Abstract
Serum IL-6 is increased in patients with acute kidney injury (AKI) and is associated with prolonged mechanical ventilation and increased mortality. Inhibition of IL-6 in mice with AKI reduces lung injury associated with a reduction in the chemokine CXCL1 and lung neutrophils. Whether circulating IL-6 or locally produced lung IL-6 mediates lung injury after AKI is unknown. We hypothesized that circulating IL-6 mediates lung injury after AKI by increasing lung endothelial CXCL1 production and subsequent neutrophil infiltration. To test the role of circulating IL-6 in AKI-mediated lung injury, recombinant murine IL-6 was administered to IL-6-deficient mice. To test the role of CXCL1 in AKI-mediated lung injury, CXCL1 was inhibited by use of CXCR2-deficient mice and anti-CXCL1 antibodies in mice with ischemic AKI or bilateral nephrectomy. Injection of recombinant IL-6 to IL-6-deficient mice with AKI increased lung CXCL1 and lung neutrophils. Lung endothelial CXCL1 was increased after AKI. CXCR2-deficient and CXCL1 antibody-treated mice with ischemic AKI or bilateral nephrectomy had reduced lung neutrophil content. In summary, we demonstrate for the first time that circulating IL-6 is a mediator of lung inflammation and injury after AKI. Since serum IL-6 is increased in patients with either AKI or acute lung injury and predicts prolonged mechanical ventilation and increased mortality in both conditions, our data suggest that serum IL-6 is not simply a biomarker of poor outcomes but a pathogenic mediator of lung injury.
Keywords: acute kidney injury, lung neutrophils, lung injury
acute kidney injury (AKI) complicates up to 20% of hospital admissions (31) and 30–50% of admissions to the ICU (29). Survival is decreased in AKI and critically ill patients with AKI have mortality rates in excess of 50% (6). Patients with AKI have a high incidence of systemic complications, particularly respiratory failure (5), which may contribute to increased mortality. Patients with AKI have prolonged ventilator time and increased total wean time (32). When AKI and respiratory failure occur in conjunction, the mortality rate is 60–80% (5, 32). We (13, 15) and others (7, 9, 10, 12, 14, 17, 23, 26) showed that AKI in animals leads to lung injury, characterized by neutrophil infiltration, capillary leak, and increased chemokine production (13).
The proinflammatory cytokines IL-6 and IL-8 are elevated early in the serum of patients with AKI and are associated with prolonged mechanical ventilation (18, 19). This raises the possibility that IL-6 and IL-8 contribute to lung injury in AKI, in addition to being early biomarkers. Mice also have increased serum IL-6 and CXCL1 (murine analog of IL-8) after AKI (13) and we showed that inhibition of IL-6 reduces lung injury in mice with AKI that is associated with reduced lung CXCL1 and lung neutrophils (15). Thus, these data suggest that IL-6 mediates lung injury in AKI via upregulation of lung CXCL1, causing subsequent neutrophil infiltration. Whether the protection against lung injury is due to inhibition of circulating IL-6 action at the lung or due to inhibition of IL-6 produced in the lung and acting locally in the lung is not known. The role of circulating vs. local IL-6 and the role of CXCL1 in AKI-mediated lung injury are investigated the present study.
In the present study, we hypothesized that circulating IL-6 contributes to AKI-mediated lung neutrophil accumulation and lung injury via upregulation of lung CXCL1. To test the role of circulating IL-6 in AKI-mediated lung injury, recombinant murine IL-6 was administered to IL-6-deficient mice with ischemic AKI and lung CXCL1 and lung neutrophil content were examined; to test the role of CXCL1 in AKI-mediated lung injury, CXCL1 function was inhibited by use of CXCR2-deficient mice and anti-CXCL1 antibodies in mice with ischemic AKI or bilateral nephrectomy.
METHODS
Animals.
Eight- to ten-week-old C57BL/6 mice and CXCR2-deficient mice (C57BL/6 background; Jackson Laboratories, Bar Harbor, ME) that weighed 20 to 25 g were used. Mice were maintained on a standard diet, and water was freely available. All experiments were conducted with adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Animal Care and Use Committee of the University of Colorado, Denver.
Surgical protocol.
Surgical procedures were performed on three groups of mice: 1) sham operation, 2) ischemic AKI, and 3) bilateral nephrectomy. For all procedures, mice were anesthetized with intraperitoneal avertin (2,2,2-tribromoethanol; Aldrich, Milwaukee, WI), a midline incision was made, and the renal pedicles were identified. In the ischemic AKI group, both renal pedicles were clamped for 22 min. After clamp removal, kidneys were observed for restoration of blood flow by the return to original color. The abdomen was closed in one layer. Sham surgery consisted of the same procedure except that clamps were not applied. In the bilateral nephrectomy model, both renal pedicles were tied off with suture and then cut distal to the suture. The ureters were pinched off with forceps, and the kidneys were removed. Mice were weighed before and after procedures.
CXCL1 inhibition.
CXCL1 acts on the CXCR1 and CXCR2 receptors. Mice possess only the CXCR2 receptor. The action of CXCL1 was inhibited in 2 ways: 1) CXCR2-deficient mice were used compared with wild-type littermates and 2) CXCL1 antibody was administered compared with administration of rabbit IgG. CXCL1 antibody (R&D Systems #MAB4531) or rabbit IgG (R&D Systems #AB-105-C) was given at a dose of 100 μg in 100 μl sterile saline intravenously just before AKI, and another dose of 100 μg in 100 μl sterile saline was given intraperitoneally 1 h after AKI.
Collection and preparation of serum samples.
At death, blood was obtained via cardiac puncture. To ensure uniformity, all samples were processed identically. Blood was allowed to clot at room temperature for 2 h and then centrifuged at 3,000 g for 10 min. Serum was collected and centrifuged a s time at 3,000 g for 1 min to ensure elimination of red blood cells. Samples with notable hemolysis were discarded.
Blood urea nitrogen and serum creatinine measurement.
Serum was collected as described above. Blood urea nitrogen (BUN) and serum creatinine were measured using quantitative colorimetric assays (BioAssay systems DICT-500 and DIUR-500).
Lung neutrophil content (myeloperoxidase activity).
