Abstract
Because of its critical importance in rectoanal incontinence, we determined the feasibility to reconstruct internal anal sphincter (IAS) from human IAS smooth muscle cells (SMCs) with functional and molecular attributes similar to the intact sphincter. The reconstructs were developed using SMCs from the circular smooth muscle layer of the human IAS, grown in smooth muscle differentiation media under sterile conditions in Sylgard-coated tissue culture plates with central Sylgard posts. The basal tone in the reconstructs and its changes were recorded following 0 Ca2+, KCl, bethanechol, isoproterenol, protein kinase C (PKC) activator phorbol 12,13-dibutyrate, and Rho kinase (ROCK) and PKC inhibitors Y-27632 and Gö-6850, respectively. Western blot (WB), immunofluorescence (IF), and immunocytochemical (IC) analyses were also performed. The reconstructs developed spontaneous tone (0.68 ± 0.26 mN). Bethanechol (a muscarinic agonist) and K+ depolarization produced contraction, whereas isoproterenol (β-adrenoceptor agonist) and Y-27632 produced a concentration-dependent decrease in the tone. Maximal decrease in basal tone with Y-27632 and Gö-6850 (each 10−5 M) was 80.45 ± 3.29 and 17.76 ± 3.50%, respectively. WB data with the IAS constructs′ SMCs revealed higher levels of RhoA/ROCK, protein kinase C-potentiated inhibitor or inhibitory phosphoprotein for myosin phosphatase (CPI-17), phospho-CPI-17, MYPT1, and 20-kDa myosin light chain vs. rectal smooth muscle. WB, IF, and IC studies of original SMCs and redispersed from the reconstructs for the relative distribution of different signal transduction proteins confirmed the feasibility of reconstruction of IAS with functional properties similar to intact IAS and demonstrated the development of myogenic tone with critical dependence on RhoA/ROCK. We conclude that it is feasible to bioengineer IAS constructs using human IAS SMCs that behave like intact IAS.
Keywords: internal anal sphincter, smooth muscle tone, internal anal sphincter reconstructs, Rho kinase, protein kinase C, rectoanal incontinence
the basal tone in the internal anal sphincter (IAS) smooth muscle plays a critical role in rectoanal continence, and its failure leads to incontinence and other rectoanal motility disorders (2, 3, 31). Presently, there is no satisfactory treatment for any of these debilitating conditions (18). Surgical replacement of IAS generated via tissue engineering offers a viable option for such conditions.
Earlier studies were successful in bioengineering the IAS starting with the smooth muscle cells (SMCs) from different animal species (15, 30). More recent studies have tissue engineered the IAS from the human IAS SMCs as well (29, 38). Data from those reconstructs suggest the basal tone to be primarily protein kinase C (PKC)-mediated rather than RhoA/Rho kinase (ROCK). These data were based on near obliteration of the IAS reconstructs' tone by PKC inhibitors and a limited effect of RhoA/ROCK inhibitors. However, presently, there are no data that directly compare the functional and molecular control mechanisms for the basal tone of these reconstructs with those of intact human IAS.
PKC-predominant control of basal tone of the earlier prepared IAS reconstructs (38) is in sharp contrast with the recent data in intact human IAS, wherein the basal tone has been shown to be RhoA/ROCK predominant (33). Although the exact reason for the differences is not known, dedifferentiation of the phenotypic tonic SMCs to the phasic type may be one of the reasons. Previous studies have shown that, in the phasic smooth muscles, the PKC pathway (primarily via PKCα) is the major determinant of the sustained contraction (1, 11, 37). The sustained smooth muscle contraction or the basal tone via the RhoA/ROCK pathway is maintained primarily because of the phosphorylation of regulatory binding subunit (MYPT1) and catalytic subunit PPicδ of myosin light chain phosphatase (MLCP) and of protein kinase C-potentiated inhibitor or inhibitory phosphoprotein for myosin phosphatase (CPI-17), an endogenous inhibitor of MLCP (10, 26, 32).
The purpose of the present investigation was to develop IAS reconstructs from human IAS SMCs with functional and molecular attributes similar to the intact IAS. For this, we used special precautions to prevent dedifferentiation of the cells to maintain their tonic phenotypes. To validate this, we compared the molecular attributes of the SMCs redispersed from the IAS reconstructs with those of the freshly isolated SMCs from intact human IAS and rectal smooth muscles (RSM). We used PKC and RhoA/ROCK-selective inhibitors and activators, Western blotting, and immunocytochemical approaches.
MATERIALS AND METHODS
Tissue samples preparation.
Human IAS and RSM tissue samples were obtained from five subjects undergoing total removal of anorectum, via the departments of Surgery and Pathology. The studies were approved by the Institutional Review Board of Thomas Jefferson University. Tissue samples were kept in oxygenated and 0.22 μm filtered Krebs physiological solution (KPS) at 4°C. The composition of KPS was (in mM): 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4, 1.01 NaH2PO4, 25 NaHCO3, and 11.10 glucose. The IAS and RSM tissues were carefully cleaned of the extraneous serosal, longitudinal smooth muscle layer and mucosal tissues using sharp dissection. Identification and the health of the IAS were established by the development of the basal tone of the smooth muscle strips and their relaxation with the electrical field stimulation as described before (33). The IAS smooth muscle strips at optimal (Lo) were ∼1 mm wide, 1 mm thick, and 10.5 mm long (1 × 1 × 10.5 mm) oriented in the direction of the circular smooth muscle layer.
Isolation of SMCs and cell culture.
