MARCKS associates with the apical membrane of 2F3 cells, while the phosphorylated form of MARCKS is predominantly cytoplasmic. After ionomycin treatment (Fig. 9B), there appears to be less MARCKS associated with the membrane and more MARKCS in the cytoplasm.
Fig. 9.
Confocal microscopy illustrating the translocation of MARCKS in response to inomycin treatment. The cellular distribution of MARCKS (green) and phosphorylated MARCKS (red) is best seen in the z-stack images constituting the apical membrane (right) and basolateral membrane (bottom). A: endogenous MARCKS is shown to be strongly expressed at the apical membranes of live Xenopus 2F3 cells. B: after treatment with ionomycin to increase intracellular calcium and promote MARCKS translocation, endogenous MARCKS is reduced in the apical membrane and increased in the cytoplasm of live Xenopus 2F3 cells treated with inomycin.
MARCKS redistributes from low- to high-density cellular fractions after treatment with the cytoskeletal disrupting agent cytochalasin E.
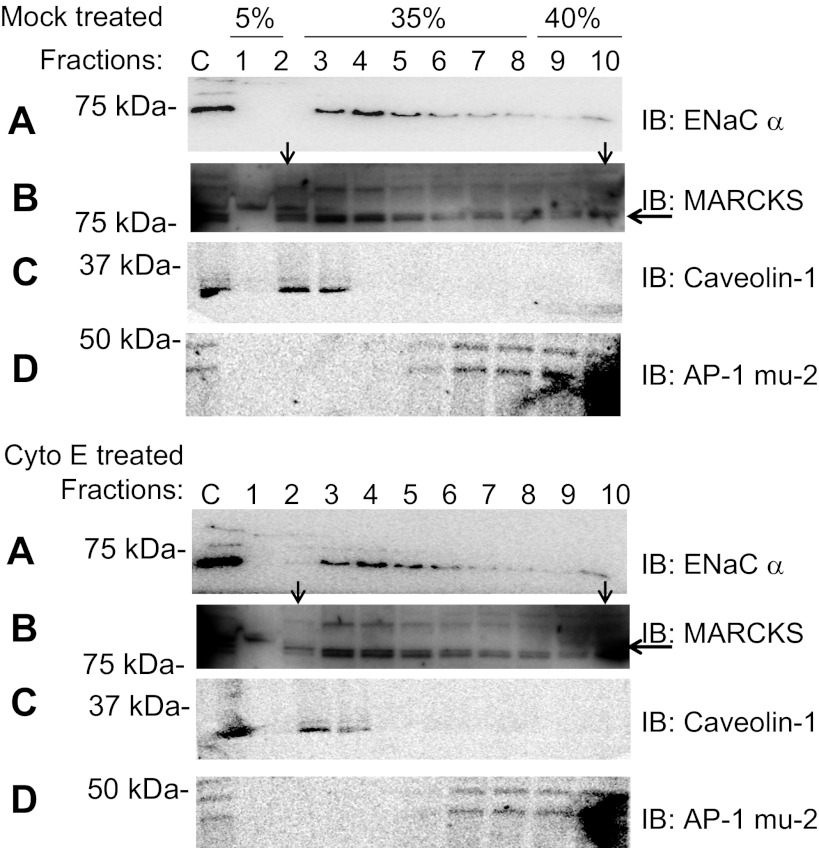
We have previously shown that the actin cytoskeleton is essential for ENaC activity (41a). In that report, we show that disruption of the actin cytoskeleton by cytochalasin E causes a decrease in ENaC channel activity in patches without affecting the total number of ENaC in the apical membrane. Because MARCKS is thought to associate with the actin cytoskeleton, we investigated whether MARCKS is affected by cytochalasin E treatment. Figure 10 shows that MARCKS is present in light fractions but shifts to higher density fractions after cytochalasin E treatment.
Fig. 10.
Subcellular fractionation and Western blot analysis showing the effect of cytochalasin E on Xenopus ENaC and MARCKS. Western blots of Xenopus ENaC α-subunit (A) or MARCKS (B) after treatment of the apical side of Xenopus 2F3 cells grown on permeable inserts with vehicle alone (top) or cytochalasin E (bottom). MARCKS but not Xenopus ENaC-α redistributes from low- to high-density sucrose fractions with cytochalasin E treatment. Western blots are shown of caveolin-1 (C) as a lipid raft marker or μ-2 (D) as a non-lipid raft marker after treatment of the apical side of Xenopus 2F3 cells grown on permeable inserts with mock or cytochalasin E.