One fourth of lung was homogenized in 1 ml of cold hexdecyltrimethlylammonium bromide buffer [50 mM KPO4 and 0.5% hexdecyltrimethylammonium bromide (pH 6.0)], sonicated on ice for 10 s, and centrifuged at 14,000 g for 30 min at 4°C. Twenty microliters of supernatant were transferred into a 96-well plate, and 200 μl of 37°C O-dianisidine hydrochloride solution (16.7 mg O-dianisidine, 100 ml: 90% water and 10% 50 mM KPO4 buffer + 0.0005% H2O2) were added immediately before the optical density was read at 450 nm and again 30 s later (Benchmark microplate reader; BioRad).
Lung capillary leak (Evan's blue dye assay).
A total of 250 μl of Evan's blue dye (EBD; 5 mg/ml) was injected via tail vein 1 h before death. Lungs were perfused with 3 ml PBS via the right ventricle to remove EBD within the vasculature, excised, weighed, and homogenized in 2 ml formamide. The homogenate was incubated in a 37°C water bath overnight and then centrifuged at 14,000 g for 30 min. The optical density of supernatant was determined at 620 nm, and EBD concentration was calculated against a standard curve (mg EBD per g lung tissue).
Serum IL-6 measurement.
Serum IL-6 was measured by ELISA (R&D Systems, Minneapolis, MN).
Lung IL-6 and CXCL1 measurement.
Frozen lung was prepared for ELISA as described previously (13). Supernatants were analyzed for protein content using a Bio-Rad DC protein assay kit (Hercules, CA). IL-6 and CXCL1 were determined by ELISA (R&D Systems).
Real-time PCR.
Cytosolic RNA was isolated from mouse lung using the RNeasy kit (Qiagen, Valencia, CA). Before real-time PCR, RNA was converted to cDNA using the iScript reverse transcriptase kit (Bio-Rad) as described by the manufacturer. RT-PCR primers specific to IL-6: 5′-ACCGCTATGAAGTTCCTCTC-3′ (F), 5′-CCTCTGTGAAGTCTCCTCTC-3′ (R), and β-actin: 5′-CGTGCGTGACATCAAAGAG-3′ (F), 5′-TGCCACAGGATTCCATAC-3′ (R) were designed using Beacon Designer 5.0 software (Premier Biosoft International, Palo Alto, CA). RT-PCR was performed using 70-nM primers and the SYBR Green JumpStart Taq Readymix QPCR kit (Sigma) on a Bio-Rad I-Cycler. RT-PCR runs were analyzed by agarose gel electrophoresis and melt curve to verify that the correct amplicon was produced. β-Actin RNA was used as internal control, and the amount of RNA was calculated by the comparative CT method as recommended by the manufacturer.
Administration of IL-6 to IL-6-deficient mice.
IL-6-deficient mice (Jackson Labs Strain B6.129S2-IL6/J) underwent ischemic AKI surgery as described above. Two hundred nanograms of recombinant murine IL-6 (PeproTech #216–16) in 200 μl of PBS with 0.1% BSA were injected at 1, 2, and 3 h after AKI for a total dose of 600 ng. Vehicle-treated mice received the same volume of PBS in 0.1% bovine serum albumin (BSA) at the same time points. Serum and lungs were collected 4 h after AKI.
Cell culture experiments.
Pancreatic microvascular endothelial cells (MS1 cells) from C57BL/6 mice were obtained from American Type Culture Collection (ATCC). The line has many properties of endothelial cells including expression of both Factor VIII-related antigen and vascular endothelial growth factor receptor. Immortalized murine pancreatic endothelial cells (MS1 cells, ATCC #CRL-2279) were cultured in six-well plates (Becton Dickinson #3046). Cells were grown over 3 days in 2 ml of DMEM media with l-glutamine, sodium pyruvate, 4.5 g/l glucose, 5% fetal calf serum, and 1% penicillin/streptomycin. IL-6 or vehicle was administered when cells reached 80% confluence, as assessed by phase contrast microscopy. Before administration of IL-6 or vehicle, used media were discarded, and 1 ml fresh media was instilled. In IL-6-treated cells, recombinant murine IL-6 (Peprotech #216–16) was instilled at a dose of 1 ng in 0.5 μl of sterile PBS with 0.1% BSA, and soluble IL-6 receptor (R&D Systems, #1830SR) was given at a dose of 4 ng in 4 μl of sterile PBS with 0.1% BSA. Vehicle-treated cells were instilled with 4.5 μl of PBS with 0.1% BSA. Media were collected at 4 h, and cells were scraped and homogenized in a lysis buffer consisting of 100 μl PBS, 25 μl Triton X-100 buffer, and 1 μl of protease inhibitor (Sigma #P9340).
Immunofluorescence.
Mice underwent ischemic AKI or sham surgery as described above, and after 4 h, lungs were expanded with a 1:1 mixture of OCT (Tissue-Tek, Sakura, Finetek) and PBS and were snap-frozen in liquid nitrogen after being embedded in OCT. Tissue was cut into 5-μm sections and mounted on glass slides. Slides were treated with 70% acetone/30% methanol for 10 min, fixed in 3% paraformaldehyde, and then blocked in 10% serum of the same species as the host of the secondary antibody. Incubation with primary antibody was performed for 60 min (1:25 goat anti-mouse CXCL1, Santa Cruz #16961; 1:500 rabbit anti-mouse von Willebrand Factor, abcam #6994; 1:50 rat anti-mouse Cd11b, Serotec MCA711GT). Secondary antibody incubation was also performed for 60 min (1:250 donkey anti-goat Alexa 488, Invitrogen A11055; 1:250 donkey anti-rabbit Alexa 568, Invitrogen A10042; 1:250 donkey anti-rat CD11b Cy3, Jackson Immunoresearch 712–165-153). Slides were covered with an antifading mounting medium (Vector Laboratories) for confocal microscope analysis. Samples were imaged with a ×40 water-immersion objective by using a laser-scanning confocal microscope (model LSM510; Zeiss). Data were analyzed using the LSM Image Analyzer postacquisition software (Zeiss).
RESULTS
Serum and lung IL-6 after bilateral nephrectomy and ischemic AKI.