SMCs from the IAS were isolated as described previously (35). Briefly, the circular IAS tissues were cut into ∼1-mm cubes, and the SMCs were dispersed using repeated short-term incubations with oxygenated KPS containing 0.1% collagenase type I and 0.01% soybean trypsin inhibitor at 37°C for 1 h. Each time, the dispersing solution was replaced with fresh KPS containing 0.1% collagenase and 0.01% soybean trypsin inhibitor after brief centrifugation. Finally, the cell suspension was filtered through a 500-μm Nitex mesh. The tissue trapped on the mesh was rinsed with 25 ml (5 × 5 ml) of collagenase-free KPS and incubated in collagenase-free KPS at 37°C (for a few min to 1 h). The filtrate containing the cells was centrifuged at 350 g for 10 min at room temperature (RT). The cells in the pellet were resuspended on collagen-coated plates in DMEM growth medium with 5% FBS, 5% penicillin-streptomycin, 50 μg/ml gentamicin, 2 μg/ml amphotericin B, and 50 μg/ml of sodium ascorbate (4) in 100-mm tissue culture dishes (Corning) at 37°C and 5% CO2 in an incubator with regulated humidity.
Cell lysate preparation and Western blot analysis.
The SMCs were rinsed with PBS and suspended in ice-cold homogenization buffer (10 mM Tris·HCl, pH 7.5, 5 mM MgCl2, 2 mM EDTA, 250 mM sucrose, and 1 mM dithiothreitol, and 1% Triton X-100). The extract was centrifuged at 800 g for 10 min, and protein concentration in the resultant supernatant was determined using the BCA Protein Assay Reagent Kit (Pierce, Rockford, IL). Twenty micrograms of protein in 20 μl of lysates were mixed with 2× Laemmli sample buffer (with final concentrations of 62.5 mM Tris, 1% SDS, 15% glycerol, 0.005% bromphenol blue, and 2% mercaptoethanol) and placed in a boiling water bath for 5 min. Proteins in the samples separated by SDS-polyacrylamide gel [7.5% gel for ROCK II, phospho (p)-Thr696MYPT1 and MYPT1; 15% gel for RhoA, 20-kDa myosin light chain (MLC20), p-Thr18/Ser19MLC20, CPI-17, and p-Thr38CPI-17] were electrophoretically transferred to a polyvinylidene difluoride membrane using the iBlot Dry Blotting System (Invitrogen, Carlsbad, CA) at RT.
To block nonspecific antibody binding, the membrane was soaked for 1 h at RT in LI-COR blocking buffer, after which it was incubated with the specific primary antibodies (1:1,000 for PKCα, RhoA, ROCK II, CPI-17, p-CPI-17, p-MYPT1, MYPT1, MLC20, and p-MLC20 and 1:20,000 for α-actin) diluted in LI-COR buffer containing 0.1% Tween 20 for 1 h at RT. After being washed with TBS-Tween 20 (TBS-T) three times for 10 min, the membranes were incubated with the IRdye680-and IRdye800-conjugated secondary antibody from LI-COR Biosciences in the dark (bovine anti-rabbit 1:10,000 for PKCα, RhoA, ROCK II, MYPT1, p-MYPT1, MLC20, and bovine anti-goat; 1:5,000 for CPI-17, p-CPI-17, and p-MLC20). The membranes were washed three times for 10 min with TBS-T, kept in PBS buffer on a shaker for 10 min at RT in the dark, and scanned by a LI-COR Infrared scanner from LI-COR Biosciences (see Figs. 6 and 7).
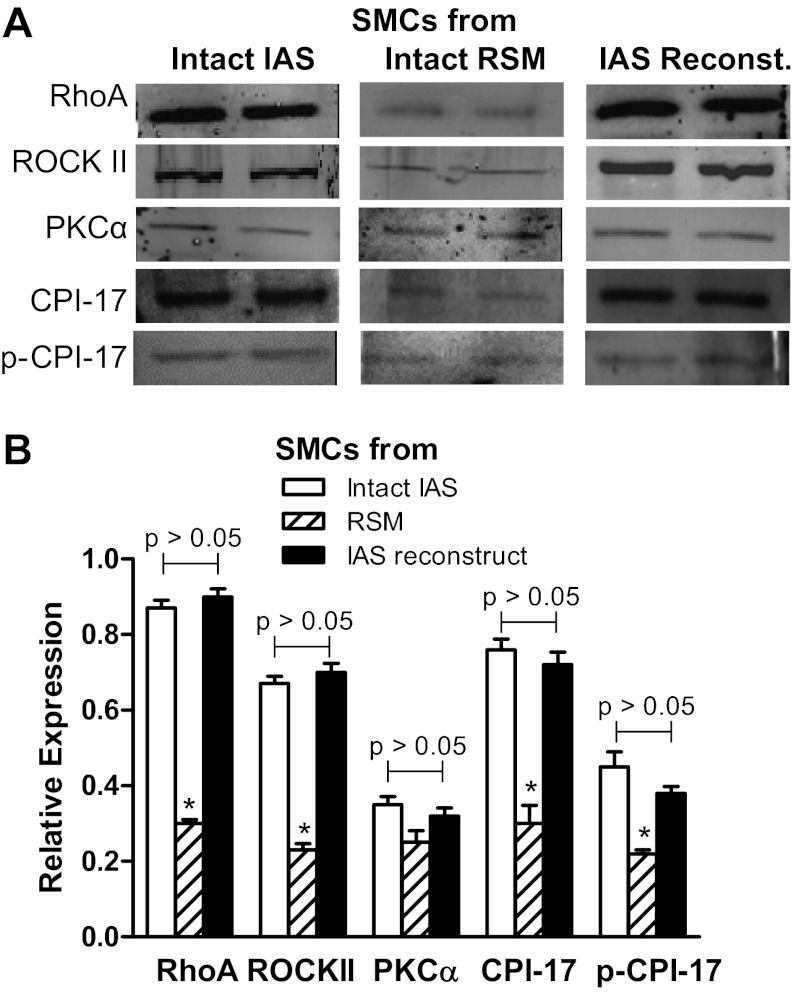
Fig. 6.
A: Western blots (WB) of RhoA, ROCKII, PKCα, protein kinase C-potentiated inhibitor or inhibitory phosphoprotein for myosin phosphatase (CPI-17), and phospho (p)-CPI-17 in the smooth muscle cells (SMCs) isolated from intact IAS, intact rectal smooth muscle (RSM), and the IAS reconstructs. B: respective quantitative data. Data show significantly higher levels of above signal transduction protein with the exception of PKCα, in the cells from intact IAS, and constructs compared with those from intact RSM (*P < 0.05; n = 5).