Amiloride-sensitive transepithelial current decreases upon PMA treatment.
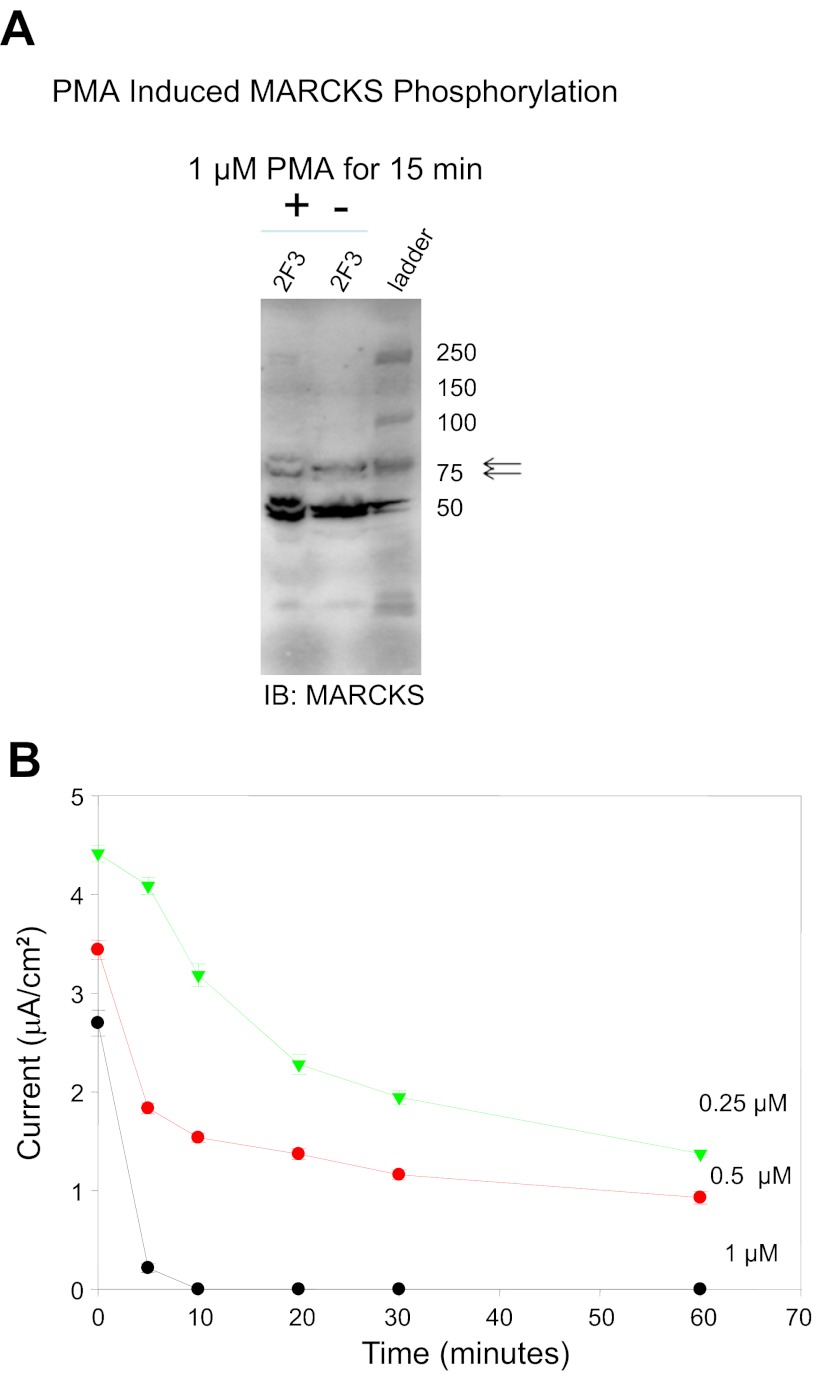
MARCKS cycles from the membrane to the cytosol in response to PKC phosphorylation of the effector domain. When phosphorylated, MARCKS does not bind PIPs; therefore, we would expect that phosphorylation would reduce the ability of MARCKS to activate ENaC by reducing PIP2/3 at the membrane. To demonstrate a role for MARCKS in the regulation of ENaC, we began by inducing MARCKS phosphorylation by activating PKC with PMA. Phosphorylation of MARCKS was monitored by Western blot analysis. PMA induced MARCKS phosphorylation within 15 min, as indicated by an increase in the top band of the immunoreactive doublet shown in Fig. 11A. Amiloride-sensitive sodium current was found to decrease in a time- and dose-dependent manner with PMA treatment over the course of a 1-h treatment (Fig. 11B).