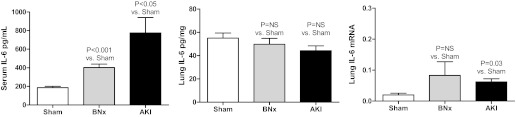
To determine whether lung IL-6 production increased after bilateral nephrectomy or ischemic AKI and whether the lung was a source of circulating IL-6, serum and lung IL-6 were determined 2 h after sham operation, bilateral nephrectomy, or ischemic AKI (Fig. 1). Serum IL-6 was increased 2 h after bilateral nephrectomy and ischemic AKI vs. sham operation; however, lung IL-6 as determined by ELISA was similar 2 h after bilateral nephrectomy and ischemic AKI vs. sham operation, suggesting that lung IL-6 protein is not produced early after bilateral nephrectomy or ischemic AKI and is not a source of circulating (serum) IL-6 at 2 h.
Fig. 1.
Serum and lung IL-6 after acute kidney injury (AKI). Serum (ELISA) and lung IL-6 (ELISA and mRNA) were determined 2 h after sham operation (Sham), bilateral nephrectomy (BNx), or ischemic AKI (AKI). Left: serum IL-6 was increased 2 h after BNx and ischemic AKI vs. sham operation (n = 4). Lung IL-6 as judged by ELISA (n = 4; middle) did not increase after BNx or ischemic AKI. Right: mRNA for IL-6 did not significantly increase after BNx (n = 8), but it was slightly increased after ischemic AKI (n = 8) vs. sham operation (n = 3).
Specifically, serum IL-6 (pg/ml) was 187 ± 14 after sham operation, 403 ± 37 after bilateral nephrectomy (P = 0.0015 vs. sham; n = 4), and 775 ± 165 after ischemic AKI (P = 0.038 vs. sham; n = 4). Lung IL-6 was (pg/mg) was 55 ± 4 after sham operation, 50 ± 5 after bilateral nephrectomy (P = NS vs. sham; n = 4), and 44 ± 4 after ischemic AKI (P = NS vs. sham; n = 4).
Lung mRNA for IL-6 was minimally increased by 2 h after ischemic AKI, but not bilateral nephrectomy, suggesting that IL-6 production is beginning to occur after ischemic AKI and thus, the lung may be a source of IL-6 production after ischemic AKI at a later time point.
Of note, the increase in lung mRNA after ischemic AKI is relatively small. To compare the magnitude of the increase, IL-6 mRNA in the lung and kidney was determined 2 h after sham operation and ischemic AKI and normalized to each other (n = 5); lung mRNA for IL-6 was 2.0 ± 0.6 after sham operation and 6.2 ± 1.0 after ischemic AKI representing an approximately threefold increase; kidney mRNA for IL-6 was 26.5 ± 7.8 after sham and was 700 ± 33 after ischemic AKI representing an ∼28-fold increase.
Administration of IL-6 to IL-6-deficient mice with ischemic AKI.
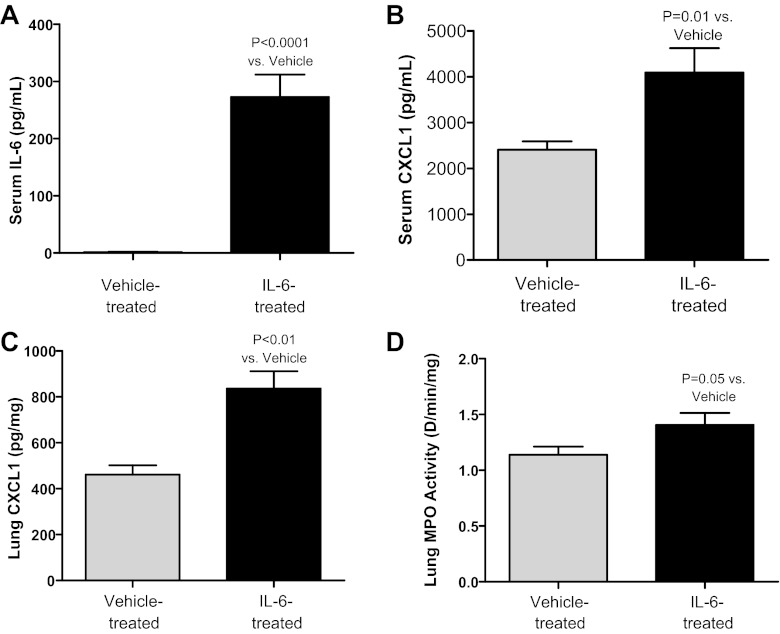
To specifically determine whether circulating IL-6 can cause AKI-mediated lung injury, 200 ng of recombinant murine IL-6 or vehicle were given intravenously at 1, 2, 3 h postischemic AKI to IL-6-deficient mice and serum creatinine, BUN, serum IL-6, serum CXCL1, lung CXCL1, and lung MPO activity were determined at 4 h. Serum creatinine and BUN were similar, demonstrating that renal function was not affected by IL-6 administration. Notably, however, serum IL-6, serum CXCL1, lung CXCL1, and lung MPO activity were all significantly increased in IL-6-treated mice vs. vehicle-treated mice (Fig. 2). In this experiment, no other source of IL-6 exists in the IL-6-deficient mice except the IL-6 given exogenously by the intravenous route; thus, these data strongly suggest circulating IL-6 can increase CXCL1 production and neutrophil recruitment in the lung.
Fig. 2.
Administration of intravenous IL-6 to IL-6-deficient mice with ischemic AKI. Two hundred nanograms of recombinant IL-6 or vehicle were given intravenously every hour for 3 h after ischemic AKI to IL-6-deficient mice, and serum IL-6, serum CXCL1, lung CXCL1, and lung myeloperoxidase (MPO) activity were determined at 4 h (n = 9–10). Serum IL-6 (A), serum CXCL1 (B), lung CXCL1 (C), and lung MPO activity (D) were all increased after intravenous administration of IL-6 vs. vehicle.
Specifically, serum creatinine was 0.9 ± 0.12 in vehicle-treated and 0.8 ± 0.11 in IL-6-treated mice (P = NS; n = 5) and BUN was 46 ± 6 in vehicle-treated and 53 ± 4 in IL-6-treated mice (P = NS; n = 5). Serum IL-6 was 0 ± 0 in vehicle-treated and was 273 ± 39 in IL-6-treated mice (P < 0.001; n = 10). Serum CXCL1 was 2,405 ± 183 in vehicle-treated and was 4,091 ± 531 in IL-6-treated mice (P = 0.01; n = 10). Lung CXCL1 was 461 ± 41 in vehicle-treated and 836 ± 76 in IL-6-treated mice (P < 0.01; n = 9–10). Lung MPO activity was 1.1 ± 0.1 in vehicle-treated and 1.4 ± 0.1 in IL-6-treated mice (P = 0.05; n = 9–10).