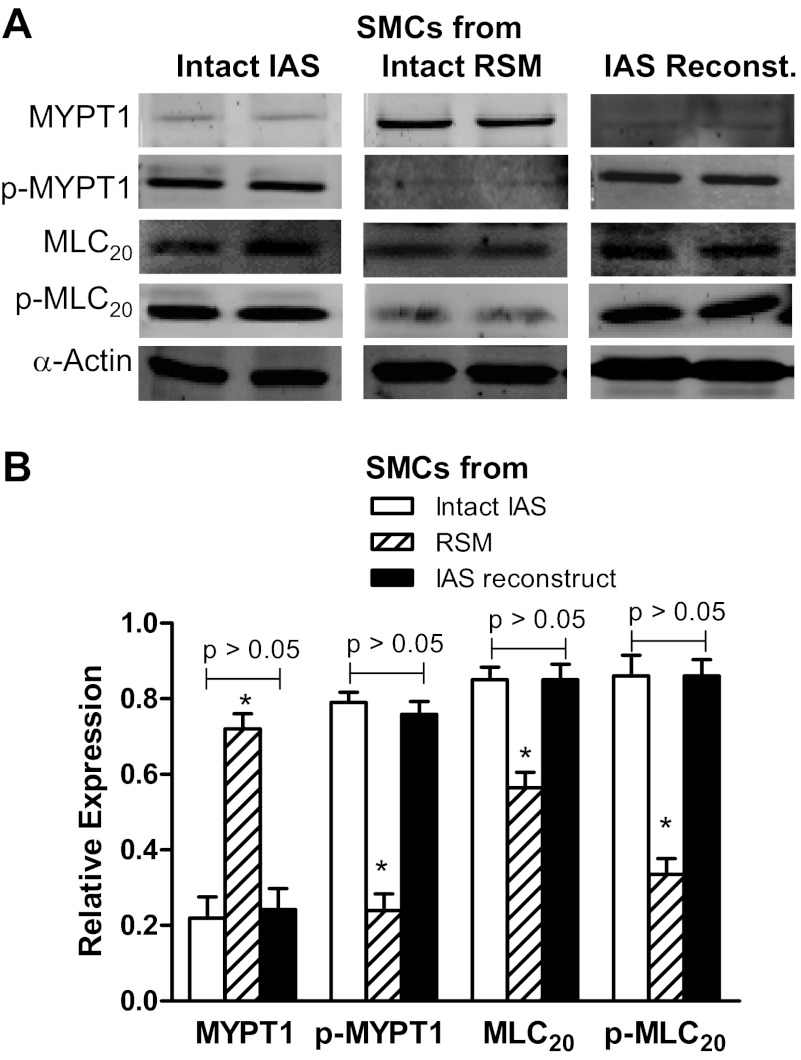
Fig. 7.
A: WB showing relative expression of MYPT1, p-MYPT1, p-20-kDa myosin light chain (MLC20), and MLC20 in the SMCs isolated from intact IAS, intact RSM, and the IAS reconstructs. B: quantitative data for these signal transduction proteins shows higher expression levels of these proteins in the IAS SMCs with the exception of MYPT1 (*P < 0.05; n = 5). The expression levels of MYPT1 were significantly lower (*P < 0 .05; n = 5) in the cells from intact IAS and the reconstructs compared with those from intact RSM.
Preparation of Sylgard molds and bioengineering of IAS reconstructs.
A custom-designed post (5 mm OD) was embedded in the center of 35-mm tissue culture dishes in which 2 ml of Sylgard solution were poured and kept at 37°C for solidification as described before (38). These molds were sterilized using 70% ethanol followed by ultraviolet (UV) exposure for 30 min. The cultured SMCs (107 cells/ml) were harvested, centrifuged, and mixed with rat tail collagen I solution (Invitrogen) containing 10× PBS and 1.0 N NaOH in sterile water, according to the manufacturer's instructions. Thus a total 1 ml of solution was mixed with the cell pallet and plated around the post in the sterile Sylgard molds (24). These dishes were kept at 37°C for 15–45 min, for the appropriate solidification of collagen, and DMEM (with 15% serum) was added. The reconstituted rings were incubated for 24 h, and media was replaced with SMC differentiation media (Biocoat, from BD Biosciences, Sparks, MD). The SMCs transformed into the IAS reconstructs in 72–96 h. These reconstructs were used for isometric tension measurement as described below.
Measurement of isometric tension in IAS reconstructs.
The above reconstructs were transferred to 2-ml muscle baths containing oxygenated KPS at 37°C for the recording of isometric tone via force transducers (FORT10; WPI, Sarasota, FL), using the PowerLab/8SP data acquisition system and recorded using Chart 4.1.2 (AD Instruments, Colorado Springs, CO). Initial average dimensions of the IAS reconstruct rings were inside diameter 5 mm, outside diameter 7 mm with overall thickness of ∼4 mm. Once in the organ baths, these reconstructs were stretched to 150% (usually achieved by initial stretch of 2 mN) of their initial lengths to provide the Lo. This was followed by the equilibration period of 90 min. These procedures have been explained before for the smooth muscle strips (21, 28, 33) and subsequently applied for the IAS reconstructs in a recent study (38). At Lo, the reconstructs were 2 mm wide, 2 mm thick, and 10.5 mm high (2 × 2 × 10.5). The success of the IAS reconstructs was defined by the development of spontaneous tone that decreased precipitously in KPS containing 0 Ca2+ and responded appropriately to bethanechol, isoproterenol, KCl, phorbol 12,13-dibutyrate (PDBu), Gö-6850, and Y-27632 in a concentration-dependent manner. All increases and decreases in the basal tone were expressed on percentile maximal basis by bethanechol (10−4 M) (36) and EGTA and 0 Ca2+ (5, 33), respectively. At the end of each experiment, wet weights of strips and reconstructs were recorded. Force/cross-sectional area (CSA) was also calculated by dividing the force generated (by the smooth muscle strips or the reconstructs) by the CSA. CSA was calculated by using the equation by Jiang et al. (17).