Fig. 11.
Measurements of transepithelial current in Xenopus 2F3 cells treated with PMA. A: Western blot analysis showing PMA-induced phosphorylation of MARCKS after 15 min using a polyclonal antibody specific for MARCKS (arrows) and MARCKS-related protein (MRP; bottom bands). The top band of the doublet represents the phosphorylated form of MARCKS. B: transepithelial resistance and voltage were measured after application of PMA on the apical side of Xenopus 2F3 cells, and transepithelial current was calculated. PMA treatment resulted in a dose- and time-dependent decrease in transepithelial current.
PKC inhibition leads to an increase in ENaC activity.
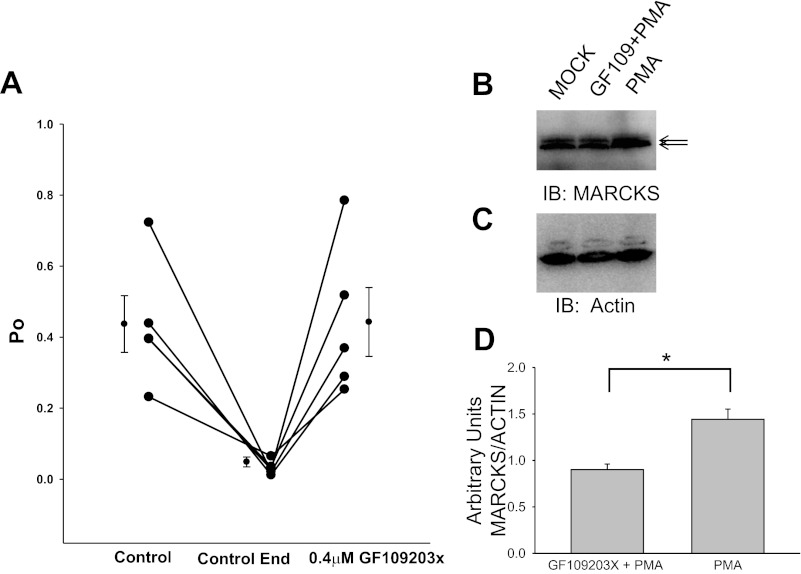
The role of MARCKS in the regulation of ENaC was further supported by single-channel patch-clamp studies. A pharmacological inhibitor of PKC was used to prevent phosphorylation of MARCKS and thus allow its unphosphorylated form to sequester PIPs at the membrane for presentation to ENaC. Inhibition of PKC resulted in an elevation in ENaC activity (Fig. 12).
Fig. 12.
Effect of the PKC inhibitor GF109203X on the open probability (Po) of Xenopus ENaC. A: a 10-min baseline recording of ENaC activity (control to control end) shows typical channel run-down. GF109203X was added to the apical bath solution, and Xenopus ENaC activity was monitored for several minutes after the control recording. The addition of 0.4 μM GF109203X resulted in the recovery of ENaC activity, as measured at the level of Po of the channels. B: representative Western blot of 3 independent experiments showing protein levels of MARCKS after Xenopus 2F3 cells were treated with vehicle (MOCK), PKC inhibitor (GF109203X) for 5 min, then PKC activator (PMA) for an additional 15 min, or PMA alone for 15 min. The top band of the doublet represents the phosphorylated form of MARCKS. C: representative Western blot corresponding to B showing total actin protein as a loading control. D: densitometric analysis of B and C showing the PMA-induced increase in phospho MARCKS protein is blocked by treatment with the PKC inhibitor GF109203X. Values are means ± SE (*P < 0.05).