Lung endothelial CXCL1 production after ischemic AKI, in vivo.
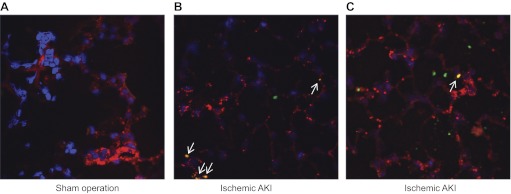
Since circulating IL-6 is in contact with the pulmonary endothelium, it might be expected that IL-6 acts at the pulmonary endothelium to increase CXCL1 production. Therefore, to determine whether the pulmonary endothelium contributes to lung CXCL1 production after AKI, dual label immunofluorescence was performed for CXCL1 and the endothelial marker von Willebrand factor (vWF) in lungs 4 h after ischemic AKI or sham operation. As shown in Fig. 3, no CXCL1 was seen after sham operation, and colocalization with CXCL1 and vWF was seen 4 h after AKI, suggesting that the pulmonary endothelial cell is a source of lung CXCL1 (n = 3). These data suggest that endothelial cells can increase production and release of CXCL1 after exposure to IL-6, although a minority of pulmonary endothelial cells demonstrates colocalization of CXCL1 and vWF.
Fig. 3.
Pulmonary endothelial cell production of CXCL1 in ischemic AKI. Immunofluorescence was performed on lungs 4 h after sham operation or ischemic AKI and labeled for CXCL1 (green), von Willebrand Factor (red), and DAPI (blue; n = 3). A: no CXCL1 was seen after sham operation. B and C: after ischemic AKI, CXCL1 was found in the lungs (green) and colocalization (yellow) was found between CXCL1 and von Willebrand Factor (white arrows); 2 separate lung fields are shown (B and C). Blue: Dapi (nuclei); red: Von Willebrand Factor (endothelial cells); green: CXCL1; yellow: endothelial cell and CXCL1 colocalization.
Endothelial cell CXCL1 production after IL-6 administration, in vitro.
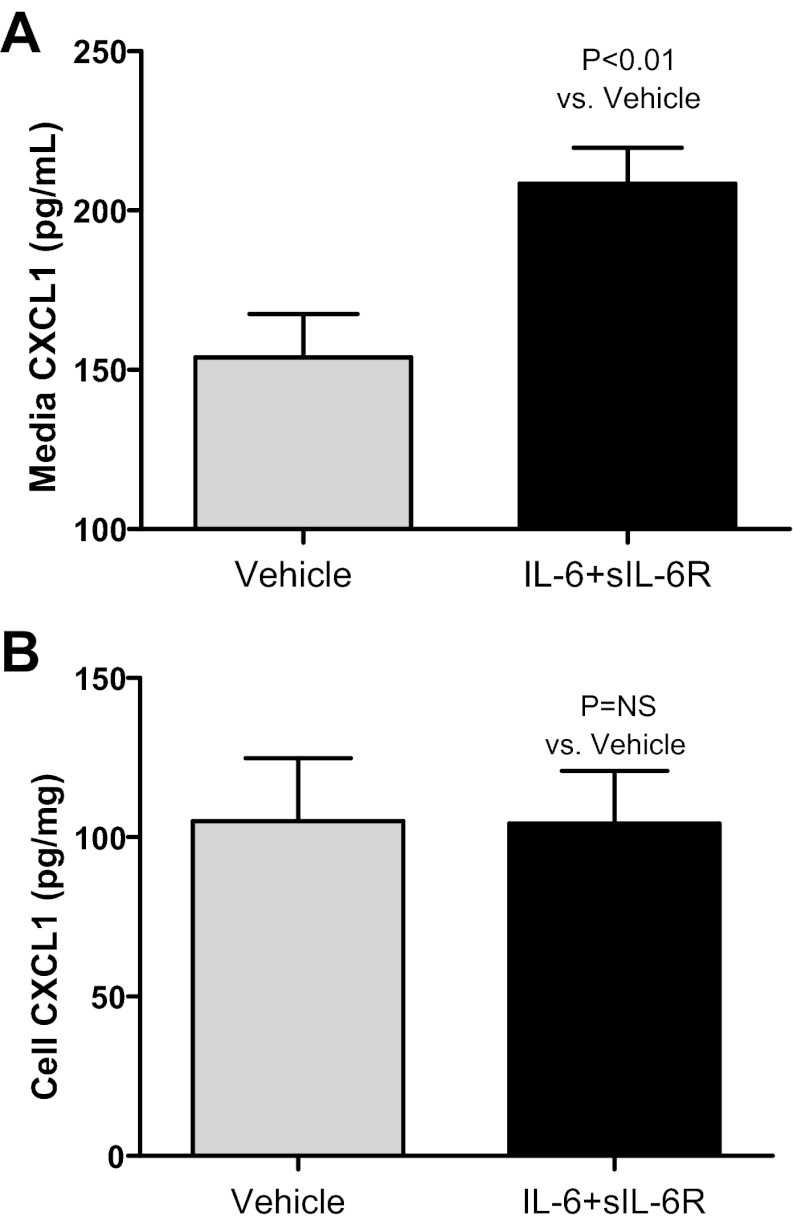
To determine whether IL-6 can directly increase endothelial production of CXCL1, IL-6 with soluble IL-6 receptor (sIL-6R) vs. vehicle was added to endothelial cells in culture (Fig. 4). Endothelial cells lack the IL-6 receptor; however, IL-6 signaling may occur in endothelial cells via the gp130 receptor via trans-signaling when complexed with the sIL-6R; thus, sIL-6R was added with the IL-6 in this experiment. CXCL1 was determined in the media and cells after 4 h. Media CXCL1 (pg/ml) was 154 ± 14 in vehicle-treated cells and 208 ± 11 in IL-6-treated cells (P = 0.006; n = 10). Cell homogenate CXCL1 (pg/mg) was 105 ± 20 in vehicle-treated cells and 104 ± 17 in IL-6-treated cells (P = NS; n = 10).
Fig. 4.
IL-6-mediated production of CXCL1 by endothelial cells, in vitro. Recombinant murine vehicle vs. IL-6 + soluble IL-6 receptor (sIL-6R; IL-6 + sIL-6R) were added to cultured MS1 endothelial cells and CXCL1 was determined in the media and cells after 4 h (n = 10). A: media CXCL1 increased with IL-6 + sIL-6R. B: cell CXCL1 was similar in vehicle-treated and IL-6 + sIL-6R.