Density of both SM strips and reconstructs was considered as 1 (mg/mm3).
IAS reconstructs section cutting, redispersion of SMCs from the IAS reconstructs, and immunofluorescence analysis.
The reconstructs were rapidly frozen over dry ice and set into blocks of 1 cm by 2 cm (horizontal direction) in optimal cutting temperature compound (Sakura Finetech, Torrance, CA). The reconstructs were then frozen sectioned (10 μm) in the horizontal direction. The sections were collected on chrome-alum-coated glass slides (the coating served to improve adhesion to the slide). The sections were fixed with ice-cold fixative (4% paraformaldehyde and 0.2% picric acid in PBS, pH 7.4) for 10 min followed by a thorough wash in PBS. To determine immunofluorescence, the sections were incubated with monoclonal primary antibodies to α-actin (1:800 dilution in 0.5% BSA/0.2% Triton X-100) (Sigma Aldrich) overnight at RT in a humid chamber (Santa Cruz Biotechnology). The sections were then rinsed in PBS (3 times) and incubated for 60 min in secondary antibodies conjugated to fluorescein isothiocyanate (FITC, 1:200; Santa Cruz Biotechnology) and visualized and photographed using a Nikon Eclipse 80i microscope.
Cells from the reconstructs were dispersed using collagenase I and soybean trypsin inhibitor treatment as described above and plated in chambered slides for attachment. Once the cells were properly attached, they were treated with C3 exoenzyme (2.5 μg/ml) and Y-27632 and Gö-6850 (each 10−6 M). The cells before and after pretreatment with inhibitors in chambered slides were fixed with 4% paraformaldehyde for 15 min and washed with PBS for three times. These cells were kept in blocking buffer (PBS containing 0.5% FBS and 0.2% Triton X-100) for 15 min followed by their overnight incubation in a humid chamber at 4°C in primary antibodies (1:100, diluted in PBS containing 0.5% FBS and 0.2% Triton) for PKCα, RhoA, ROCK II (Santa Cruz), and α-actin (36).
The cells were then stained with secondary antibodies (FITC and Texas red conjugated) and with 4′,6-diamidino-2-phenylindole (DAPI) for nucleic acid staining as described before (36). The slides were then air-dried and cover slipped with Prolong Gold mounting medium (Invitrogen). Slides were kept overnight at 4°C for appropriate polymerization of the mounting medium and then sealed with clear nail polish. Microscopic images were taken by using a Nikon Eclipse 80i microscope and a Carl Zeiss LSM 510 UV META inverted confocal microscope (Carl Zeiss Microimaging, Thornwood, NY) with a Plan-Apo 40 oil immersion lens at RT and Zeiss AIM 4.2 SP1 software (Bioimaging Facility of the Kimmel Cancer Center, Thomas Jefferson University). Images were analyzed by using Nikon imaging software (NIS elements 3.1) (Nikon Instruments, Melville, NY).
Chemicals and reagents.
Rat tail collagen I was obtained from Invitrogen. The Sylgard 184 silicone elastomer kit was purchased from Dow Corning. Bethanechol and isoproterenol, α-actin, and MLC20 antibodies were from Sigma (St. Louis, MO); KCl, PDBu, Gö-6850, and Y-27632 were purchased from Biomol (Plymouth Meeting, PA). PKCα, RhoA, ROCK II, p-MYPT1, p-Thr38CPI-17, CPI-17, and p-MLC20 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA); IRdye680- and IRdye800-conjugated mouse, goat, and rabbit secondary antibodies were obtained from LI-COR Biosciences (Lincoln, NV).
Data analysis.
Dose-response curves for bethanechol, isoproterenol, KCl, PDBu, Gö-6850, and Y-27632 were constructed on a cumulative basis. Results were expressed as means ± SE and graphed using GraphPad Prism 5.0 (Graph Pad Software, La Jolla, CA). Statistical significance between two different groups was tested by using ANOVA and by unpaired t-test. A P value <0.05 was considered statistically significant.
RESULTS
Development of basal tone in IAS reconstructs: effect of bethanechol (muscarinic receptor agonist) and isoproterenol (β-adrenoceptor agonist).
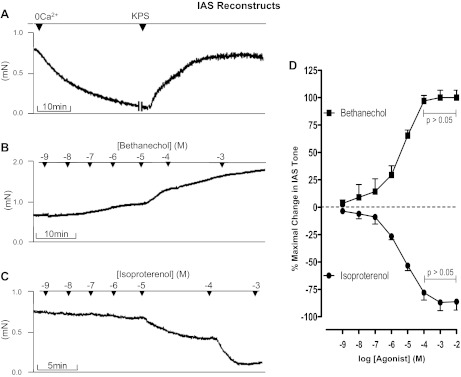
IAS reconstructs developed spontaneous tone. The basal tone in the reconstructs was 0.68 ± 0.26 mN. These reconstructs relaxed in KPS containing 0 Ca2+ and regained their original tone following the replacement with normal KPS (Fig. 1A). Likewise, these reconstructs demonstrated reproducible decreases and increases in the basal tone following introduction of relaxing and contractile agonists, in a concentration-dependent manner (Figs. 1–5). Average wet weight of reconstructs was 8.77 ± 1.73 mg, and force/CSA was 0.83 ± 0.12 mN/mm2.
Fig. 1.
Responses of the internal anal sphincter (IAS) reconstructs to 0 Ca2+ (A), bethanechol (B), and isoproterenol (C). D: quantitative data of the effects of concentration-dependent increase in the tone by bethanechol and relaxation following isoproterenol. KPS, Krebs physiological solution.