DISCUSSION
Each of the three ENaC subunits has a molecular mass of between 70 and 85 kDa and shares ∼30% homology at the amino acid level (8–10). The carboxy terminus of each ENaC subunit is ∼10 kDa in size, and the amino acid sequence is considerably different between subunits. We chose to make recombinant GST fusion proteins that included the entire carboxy-terminal domain of the ENaC α- and β-subunits to serve as immunogens for polyclonal antibody production. The reason for doing so was to produce an immunogen that would be highly immunogenical and to yield antibodies that would be specific for the respective ENaC subunit. The GST tag allowed the fusion protein to be batch purified and obtained in high yields. We demonstrated that the GST tag could be cleaved off by thrombin, but we chose to use the GST fusion protein instead of only the recombinant ENaC protein because we found proteolytic cleavage with thrombin resulted in a significant loss in protein. Also, GST migrates on SDS-PAGE gels at 26 kDa, which is outside the range for immunoreactive bands corresponding to uncleaved and cleaved ENaC. Because of the nature of polyclonal antibody production, it was expected that different rabbits would respond differently when injected with the immunogen and each rabbit would generate a unique set of antibodies. As expected and consistent with other reports, we observed multiple immunoreactive bands when probing Western blots using our ENaC α- and β-antibodies. Human lung ENaC-α has been reported to migrate at 65 and 95 kDa (28), mouse lung ENaC-α was reported at 85–90 kDa and at 65 kDa (40), and frog kidney ENaC-α was reported to migrate at 86, 97, and 100 kDa (29). We observed an immunoreactive band at ∼75 kDa corresponding to the frog and human ENaC α-subunit that was absent in parallel studies in which the signal was competed away in the presence of the immunogen with the antibody. Since the higher molecular weight bands at ∼150 kDa were also competed away, this suggests these immunoreactive bands represent an unprocessed, immature, or posttranslationally modified (e.g., glycosylated) form of ENaC. Mouse lung ENaC-β was reported to migrate at 95 kDa (40), and frog kidney ENaC-β was reported to migrate at 78, 86, 97, and 100 kDa (29). As expected, we observed immunoreactive bands at ∼75 and 50 kDa corresponding to the mouse, frog, and human ENaC β-subunit that was absent in parallel studies in which the signal was competed away in the presence of the immunogen with the antibody. In other experiments, we observed a few predominant immunoreactive bands when we used our ENaC α-antibody to probe Western blots containing proteins from frog lung tissue lysate that were less detectable in frog muscle and absent in frog skin tissue lysates. These bands may also represent unprocessed, immature, or posttranslationally modified forms of ENaC-α.
Although PIPs are not abundant in the membranes of cells, they play an important role as signaling molecules. ENaC, in addition to being rare, is limited to the apical membrane of polarized epithelial cells, but is important for maintaining water and salt homeostasis. There is strong evidence for a direct regulation of ENaC by PIPS, but if PIP2 or PIP3 and ENaC were freely diffusible in the lateral plane of the apical membrane, the molecules would only rarely associate with one another. Since ENaC activity depends on association with PIP2, it is surprising that ENaC is as active as that observed in cell-attached patches. Therefore, some mechanism such as an adaptor protein for bringing ENaC together with PIP2 is necessary. Because functional ENaC is at the apical membrane of polarized epithelial cells and PIPs are normally present at the inner leaflet of the plasma membranes, the adapter protein that could allow PIPS to bind and regulate ENaC would have to meet certain criteria. First, the adaptor protein would have to be present at the cytoplasmic face of the plasma membrane of polarized epithelial cells in close proximity to ENaC. Second, the adaptor protein should be capable of binding and sequestering PIPs but also, regardless of the affinity of MARCKS for PIPs, the affinity must be lower than the affinity of ENaC for PIPs if MARCKS is to act as a source of PIPS to activate ENaC. The adaptor protein does not necessarily have to associate directly with ENaC, but it should be regulated in such a way that it is synchronized with ENaC function.
Several proteins, including GAP43, CAP23, and MARCKS, have been found to sequester PIPs (21), but only MARCKS is present in sodium-transporting epithelial tissue and is, therefore, a candidate for ENaC regulation. MARCKS has been implicated in the regulation of several cellular processes including cell attachment and spreading (12, 30, 32), exocytosis (11, 13, 42), phagocytosis (3), cell proliferation (48), and regulation of cytoskeleton dynamics (39). The MARCKS family is composed of MARCKS and MARCKS-related protein (MRP), also known as MacMARCKS or F52 (22, 24, 45). MRP shares ∼50% homology to MARCKS, but is restricted to reproductive tissues and the brain (24, 45). Therefore, we focused on MARCKS to investigate its role in mediating the PIP-dependent regulation of ENaC.