Lung injury in CXCR2-deficient mice with AKI.
Data above suggest that IL-6 upregulates endothelial CXCL1 production and we previously demonstrated that strategies to inhibit IL-6 resulted in less lung injury after ischemic AKI and bilateral nephrectomy that was associated with a reduction in lung CXCL1 (16). CXCL1 is a potent neutrophil chemokine. Therefore, to determine whether CXCL1 itself mediates lung inflammation after AKI, CXCR2-deficient mice were studied. CXCL1 and CXCL2 (also known as MIP-2) both signal through the CXCR2 receptor. As shown in Fig. 5, lung MPO activity was reduced and lung CXCL1 was reduced in CXCR2-deficient mice 4 h after either ischemic AKI or bilateral nephrectomy demonstrating that CXCL1 or MIP-2 can mediate lung inflammation.
Fig. 5.
Lung inflammation in CXCR2-deficient mice with ischemic AKI or BNx. Lung MPO activity and lung CXCL1 were determined 4 h after ischemic AKI (AKI) or BNx in CXCR2-deficient (−/−) mice (n = 6–8). A, B: lung MPO was significantly reduced in CXCR2 −/− mice, compared with wild-type littermates (WT), after both BNx and ischemic AKI. C, D: lung CXCL1 was significantly reduced in CXCR2 −/− mice, compared with WT littermates, after both ischemic AKI and BNx.
Specifically, lung MPO (ΔU·min−1·mg−1) was 1.2 ± 0.1 in wild-type littermates with ischemic AKI and 0.6 ± 0.2 in CXCR2-deficient mice with ischemic AKI (P < 0.0001 vs. wild-type); and it was 1.5 ± 0.1 in wild-type littermates with bilateral nephrectomy and 0.7 ± 0.0 in CXCR2-deficient mice with bilateral nephrectomy (P = 0.0001 vs. wild-type; n = 6–8).
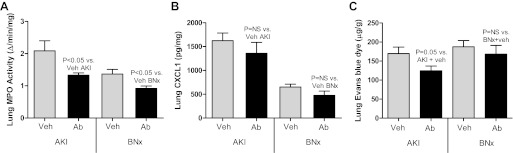
Lung injury after CXCL1 antibody administration in AKI.
CXCL1 and CXCL2 (also known as MIP-2) both signal through the CXCR2 receptor. To determine whether the reduction in lung inflammation in CXCR2-deficient mice with AKI is specifically due to reduction in the activity of the CXCL1, vehicle (rabbit IgG) or CXCL1 antibody was administered to mice with bilateral nephrectomy or ischemic AKI, and lungs were collected 4 h after AKI. As shown in Fig. 6, lung injury as judged by lung MPO activity and lung EBD accumulation (a marker of lung capillary leak) was improved in mice treated with anti-CXCL1 antibody with ischemic AKI vs. vehicle-treated mice with ischemic AKI. In mice with bilateral nephrectomy, antibody treatment significantly reduced lung MPO activity; however, lung capillary leak (EBD accumulation) was similar. Lung CXCL1 was similar with antibody treatement vs. vehicle treatment after both ischemic AKI and bilateral nephrectomy.
Fig. 6.
Lung inflammation and lung capillary leak in CXCL1 antibody-treated mice with ischemic AKI or BNx. Lung MPO activity, lung CXCL1, and lung Evan's blue dye accumulation were assessed 4 h after ischemic AKI (AKI) or BNx in mice treated with vehicle (Veh; rabbit IgG) or CXCL1 antibody (Ab). A: lung MPO activity was reduced in CXCL1 antibody-treated mice with either ischemic AKI or BNx vs. vehicle-treated (n = 9). B: lung CXCL1 was similar in vehicle-treated and CXCL1 antibody-treated mice with either ischemic AKI or BNx (n = 8). C: lung Evan's blue dye was accumulation (lung capillary leak) was reduced in CXCL1 antibody-treated mice with ischemic AKI, but not with BNx vs. vehicle-treated ischemic AKI or BNx, respectively (n = 5–8).
Specifically, lung MPO activity (ΔU·min−1·mg−1) was 0.9 ± 0.1 in sham operation plus rabbit IgG, 0.7 ± 0.1 in sham operation plus CXCL1 antibody (P = NS vs. sham plus rabbit IgG), 2.1 ± 0.9 in ischemic AKI plus rabbit IgG, 1.3 ± 0.2 in ischemic AKI plus CXCL1 antibody (P = 0.03 vs. ischemic AKI plus rabbit IgG; n = 9), 1.4 ± 0.1 in bilateral nephrectomy plus rabbit IgG, and 0.9 ± 0.1 in bilateral nephrectomy plus CXCL1 antibody (P = 0.02 vs. bilateral nephrectomy with rabbit IgG; n = 6–7). Lung EBD (μg/g lung wt) was 169.6 ± 17 in AKI plus rabbit IgG and was 124.5 ± 12 in AKI plus CXCL1 antibody (P = 0.05; n = 5–8).
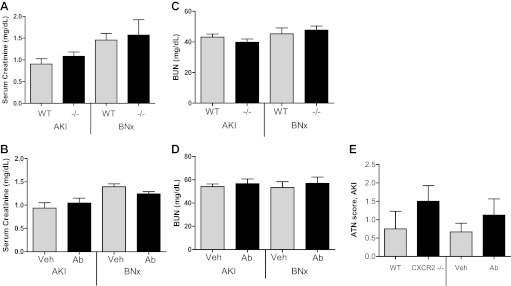
Renal function and renal histology in CXCR2-deficient mice and CXCL1-inhibited mice after AKI.
To determine whether the protection in lung injury in CXCR2-deficient mice and CXCL1-inhibited mice was due to protection from AKI, BUN and serum creatinine were measured, and kidney histology was assessed by periodic acid Schiff staining 4 h after AKI. As shown in Fig. 7, serum creatinine, BUN, and acute tubular necrosis (ATN) scores were similar in wild-type vs. CXCR2-deficient mice with AKI as well as vehicle-treated vs. CXCL1 antibody-treated mice with AKI (n = 9–12 for creatinine and BUN; n = 4–9 for ATN scores).
Fig. 7.