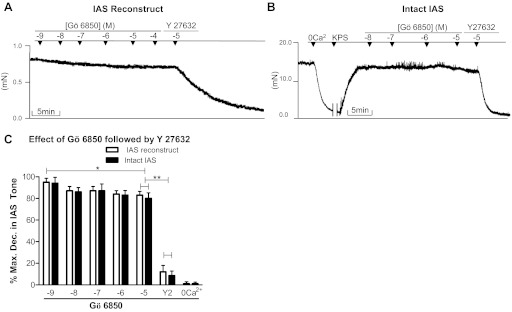
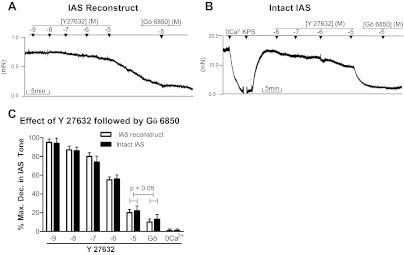
Fig. 5.
A and B: original tracings showing the effects of Gö-6850 (10−9 to 10−5 M) [protein kinase C (PKC) inhibitor] followed by Y-27632 [Rho kinase (ROCK) inhibitor] (10−5 M) on IAS reconstructs and intact IAS, respectively. C: quantitative data comparison. Data in Figs. 4 and 5 show that Y-27632 produces 80.45 ± 3.29% decreases in tone, whereas Gö-6850 produces only 17.76 ± 3.50%. As shown, addition of Gö-6850 only causes minimal but significant (*P < 0.05, n = 4–6) effect, and administration of Y-27632 following Gö-6850 produces further significantly higher relaxation (**P < 0.05).
Bethanechol produced a concentration-dependent increase in the basal tone in the IAS reconstructs. In the maximal effective concentration of 10−4 M, bethanechol produced a 96.99 ± 4.94% increase in the tone, a response not significantly different from that of 10−3 M bethanechol (P > 0.05). An original tracing of bethanechol response is given in Fig. 1B, and quantitative data are given in Fig. 1D. Conversely, isoproterenol produced a concentration-dependent decrease in the tone in IAS reconstructs (original tracing in Fig. 1C and quantitative data in Fig. 1D). The maximal effective concentration of isoproterenol (10−4 M) produced 77.98 ± 6.77% relaxation, not significantly different from that of 10−3 M (P > 0.05).
Effect of K+ depolarization and PKC activator (PDBu) on basal tone in human IAS reconstructs.
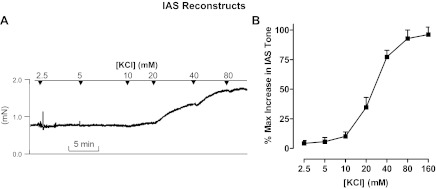
K+ depolarization with KCl produced a significant and concentration (2.5–160 mM)-dependent increase in the basal tone of IAS reconstructs (P < 0.05; n = 5). Typical tracings showing the effects of KCl are given in Fig. 2A, and quantitative data are given in Fig. 2B.
Fig. 2.
A: tracing showing the effect of IAS reconstructs to KCl. B: counterpart quantitative data showing that K+ depolarization causes a concentration-dependent contraction in the IAS reconstructs. Brackets denote concentration.
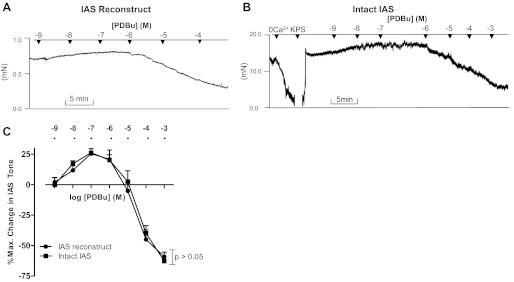
In contrast, the IAS responses of PDBu were concentration dependent; a concentration range (10−9 to 10−7 M) produced an increase in the basal tone, whereas higher concentrations (10−6 to 10−3 M) produced a decrease. PDBu in the concentration of 10−7 M produced an increase in the IAS tone of 25 ± 2.45%. Typical tracings of this biphasic response of PDBu in the reconstructs are shown in Fig. 3A, and quantitative data are shown in Fig. 3C. PDBu in the concentrations of 10−3 M produced 59.09 ± 3.07% relaxation. It is noteworthy that PDBu produced a biphasic response in intact human IAS also, similar to that in the reconstructs (Fig. 3, B and C).
Fig. 3.
A and B: tracings showing the effects of IAS reconstructs and intact IAS to phorbol 12,13-dibutyrate (PDBu). In the tracing shown here as well as in Figs. 4B and 5B, the effect of 0 Ca2+ determined the basal tone in the IAS and percent maximal relaxation. C: quantitative data showing resemblance of the PDBu effect in intact IAS with the IAS reconstructs, as the two curves obtained from the IAS reconstructs and intact IAS are not significantly different from each other (P > 0.05; n = 5).
Effects of PKC (Gö-6850) and ROCK (Y-27632) inhibitors given individually and in combination, on basal tone in human IAS reconstructs.
In contrast with the results from the previous reports, data in the presently prepared constructs demonstrated a prominent inhibitory effect of the ROCK inhibitor Y-27632 and only a modest effect with the PKC inhibitor Gö-6850. The inhibitory effects with both of these inhibitors were concentration dependent (Figs. 4, A and C, and 5, A and C). The maximal inhibitory effect of Y-27632 (10−5 M) was 80.45 ± 3.29%. When the maximal effective concentration of Y-27632 was combined with Gö-6850, there was a limited additional decrease in the tone to 90.10 ± 1.34% (Fig. 4, A and C). The above effect of Y-27632 was significantly greater than the maximal effect with Gö-6850 of 17.76 ± 3.50% (P < 0.05; n = 5; Fig. 5C). As shown in the tracing (Fig. 5A), further increase in the concentration of Gö-6850 to 10−4 M did not cause a further decrease in the tone. However, when the maximal effective concentration of Gö-6850 was combined with Y-27632 (10−5 M), there was a significantly higher decrease to 88.94 ± 2.03% (P < 0.05; n = 5; Fig. 5, A and C). Typical tracings of the effects of Y-27632 and Gö-6850 alone and in combination are given in Figs. 4A and 5A, respectively.
Fig. 4.