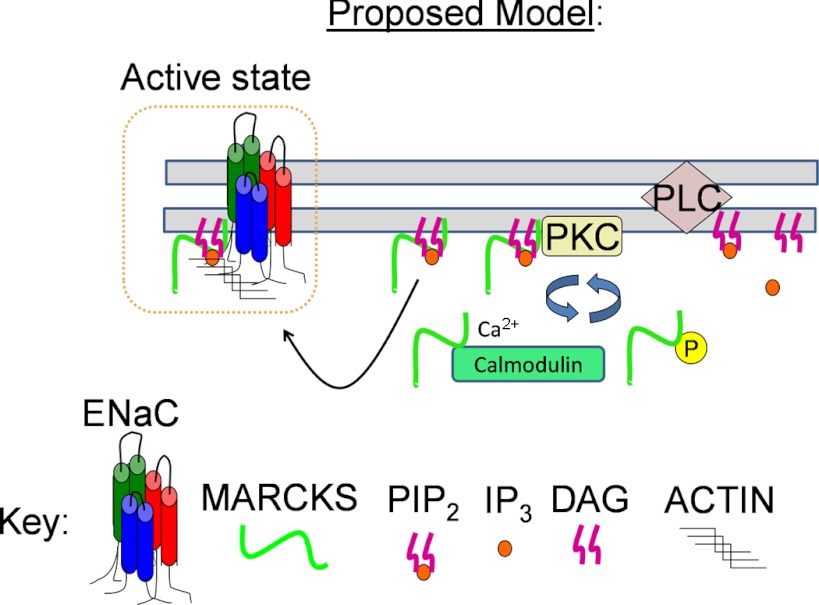
These findings suggest that MARCKS could function as an adapter protein that could regulate PIP availability at the apical membrane where ENaC is present. Therefore, we hypothesized a role for MARCKS in sequestering and presenting PIPs to promote ENaC activity (Fig. 13). MARCKS is an ideal PIP-sequestering protein because it is abundantly expressed and can be localized to the cytoplasmic face of the plasma membrane, and binds PIPs with an affinity that allows it to release PIPs in the presence of ENaC.
Fig. 13.
Proposed model illustrating that MARCKS acts as a reversible source of PIP2 at the plasma membrane for regulating Xenopus ENaC activity. MARCKS attaches to the cytoplasmic face of the plasma membrane of a quiescent cell, i.e., low free intracellular calcium concentration ([Ca2+]i) and low PKC activity, by its N-terminal myristate and its basic effector domain. MARCKS is displaced from the membrane and resides in the cytoplasm after being phosphorylated by PKC at serine residues in the basic effector domain or upon an increase in free [Ca2+]i, as Ca/CaM binds to the basic effector domain. PIPs bind and activate Xenopus ENaC after MARCKS is displaced from the membrane. PIPs may also stabilize Xenopus ENaC to the actin cytoskeleton and lipid rafts. DAG, diacylglyceride; IP3, inositol-1,4,5-trisphosphate.
Ali et al. (2) have already identified and characterized MARCKS in the renal epithelial cell line A6, derived from the Xenopus kidney. We used a clone of this cell line, 2F3, as our experimental model to investigate the role of MARCKS in mediating PIP-dependent regulation of ENaC. 2F3 cells overexpress ENaC when cultured on permeable supports and, like A6 cells, represent an excellent model to use in patch-clamp studies.
MARCKS is an interesting protein for several reasons. MARCKS has a predicted molecular mass of ∼32 kDa, but migrates slowly and close to 75 kDa with SDS-PAGE probably due to its rod-shaped structure, unusual pI, composition of amino acids residues, and ability to bind SDS molecules. Besides sequestering and acting as a source of PIPs at the cytosolic surface of the apical membrane, it can also translocate from its association with the apical membrane to the cytosol, after which it no longer associates with PIPs. Thus MARCKS-mediated delivery of PIPs to ENaC can be regulated by controlling the translocation of MARCKS. There are several mechanisms that promote the translocation of MARCKS. The PKC-mediated phosphorylation of serines within the basic effector domain displaces MARCKS from the cytoplasmic face of the plasma membrane since the extra negative charges reduce the strength of the electrostatic interactions between negative head groups on phospholipids and the cationic residues in the effector domain. The binding of MARCKS to cytoplasmic proteins such as calmodulin also results in the displacement of MARCKS from the plasma membrane. Our findings that MARCKS interacts with the cytoskeleton are consistent with the reports of others (1, 6). MARCKS is known to bind to F-actin, and such an interaction would stabilize the cellular localization of MARCKS in a way to more easily allow translocation and promote sequestration of PIPs.