Renal function and renal injury in CXCL1-inhibited mice after ischemic AKI and BNx. Renal function was assessed by serum creatinine and blood urea nitrogen (BUN) 4 h after ischemic AKI (AKI) or BNx in WT, CXCR2-deficient (−/−) mice, vehicle-treated (Veh), or CXCL1 antibody-treated (Ab) mice with ischemic AKI or BNx. Renal injury was assessed at 4 h by acute tubular necrosis score (ATN) on kidney histology in WT, CXCR2 −/−, vehicle-treated, and Ab-treated mice with ischemic AKI. Serum creatinine (A) and BUN (B) were similar in WT and CXCR2 −/− mice with AKI or BNx (n = 9–12). Serum creatinine (C) and BUN (D) were similar in Veh and CXCL1 Ab-treated mice with AKI or BNx (n = 9–12). E: ATN scores were similar in WT, CXCR2 −/−, Veh-treated, and CXCL1 Ab-treated mice with ischemic AKI (n = 4–9).
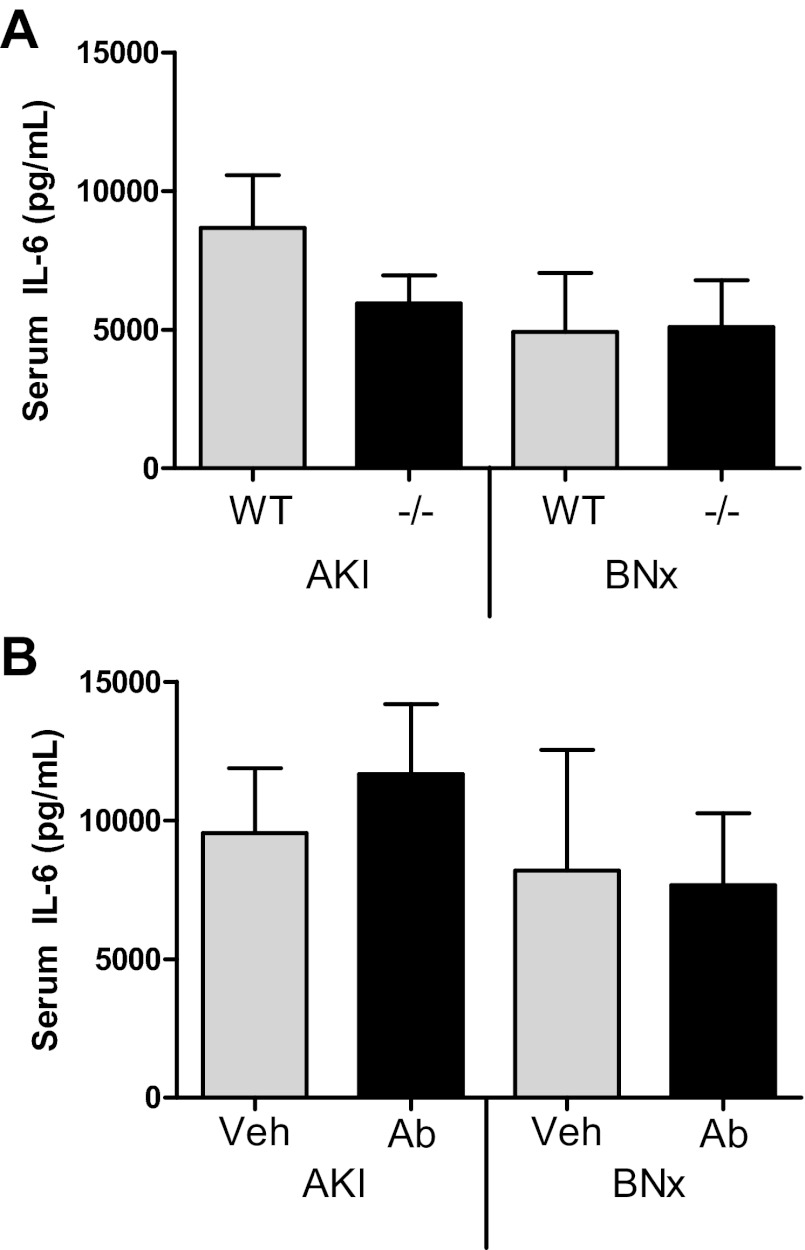
Serum IL-6 in CXCL1-inhibited mice after AKI.
To determine whether the protection from AKI-mediated lung injury by CXCL1 inhibition was due to decreased circulating IL-6, serum IL-6 was measured. As shown in Fig. 8, serum IL-6 was similar in wild-type vs. CXCR2-deficient mice with ischemic AKI (n = 11) or bilateral nephrectomy (n = 7) as well as vehicle-treated vs. CXCL1 antibody-treated mice with ischemic AKI (n = 11) or bilateral nephrectomy (n = 7–10).
Fig. 8.
Serum IL-6 in CXCL1-inhibited mice after AKI. Serum IL-6 was measured 4 h after ischemic AKI (AKI) or BNx in WT, CXCR2-deficient (−/−) mice, Veh-treated, or CXCL1 Ab-treated mice. A: serum IL-6 was similar in WT and CXCR2 −/− mice with AKI (n = 11) or BNx (n = 7). B: serum IL-6 was similar in Veh and CXCL1 Ab-treated mice with AKI (n = 11) or BNx (n = 7–10).
DISCUSSION
Acute lung injury (ALI) is a heterogeneous disease with multiple causes that are either direct or indirect. Direct etiologies of ALI induce lung injury from the alveolar side of the alveolar-capillary barrier, and they include pneumonia and aspiration of gastric contents. Direct injury results in the production of cytokines and chemokines by alveolar macrophages and other pulmonary cells leading to neutrophil infiltration and subsequent lung tissue injury. Indirect etiologies of ALI are from systemic insults such as pancreatitis, sepsis, or trauma (33). Presumably, circulating factors in these diseases have a deleterious effect on the lung resulting in a similar clinical picture of ALI as caused by direct pulmonary injury. Animal and human data have demonstrated the proinflammatory cytokines IL-1β and TNF-α as key circulating mediators of lung injury after sepsis, trauma, and pancreatitis as inhibition of these cytokines protects against lung injury in these models; since injection of these cytokines results in lung injury, it suggests that these cytokines circulate and act at the lung to mediate lung injury. In the present study, we focused on IL-6 as a potential circulating mediator of lung injury after AKI.