A and B: typical tracings showing the effects of Y-27632 (10−9 to 10−5 M) followed by the addition of a maximal effective dose of Gö-6850 (10−5 M) in the IAS reconstructs and intact human IAS smooth muscle strips, respectively. The counterpart quantitative data of these series of experiments is given in C, showing no significant differences between the two types of smooth muscle preparations (P > 0.05; via ANOVA). Data show that, following the maximal effect of Y-27632, Gö-6850 produces further limited but significant decrease in the IAS tone (P < 0.05).
Effect of PKC inhibitor (Gö-6850) and ROCK inhibitor (Y-27632) on the basal tone of intact IAS smooth muscle strips.
Employing intact human IAS smooth muscle strips, percent maximal decreases in the IAS tone following Y-27632 and Gö-6850 were not significantly different from those of the reconstructs (P > 0.05; n = 5; Figs. 4, A and C, and 5, A and C). Likewise, their combined effects were also not significantly different from those of the reconstructs (P > 0.05; n = 5). Typical tracings of such experiments are given in Figs. 4B and 5B, and counterpart quantitative data are given in Figs. 4C and 5C. In such experiments, the effect of 0 Ca2+ was examined first to determine the actual levels of active basal tone, before the administration of Y-27632 and Gö-6850. As shown after the maximal decrease in the IAS tone with 10−5 M Gö-6850 (20.76 ± 3.45), addition of Y-27632 caused a further precipitous decrease in the tone to 92.45 ± 3.45% (P < 0.05; n = 5; Fig. 5, B and C). However, when the protocol was reversed, Y-27632 produced 80.45 ± 4.35% relaxation, and addition of Gö-6850 caused no further significant increase (P > 0.05; n = 5; Fig. 4C).
Western blot analyses of SMCs used for the seeding of human IAS, and following their redispersion from the reconstructs and their comparison with those from intact RSM.
Western blot analysis revealed similarities for the characteristic higher expression levels of RhoA/ROCK and other signal transduction proteins in the SMCs freshly dispersed from intact IAS and from the IAS reconstructs. Data show that there were no significant differences between the expressions of RhoA/ROCK, PKCα, CPI-17, and p-CPI17 in the intact vs. reconstructs' IAS SMC (P > 0.05; n = 5; Fig. 6, A and B). Similarly, there were no significant differences between the expressions of MYPT1, p-MYPT1, MLC20, and p-MLC20 (P > 0 .05; n = 5; Fig. 7, A and B). Interestingly, these expression levels in the SMCs from intact and reconstructs were significantly different from those of the RSM (P < 0.05; n = 5; Figs. 6, A and B, and 7, A and B).
Immunocytochemical analyses of SMCs in IAS reconstruct.
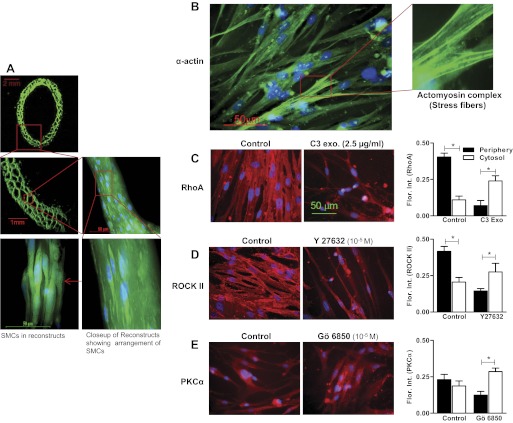
Immunocytochemical analyses showed high expressions of the smooth muscle marker smooth muscle α-actin both in IAS reconstructs (Fig. 8A) and in the SMCs redispersed from the IAS reconstructs (Fig. 8B). Immunofluorescence intensity studies in redispersed cells revealed that, in the basal state, RhoA and ROCK II were significantly higher at the periphery than the cytosol of the SMCs (P < 0.05; n = 5; Fig. 8, C and D). C3 exoenzyme, Y-27632, and Gö-6850 caused significant redistribution of RhoA, ROCK II, and PKCα, respectively, from the periphery to the cytosol (P < 0.05; n = 5; Fig. 8, C–E). Interestingly, PKCα immunofluorescence intensity (in comparison with RhoA and ROCK II) was significantly less at the periphery (P < 0.05; n = 5; Fig. 8E). DAPI was used to stain cell nuclei both in IAS reconstructs and in redispersed cells immunocytochemical and immunohistochemical analyses. These data suggest that RhoA/ROCK are constitutively active in these SMCs similar to those in the freshly isolated SMCs.
Fig. 8.
A: α-actin staining of IAS reconstructs section (10 μm). Photographs were taken at lower (top) and higher (bottom) magnifications to examine the cellular organization in the IAS reconstructs. Note a honeycomb-like arrangement of collagen matrix and close network of SMCs running parallel to each other. B: immunofluorescence and confocal microscopic images showing the expression of smooth muscle α-actin (and actomyosin complex in the enlarged inset) in the SMC redispersed from the IAS reconstructs. C and D: immunofluorescence analysis reveals higher distribution of RhoA/ROCK in the periphery of the cells (redispersed from the IAS reconstructs) in the basal state (*P < 0.05) and their redistribution toward the cytosol following C3 exoenzyme and Y-27632. E: quantitative analysis of the distribution of PKCα (in the SMCs redispersed from the reconstructs) before and after Gö-6850 reveals a similar trend. Data show preservation of the phenotypic tonic characteristics of the SMC during the process of transformation into reconstructs.
DISCUSSION
The present studies for the first time demonstrate successfully developed IAS reconstructs with functional and molecular attributes similar to the intact IAS. The IAS reconstructs were prepared directly from the SMCs isolated from intact IAS. The IAS reconstructs develop high basal tone and respond to different agonists and antagonists in a manner similar to the intact IAS, and the basal tone is regulated primarily via the RhoA/ROCK pathway. To determine the issue of dedifferentiation of the SMCs during the IAS reconstructs transformation, we compared the molecular attributes of the seeded cells with those redispersed from the reconstructs.