Calcium ionophores provide a permeability pathway for the movement of Ca2+ into the cell from the external medium and can, thereby, increase intracellular calcium. The introduction of the calcium ionophore ionomycin to cells results in an increase in intracellular calcium, which presumably results in the activation of PKC and activation of calmodulin. We found that the addition of ionomycin quickly and significantly resulted in the displacement of MARCKS from the membrane into the cytoplasm.
It is not surprising to hypothesize that the positive charges within the intracellular domain of a transmembrane protein such as ENaC could interact and be regulated by anionic phospholipids that are normally present at the inner leaflet of the plasma membrane. A common strategy to address this hypothesis is to create constructs that are devoid of the region suspected in the binding. However, care should be taken when making conclusions regarding direct binding and protein function when using expression constructs that consist of a truncated protein. One reason is that it is possible the protein is not functional due to a large portion being absent. Another reason could be due to the protein's adopting a conformation other than its native conformation, which could influence the correct insertion of the protein into the membrane or mask crucial amino acid residues from posttranslational modifications (e.g., glycosylation, ubiquination, phosphorylation).
We performed PIP strip overlay binding assays but used recombinant fusion proteins as the source of ENaC and MARCKS instead of eluting proteins bound to beads from immunoprecipitation studies. This strategy is better suited to obtain more accurate observations because it eliminates the possibility of the binding being attributed to nonspecific proteins pulled down by the antibody or beads or inconsistencies in the transfection of the expression constructs. Interestingly, our PIP strip overlay binding assays demonstrated the N termini of ENaC-β PIP2 and PIP3, both of which stimulate ENaC activity in excised patches of the apical membrane (27, 46). It is possible that Xenopus ENaC-β preferentially bound to PIP2 and PIP3 and Xenopus ENaC α- or γ-subunits did not bind because of differences within the amino-terminal domain of the three subunits. Another reason for only the N terminus of Xenopus ENaC-β binding PIP2 and PIP3 may be due to various amino acid residues being more accessible to bind PIPs. ENaC β- and γ-subunits also bound strongly to PA. PA was recently shown to inhibit ENaC activity (47). These results suggest that hydrophobicity and anionic character are important for binding and that the inositol phosphate head group is important for ENaC activation. This observation also indicates differential regulation of ENaC by anionic phosphoinositides. Further studies will be needed to determine under what conditions different phospholipids, PIP2, PIP3, or PA, bind ENaC.
Phosphoinositides including PIP2, PIP3, and PA have been shown to differentially regulate ENaC. Compared with PIP3, PIP2 levels are higher and it is more stable. This suggests the generation of PIP2 is more tightly controlled both spatially and temporally and would probably be the molecule primarily responsible for the regulation of ENaC during rearrangements of the actin cytoskeleton. The levels of PIPs are directly proportional to ENaC activity, as low levels correlate with a reduction in ENaC activity and high levels correlate with an elevation in ENaC activity. Conversely, the levels of PA are inversely proportional to ENaC activity.
Our results indicate MARCKS may be the missing link in the regulation of ENaC function (i.e., sodium transport) by anionic phospholipids (Fig. 13). Future studies should investigate the stimuli within the cell that determine the duration and timing of MARCKS cycling between the membrane and the cytoplasm. Like ENaC, MARCKS is subject to proteolytic cleavage by various proteases, and future studies may address whether this type of processing of MARCKS also affects ENaC function. Finally, it may be intriguing to speculate a role for MARCKS in the PIP-dependent regulation of ENaC in various diseases such as cancer.
GRANTS
This research was supported by National Institutes of Health Grants F32 DK093255-01 (to A. A. Alli), T32 DK007771 (to C. A. Parkos), 5R01 DK067110 (to H.-P. Ma), and R37 DK037963 (to D. C. Eaton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Abdel A. Alli and D.C.E. provided conception and design of research; Abdel A. Alli, H.F.B., Alia A. Alli, Y.A., J.Z.S., and O.K.A.-K. performed experiments; Abdel A. Alli, H.F.B., Alia A. Alli, and D.C.E. analyzed data; Abdel A. Alli, H.M., L.Y., and D.C.E. interpreted results of experiments; Abdel A. Alli, H.F.B., Alia A. Alli, and D.C.E. prepared figures; Abdel A. Alli and D.C.E. drafted manuscript; Abdel A. Alli and D.C.E. edited and revised manuscript; Abdel A. Alli, H.F.B., Alia A. Alli, Y.A., J.Z.S., H.M., L.Y., O.K.A.-K., and D.C.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. T. S. Karpova, L. He, and P. E. Lipsky for kindly providing us with the CFP-YFP construct for our FRET studies. The authors thank B. J. Duke for maintaining 2F3 cells in culture. We thank the Proteomics Core Facility at the Moffitt Cancer Center, Tampa, FL, for mass spectrometry services.