Like in other models of indirect lung injury, data in animal models of AKI suggest that inflammatory mediators cause lung injury as anti-inflammatory treatment with IL-10 (13), α-melanocyte-stimulating hormone (7), or a p38 MAPkinase inhibitor (17) protects against AKI-mediated lung injury. IL-6 is the first, and, to date, only specific mediator of lung injury after AKI that has been identified. Methods to inhibit IL-6 using IL-6-deficient mice or IL-6 antibody protected against lung injury after either ischemic AKI or bilateral nephrectomy; whether the protection against lung injury was due to inhibition of circulating IL-6 acting at the lung or due to inhibition of pulmonary IL-6 and IL-6 acting locally in the lung was not known.
Therefore, in the present study, we sought to determine whether circulating IL-6 vs. locally produced lung IL-6 contributed to lung injury after AKI. Although mRNA for IL-6 was minimally increased in the lung after both ischemic AKI and bilateral nephrectomy, IL-6 protein was not increased suggesting that the lung is not a major source of serum IL-6 after AKI. Data to date demonstrate that increased production as well as decreased renal clearance of IL-6 contribute to increased serum IL-6 after bilateral nephrectomy and ischemic AKI. For example, splenic and hepatic IL-6 increase 2 h after either ischemic AKI (2) or bilateral nephrectomy (3), and increased renal IL-6 production occurs after ischemic AKI (2). Furthermore, decreased renal clearance of IL-6 occurs after bilateral nephrectomy (3) or ischemic AKI (8) as injection of intravenous IL-6 in either model results on prolonged serum appearance compared with mice with normal kidney function.
To determine whether circulating IL-6 could contribute to lung injury after AKI, recombinant murine IL-6 was administered intravenously to IL-6-deficient mice with AKI. We found that serum IL-6, serum CXCL1, lung CXCL1, and lung MPO activity were all increased in the mice administered IL-6. Since the only source of IL-6 in the IL-6-deficient mice is what was injected intravenously, these data strongly suggest that circulating IL-6 acts systemically to increase CXCL1 production and acts specifically at the lung to increase CXCL1 production and promote neutrophil infiltration. It should be noted that the serum level of IL-6 achieved with intravenous injection was at a physiologic level, if not slightly lower, than that typically achieved after AKI; specifically, serum IL-6 was 273 pg/ml 4 h after AKI with three intravenous injections in this study while we previously demonstrated that serum IL-6 is greater than 600 pg/ml at 4 h after either ischemic AKI or bilateral nephrectomy (16).
Serum IL-6 is increased in patients with AKI (19) and predicts longer duration of mechanical ventilation (18) as well as increased mortality (28). Serum IL-6 is also increased in patients with ALI and, as with AKI, higher levels predict longer duration of mechanical ventilation (25) and increased mortality (21, 25). Thus, our data are clinically relevant and suggest that serum IL-6 is not simply a biomarker of lung injury but that serum IL-6 affects lung by increasing CXCL1 production and neutrophil infiltration.
CXCL1 is a neutrophil chemokine that is known to play an important role in neutrophil recruitment in a variety of direct and indirect lung injury models including sepsis, pancreatitis, and ventilator-induced lung injury (4). In patients, increased CXCL1 analogs such as IL-8 have been shown to correlate with adverse outcomes in both AKI and ALI. Specifically, serum IL-8 is increased in patients with AKI and is associated with prolonged mechanical ventilation (18) as well as increased mortality (28). In patients with ALI, IL-8 is increased in the serum (20) and bronchoalveolar lavage fluid (21) and is predictive of increased mortality. Since we previously showed that lung inflammation (as judged by lung MPO activity) is associated with reduced lung CXCL1 after AKI in a variety of settings (1, 2, 16), we hypothesized that circulating IL-6 acts at the lung to increase CXCL1 production that then leads to increased lung neutrophil accumulation and lung tissue injury; a role of CXCL1 in AKI-mediated lung injury has not previously been examined.
Therefore, to determine whether CXCL1 directly played a role in AKI-mediated lung injury, CXCR2-deficient mice were studied and were found to have markedly reduced lung neutrophil content (MPO activity) after ischemic AKI or bilateral nephrectomy. Because both CXCL1 and CXCL2 (also known as MIP-2) signal through the CXCR2 receptor, we further examined the role of CXCL1 specifically by utilizing neutralizing antibodies to CXCL1 and found that lung neutrophils and capillary leak were both reduced in antibody-treated mice with ischemic AKI. Since CXCL1 is a neutrophil chemokine, these data suggest that CXCL1 mediates lung injury via neutrophil infiltration after AKI. Although not examined in this study, CXCL1 may also cause lung injury by directly affecting the lung endothelium causing apoptosis and lung capillary leak. Specifically, CXCL1 has been shown to directly cause apoptosis in endothelial cells (30) and lung endothelial apoptosis has been shown to contribute to lung capillary leak after ischemic AKI (11). Although CXCL1 inhibition protects against renal dysfunction in ischemic or cisplatin-induced AKI, our studies did not confirm this protective effect.
Although CXCL1 antibody treatment reduced lung neutrophil content after bilateral nephrectomy, lung capillary leak did not improve suggesting other factors may contribute to lung capillary leak after bilateral nephrectomy. It is possible that the inflammatory nature of lung injury after bilateral nephrectomy is less than that after ischemic AKI. Furthermore, although there are many similarities between the lung injury that occurs after ischemic AKI and bilateral nephrectomy, functional genomic analysis has demonstrated that there are differences in the genetic profiles in the lung between ischemic AKI and bilateral nephrectomy (10).
Given the proximity of circulating IL-6 to the endothelial cell, we sought to determine whether circulating IL-6 acts on the pulmonary endothelial cell to produce CXCL1. In vitro, we found that IL-6, with sIL-6R, stimulated endothelial CXCL1 production. Endothelial cells lack the IL-6 receptor but are able to respond to IL-6 via trans-signaling, a process in which IL-6 and sIL-6R form a complex, and then enter the cell (24). These data are consistent with previous studies demonstrating a role of IL-6 in endothelial CXCL1 production via trans-signaling (22, 27). Since circulating sIL-6R is increased threefold after AKI (24), we examined lung endothelial CXCL1 after AKI by immunofluorescence in vivo and found that CXCL1 colocalized in endothelial cells. Together, these data suggest that the pulmonary endothelial cell produces CXCL1 in response to circulating IL-6. Although CXCL1 was present in the lung endothelium after AKI, other cell types also contained CXCL1. In this regard, we previously showed that lung interstitial mononuclear phagocytes and alveolar mononuclear phagocytes may also contribute to lung CXCL1 production after ischemic AKI, thus, multiple lung cell types appear to be important in CXCL1 production after AKI. Factors other than IL-6 are also likely to be important in CXCL1 production as IL-6-deficient mice with AKI had detectable levels of serum and lung CXCL1 in our study.