IAS tissue engineering is both rewarding and challenging. As stated before, presently, there are no safe and successful avenues to avert the IAS dysfunction. Therefore, replacement of the dysfunctional IAS with the reconstructs will be significant progress in the field. The IAS smooth muscle being phenotypically tonic is different from the other phasic smooth muscles; therefore, there is a risk of its dedifferentiation during the tissue engineering. Recent studies in intact human IAS (33) and lower esophageal sphincter (LES) (34) have shown the myogenic basal tone to be RhoA/ROCK predominant and provide an important reference point for the distinct tonic smooth muscles.
In the present studies, the basal tone of the reconstructs ranged from 0.40 to 0.98 mN (0.68 ± 0.26 mN), ∼50 times that reported previously in one recent study (38) and ∼2 times that in the other (29). This basal tone in the reconstructs is ∼1/20th of the intact IAS under the present experimental conditions based on the force/CSA based on the methodology explained above by Jiang et al. (17) for the smooth muscle. Accordingly, the force/CSA in the case of the reconstructs and intact IAS were 0.83 ± 0.12 and 17.39 ± 2.51, respectively. It is noteworthy that, although the functional and molecular attributes of the IAS reconstructs are similar to the intact IAS, it is not possible to directly compare and duplicate the absolute values of the force developed in the two types of preparations, for a number of reasons. The exact number of SMCs in the intact IAS is definitively severalfold higher. A bulk of the mass in the reconstructs consists of the collagen matrix used as scaffold, which does not contribute to active force. Besides, the contributions of the characteristic extracellular matrix, the intercellular syncytium (e.g., specialized gap junctions and interstitial cells of Cajal), and the neurohumoral and paracrine factors are expected to play a significant role in the intact IAS tone. One can merely attempt to emulate some of these conditions during the culture of the reconstructs. Speculatively, once transplanted, the grafts are integrated in the region of interest, the number of cells may grow further, and the above-mentioned intercellular network and the neurohumoral and paracrine conditions may develop.
The basal tone in the present IAS reconstructs responds to agonists and antagonists in a manner similar to the intact IAS and is RhoA/ROCK predominant (33, 34). The maximal inhibitory concentration of the ROCK inhibitor Y-27632 nearly obliterates the basal tone in the IAS reconstructs, whereas PKC inhibition only causes a modest decrease in the basal tone. These data combined with the subsequent signal transduction machinery suggest strong resemblance with the intact IAS and are primarily RhoA/ROCK dependent (33).
The IAS reconstructs contract following K+ depolarization and the muscarinic type 3 receptor agonist bethanechol (36), and relax in response to the β-adrenoceptor agonist isoproterenol, in a concentration-dependent manner, responses similar to the intact IAS (7, 33). Likewise, the effects of the PKC activator PDBu resemble those of intact human IAS here (Fig. 3, A–C), showing a biphasic effect (contraction in the lower concentrations and relaxation in the higher), and other tonic gastrointestinal smooth muscle preparations (8, 19, 20) depending on the level of the basal tone. However, these data are different from the earlier studies in human IAS reconstructs showing a frank increase in the tone (38).
The exact reason for the observed differences between the present and earlier data in human IAS reconstructs is not understood. The possibilities include contamination of the tonic and phasic phenotypic SMC before seeding and dedifferentiation of the cells during protracted cell culturing. In the present studies, only the SMCs specifically isolated from the functionally identified IAS (33) were used for the seeding for the IAS reconstructs. The SMCs used for the seeding as well as from the fully developed IAS reconstructs have distinctly higher expression of smooth muscle α-actin and myosin, and h-caldesmon and calponin characteristic of tonic smooth muscles (later data not shown). Because of the characteristic differences between the phenotypic tonic vs. phasic SMC, one possible explanation is the dedifferentiation during the longer incubation period (15 days) and higher passage levels. A study by Woodsome et al. (39) in vascular SMCs has shown an increase in expression of PKCα at passage 10. Hong et al. (16) have also shown that longer incubation leads to depletion of distinctive smooth muscle proteins in the SMCs, which may change to a phasic type similar to the RSM. It is well known that characteristic smooth muscle contractile proteins are affected by multiple factors, such as three-dimensional culture environment (12), initial seed density (6), incubation time (12, 16, 22), and levels of RhoA expression (40). Therefore, a lower incubation period as employed in the present studies may preserve the phenotypic characteristics of SMCs and is a favorable factor in the designing of the IAS reconstructs, and speculatively in the better outcome of these reconstructs following in vivo transplantation. The cells used in this study were within the fifth passage, and the incubation time for the IAS reconstructs was 3–4 days.
In our experience, the use of collagen vs. fibronectin as scaffold matrix leads to more reproducible and robust development of basal tone in the IAS reconstructs, and their responses to different agonists. Although the exact reason for this is not known, the possibilities are that collagen has greater strength than fibrin (9) and that human IAS SMCs express higher levels of collagen-binding integrins in extracellular matrix proteins (16).
To examine the concern of dedifferentiation, we compared RhoA/ROCK vs. PKC signal transduction by Western blot studies of the SMC from intact IAS vs. the SMCs redispersed from IAS reconstructs, and the phasic phenotypic RSM. Data show striking resemblance between the intact IAS and IAS reconstructs and clear-cut differences from the RSM. The SMCs from intact IAS (used for the seeding of collagen mats) and from reconstructs have characteristically higher levels of RhoA/ROCK II, CPI-17, p-CPI-17, MLC20, and p-MLC20. In addition, the levels of MYPT1 were significantly lower in the intact IAS and IAS reconstructs vs. the phasic RSM. These data are similar to those reported in intact human and animal LES (13, 25, 34) and IAS of animals (26, 27, 33). These data reveal no significant downregulation of the RhoA/ROCK pathway-related signal transduction cascade as discussed above, suggesting a lack of dedifferentiation of the IAS SMCs during the process of IAS reconstructs transformation used in these studies. These results are consistent with our findings in intact rat and human IAS SMCs and in other reconstructs (24, 26, 27, 33).