REFERENCES
- 1. Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell 71: 713–716, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Ali N, Macala LJ, Hayslett JP. Identification and characterization of MARCKS in Xenopus laevis. Biochem Biophys Res Commun 234: 143–146, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Allen LH, Aderem A. A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J Exp Med 182: 829–840, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alli AA, Gower WR., Jr The C type natriuretic peptide receptor tethers AHNAK1 at the plasma membrane to potentiate arachidonic acid-induced calcium mobilization. Am J Physiol Cell Physiol 297: C1157–C1167, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Alli AA, Gower WR., Jr Molecular approaches to examine the phosphorylation state of the C type natriuretic peptide receptor. J Cell Biochem 110: 985–994, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J 362: 1–12, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Canessa CM, Horisberger JD, Schild L, Rossier BC. Expression cloning of the epithelial sodium channel. Kidney Int 48: 950–955, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol Cell Physiol 267: C1682–C1690, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Coffey ET, Herrero I, Sihra TS, Sanchez-Prieto J, Nicholls DG. Glutamate exocytosis and MARCKS phosphorylation are enhanced by a metabotropic glutamate receptor coupled to a protein kinase C synergistically activated by diacylglycerol and arachidonic acid. J Neurochem 63: 1303–1310, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Disatnik MH, Boutet SC, Lee CH, Mochly-Rosen D, Rando TA. Sequential activation of individual PKC isozymes in integrin-mediated muscle cell spreading: a role for MARCKS in an integrin signaling pathway. J Cell Sci 115: 2151–2163, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Eliyahu E, Shtraizent N, Tsaadon A, Shalgi R. Association between myristoylated alanin-rich C kinase substrate (MARCKS) translocation and cortical granule exocytosis in rat eggs. Reproduction 131: 221–231, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Graff JM, Young TN, Johnson JD, Blackshear PJ. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem 264: 21818–21823, 1989 [PubMed] [Google Scholar]
- 15. Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature 356: 618–622, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Helms MN, Liu L, Liang YY, Al-Khalili O, Vandewalle A, Saxena S, Eaton DC, Ma HP. Phosphatidylinositol 3,4,5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with gamma-ENaC. J Biol Chem 280: 40885–40891, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Helms MN, Liu L, Liang YY, Al-Khalili O, Vandewalle A, Saxena S, Eaton DC, Ma HP. Phosphatidylinositol 3,4,5-trisphosphate mediates aldosterone stimulation of epithelial sodium channel (ENaC) and interacts with gamma-ENaC. J Biol Chem 280: 40885–40891, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Karpova T, McNally JG. Detecting protein-protein interactions with CFP-YFP FRET by acceptor photobleaching. Curr Protoc Cytom: Chapter 12: Unit 12, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Karpova TS, Baumann CT, He L, Wu X, Grammer A, Lipsky P, Hager GL, McNally JG. Fluorescence resonance energy transfer from cyan to yellow fluorescent protein detected by acceptor photobleaching using confocal microscopy and a single laser. J Microsc 209: 56–70, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Kim J, Shishido T, Jiang X, Aderem A, McLaughlin S. Phosphorylation, high ionic strength, and calmodulin reverse the binding of MARCKS to phospholipid vesicles. J Biol Chem 269: 28214–28219, 1994 [PubMed] [Google Scholar]
- 20a.Laummonerie C, Mutterer J.2005. Colocalization_Finder. java version 1.1 ImageJ plugin.