In summary, we demonstrate for the first time that circulating, rather than local lung, IL-6 mediates lung inflammation and injury after AKI. Since serum IL-6 is increased in patients with AKI or ALI and predicts prolonged mechanical ventilation and increased mortality in both of these conditions, our data suggest that serum IL-6 is not simply a biomarker of poor outcomes but a pathogenic mediator of lung injury. We demonstrate that IL-6 upregulates endothelial CXCL1 production, in vitro and in vivo, and that inhibition of CXCL1 in ischemic AKI protects against lung injury as judged by reduced lung neutrophil content and reduced lung capillary leak. CXCL1 inhibition is clinically feasible in patients. The CXCL1 inhibitors repertaxin and reparixin are being studied in clinical trials in other settings (e.g., clinicaltrials.gov NCT identifiers NCT00248040, NCT01220856, NCT00224406). Thus, CXCL1 inhibition may be a useful therapeutic target in patients at risk for AKI and AKI-mediated lung injury.
GRANTS
This work was supported by Grant 1R01 HL095363 to S. Faubel.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.A., A.A.-H., and S.F. conception and design of research; N.A., A.A.-H., C.A., R.B., J.B., R.G.W., Z.H., and S.F. performed experiments; N.A., A.A.-H., C.A., R.B., J.B., R.G.W., Z.H., and S.F. analyzed data; N.A., A.A.-H., C.A., R.B., C.L.E., and S.F. interpreted results of experiments; N.A., A.A.-H., and S.F. prepared figures; N.A., A.A.-H., and S.F. drafted manuscript; N.A., A.A.-H., C.A., R.B., C.L.E., and S.F. edited and revised manuscript; N.A., A.A.-H., C.A., R.B., J.B., R.G.W., Z.H., C.L.E., and S.F. approved final version of manuscript.
REFERENCES
- 1.Altmann C, Andres-Hernando A, McMahan RH, Ahuja N, He Z, Rivard CJ, Edelstein CL, Barthel L, Janssen WJ, Faubel S. Macrophages mediate lung inflammation in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 302: F421–F432, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andres-Hernando A, Altmann C, Ahuja N, Lanaspa MA, Nemenoff R, He Z, Ishimoto T, Simpson PA, Weiser-Evans MC, Bacalja J, Faubel S. Splenectomy exacerbates lung injury after ischemic acute kidney injury in mice. Am J Physiol Renal Physiol 301: F907–F916, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andres Hernando A, Dursun B, Altmann C, Ahuja N, He Z, Bhargava R, Edelstein CL, Jani A, Hoke TS, Klein CL, Faubel S. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol Dial Transplant (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 110: 1703–1716, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med 155: 1505–1511, 1995 [PubMed] [Google Scholar]
- 6.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int 70: 1120–1126, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Deng J, Hu X, Yuen PS, Star RA. Alpha-melanocyte-stimulating hormone inhibits lung injury after renal ischemia/reperfusion. Am J Respir Crit Care Med 169: 749–756, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Dennen P, Altmann C, Kaufman J, Klein CL, Andres-Hernando A, Ahuja NH, Edelstein CL, Cadnapaphornchai MA, Keniston A, Faubel S. Urine interleukin-6 is an early biomarker of acute kidney injury in children undergoing cardiac surgery. Crit Care 14: R181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol 19: 547–558, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassoun HT, Grigoryev DN, Lie ML, Liu M, Cheadle C, Tuder RM, Rabb H. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol 293: F30–F40, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Hassoun HT, Lie ML, Grigoryev DN, Liu M, Tuder RM, Rabb H. Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am J Physiol Renal Physiol 297: F125–F137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidland A, Heine H, Heidbreder E, Haunschild J, Weipert J, Gilge U, Kluger G, Horl WH. Uremic pneumonitis. Evidence for participation of proteolytic enzymes. Contrib Nephrol 41: 352–366, 1984 [PubMed] [Google Scholar]
- 13.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, Tao Y, Dursun B, Voelkel NF, Edelstein CL, Faubel S. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol 18: 155–164, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Kim do J, Park SH, Sheen MR, Jeon US, Kim SW, Koh ES, Woo SK. Comparison of experimental lung injury from acute renal failure with injury due to sepsis. Respiration 73: 815–824, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int 74: 901–909, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int 74: 901–909, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int 55: 2362–2367, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, Faubel S. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care 13: R104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu KD, Glidden DV, Eisner MD, Parsons PE, Ware LB, Wheeler A, Korpak A, Thompson BT, Chertow GM, Matthay MA. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med 35: 2755–2761, 2007 [PMC free article] [PubMed] [Google Scholar]
- 20.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 107: 1062–1073, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest 108: 1303–1314, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Modur V, Li Y, Zimmerman GA, Prescott SM, McIntyre TM. Retrograde inflammatory signaling from neutrophils to endothelial cells by soluble interleukin-6 receptor alpha. J Clin Invest 100: 2752–2756, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nath KA, Grande JP, Croatt AJ, Frank E, Caplice NM, Hebbel RP, Katusic ZS. Transgenic sickle mice are markedly sensitive to renal ischemia-reperfusion injury. Am J Pathol 166: 963–972, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, Axelrod JH. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol 19: 1106–1115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 33: 1–6; discussion 230–232, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int 63: 600–606, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6: 315–325, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 65: 1357–1365, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Star RA. Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Terui Y, Ikeda M, Tomizuka H, Kasahara T, Ohtsuki T, Uwai M, Mori M, Itoh T, Tanaka M, Yamada M, Shimamura S, Ishizaka Y, Ikeda K, Ozawa K, Miura Y, Hatake K. Activated endothelial cells induce apoptosis in leukemic cells by endothelial interleukin-8. Blood 92: 2672–2680, 1998 [PubMed] [Google Scholar]
- 31.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Vieira JM, Jr, Castro I, Curvello-Neto A, Demarzo S, Caruso P, Pastore L, Jr, Imanishe MH, Abdulkader RC, Deheinzelin D. Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med 35: 184–191, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]