- 21. Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P2 at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol 149: 1455–1472, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Aderem A. MacMARCKS, a novel member of the MARCKS family of protein kinase C substrates. Cell 70: 791–801, 1992 [DOI] [PubMed] [Google Scholar]
- 23. Liu L, Duke BJ, Malik B, Yue Q, Eaton DC. Biphasic regulation of ENaC by TGF-α and EGF in renal epithelial cells. Am J Physiol Renal Physiol 296: F1417–F1427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lobach DF, Rochelle JM, Watson ML, Seldin MF, Blackshear PJ. Nucleotide sequence, expression, and chromosomal mapping of Mrp and mapping of five related sequences. Genomics 17: 194–204, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Ma HP, Chou CF, Wei SP, Eaton DC. Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflügers Arch 455: 169–180, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J Biol Chem 277: 7641–7644, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J Biol Chem 277: 7641–7644, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Mace OJ, Woollhead AM, Baines DL. AICAR activates AMPK and alters PIP2 association with the epithelial sodium channel ENaC to inhibit Na+ transport in H441 lung epithelial cells. J Physiol 586: 4541–4557, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malik B, Schlanger L, Al-Khalili O, Bao HF, Yue G, Price SR, Mitch WE, Eaton DC. Enac degradation in A6 cells by the ubiquitin-proteosome proteolytic pathway. J Biol Chem 276: 12903–12910, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Manenti S, Malecaze F, Darbon JM. The major myristoylated PKC substrate (MARCKS) is involved in cell spreading, tyrosine phosphorylation of paxillin, and focal contact formation. FEBS Lett 419: 95–98, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Markadieu N, Blero D, Boom A, Erneux C, Beauwens R. Phosphatidylinositol 3,4,5-trisphosphate: an early mediator of insulin-stimulated sodium transport in A6 cells. Am J Physiol Renal Physiol 287: F319–F328, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Myat MM, Anderson S, Allen LA, Aderem A. MARCKS regulates membrane ruffling and cell spreading. Curr Biol 7: 611–614, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Planes C, Randrianarison NH, Charles RP, Frateschi S, Cluzeaud F, Vuagniaux G, Soler P, Clerici C, Rossier BC, Hummler E. ENaC-mediated alveolar fluid clearance and lung fluid balance depend on the channel-activating protease 1. EMBO Mol Med 2: 26–37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pochynyuk O, Bugaj V, Stockand JD. Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr Opin Nephrol Hypertens 17: 533–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pochynyuk O, Tong Q, Medina J, Vandewalle A, Staruschenko A, Bugaj V, Stockand JD. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J Gen Physiol 130: 399–413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pochynyuk O, Tong Q, Staruschenko A, Ma HP, Stockand JD. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am J Physiol Renal Physiol 290: F949–F957, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Pochynyuk O, Tong Q, Staruschenko A, Stockand JD. Binding and direct activation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. J Physiol 580: 365–372, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poussard S, Dulong S, Aragon B, Jacques BJ, Veschambre P, Ducastaing A, Cottin P. Evidence for a MARCKS-PKCalpha complex in skeletal muscle. Int J Biochem Cell Biol 33: 711–721, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Randrianarison N, Clerici C, Ferreira C, Fontayne A, Pradervand S, Fowler-Jaeger N, Hummler E, Rossier BC, Planes C. Low expression of the β-ENaC subunit impairs lung fluid clearance in the mouse. Am J Physiol Lung Cell Mol Physiol 294: L409–L416, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Rasband WS. Image J. 2006. http://rsbweb.nih.gov/ij/
- 41a. Reifenberger MS, Duke BJ, Yu L, Eaton D. Regulation of ENaC by the cytoskeleton (Abstract). FASEB J Suppl: 611.4, 2010 [Google Scholar]
- 42. Salli U, Supancic S, Stormshak F. Phosphorylation of myristoylated alanine-rich C kinase substrate (MARCKS) protein is associated with bovine luteal oxytocin exocytosis. Biol Reprod 63: 12–20, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Tong Q, Stockand JD. Receptor tyrosine kinases mediate epithelial Na+ channel inhibition by epidermal growth factor. Am J Physiol Renal Physiol 288: F150–F161, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Wu M, Chen DF, Sasaoka T, Tonegawa S. Neural tube defects and abnormal brain development in F52-deficient mice. Proc Natl Acad Sci USA 93: 2110–2115, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Zhang ZR, Chou CF, Wang J, Liang YY, Ma HP. Anionic phospholipids differentially regulate the epithelial sodium channel (ENaC) by interacting with alpha, beta, and gamma ENaC subunits. Pflügers Arch 459: 377–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao Y, Neltner BS, Davis HW. Role of MARCKS in regulating endothelial cell proliferation. Am J Physiol Cell Physiol 279: C1611–C1620, 2000 [DOI] [PubMed] [Google Scholar]