Immunocytochemical analysis reveals that the phenotypic SMCs from intact IAS and the IAS reconstructs display higher expression of RhoA/ROCK in the periphery of cells, suggesting their constitutively active nature in both specimens. This was further ascertained by the redistribution of RhoA/ROCK following their respective inhibitions by C3 exoenzyme and Y-27632.
In summary, the development of bioengineered IAS reconstructs has significant implications in the replacement of the dysfunctional IAS with the functional IAS reconstructs that maintain the original phenotypic molecular characteristics. Dysfunctional IAS has been associated with rectoanal incontinence and other rectoanal motility disorders characterized with hypo- and hypertensive IAS, otherwise refractory to the conventional treatments. The approach used here starting with the seeding of the phenotypically differentiated SMCs may be more practical compared with the stem cells used in the vascular grafts to replace the damaged blood smooth muscle (14).
GRANTS
The work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grant R01DK035385 and an institutional grant from Thomas Jefferson University.
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: J.S. performed experiments; J.S. analyzed data; J.S. and S.R. interpreted results of experiments; J.S. prepared figures; S.R. conception and design of research; S.R. drafted manuscript; S.R. edited and revised manuscript; S.R. approved final version of manuscript.
REFERENCES
- 1. Ali I, Sarna SK. Selective modulation of PKC isozymes by inflammation in canine colonic circular muscle cells. Gastroenterology 122: 483–494, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bhardwaj R, Vaizey CJ, Boulos PB, Hoyle CHV. Neuromyogenic properties of the internal anal sphincter: therapeutic rationale for anal fissures. Gut 46: 861–868, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bharucha AE. Outcome measures for fecal incontinence: anorectal structure and function. Gastroenterology 126: S90–S98, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bi D, Nishimura J, Niiro N, Hirano K, Kanaide H. Contractile properties of the cultured vascular smooth muscle cells: the crucial role played by RhoA in the regulation of contractility. Circ Res 96: 890–897, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Biancani P, Walsh JH, Behar J. Vasoactive intestinal polypeptide: a neurotransmitter for relaxation of the rabbit internal anal sphincter. Gastroenterology 89: 867–874, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Birukov KG, Frid MG, Rogers JD, Shirinsky VP, Koteliansky VE, Campbell JH, Campbell GR. Synthesis and expression of smooth muscle phenotype markers in prmary culture of rabbit aortic smooth muscle cells: influence of seeding density and media and relation to cell contractility. Exp Cell Res 204: 46–53, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Burleigh DE, D'Mello A. Neural and pharmacological factors affecting motility of the internal anal sphincter. Gastroenterology 84: 409–417, 1983 [PubMed] [Google Scholar]
- 8. Chakder S, Sarma D, Rattan S. Mechanism of internal anal sphincter smooth muscle relaxation by phorbol 12,13-dibutyrate. Am J Physiol Gastrointest Liver Physiol 280: G1341–G1350, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials 25: 3699–3707, 2004 [DOI] [PubMed] [Google Scholar]
- 10. De Godoy MA, Rattan S. Role of rho kinase in the functional and dysfunctional tonic smooth muscles. Trends Pharmacol Sci 32: 384–393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eto M, Kitazawa T, Yazawa A, Mukai H, Ono Y, Brautigan DL. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C α and δ isoforms. J Biol Chem 276: 29072–29078, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Halayko AJ, Salari H, Ma X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol Lung Cell Mol Physiol 270: L1040–L1051, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol 288: G407–G416, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Harris LJ, Abdollahi H, Zhang P, McIlhenny S, Tulenko TN, DiMuzio PJ. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J Surg Res 168: 306–314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hecker L, Baar K, Dennis RG, Bitar KN. Development of a three-dimensional physiological model of the internal anal sphincter bioengineered in vitro from isolated smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 289: G188–G196, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Hong H, Stegemann JP. 2D and 3D collagen and fibrin biopolymers promote specific ECM and integrin gene expression by vascular smooth muscle cells. J Biomater Sci Polym Ed 19: 1279–1293, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang H, Halayko AJ, Rao K, Cunningham P, Stephens NL. Normalization of force generated by canine airway smooth muscles. Am J Physiol Lung Cell Mol Physiol 260: L522–L529, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Madalinski M, Kalinowski L. Novel options for the pharmacological treatment of chronic anal fissure-Role of botulinum toxin. Curr Clin Pharmacol 4: 47–52, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto T, Nishiyama M, Kobayashi T, Kasuya Y, Kamata K. Effect of phorbol 12,13-dibutyrate on smooth muscle tone in rat stomach fundus. J Smooth Muscle Res 41: 107–116, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Mitsui M, Karaki H. Contractile and relaxant effects of phorbol ester in the intestinal smooth muscle of guinea-pig taenia caeci. Br J Pharmacol 109: 229–233, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moummi C, Rattan S. Effect of methylene blue and N-ethylmaleimide on internal anal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 255: G571–G578, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Nair DG, Han TY, Lourenssen S, Blennerhassett MG. Proliferation modulates intestinal smooth muscle phenotype in vitro and in colitis in vivo. Am J Physiol Gastrointest Liver Physiol 300: G903–G913, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Oishi K, Itoh Y, Isshiki Y, Kai C, Takeda Y, Yamaura K, Takano-Ohmuro H, Uchida MK. Agonist-induced isometric contraction of smooth muscle cell-populated collagen gel fiber. Am J Physiol Cell Physiol 279: C1432–C1442, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Park SY, Song HJ, Sohn UD. Participation of Rho-associated kinase in electrical stimulated and acetylcholine-induced contraction of feline esophageal smooth muscle. Eur J Pharmacol 607: 220–225, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 291: G830–G837, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Patel CA, Rattan S. Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK. Am J Physiol Gastrointest Liver Physiol 292: G1747–G1756, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Percy WH. In vitro techniques for the study of gastrointestinal motility. In: Handbook of Methods in Gastrointestinal Pharmacology, edited by Gaginella TS. Boca Raton, FL: CRC, 1996, p. 189–224 [Google Scholar]
- 29. Raghavan S, Gilmont RR, Miyasaka EA, Somara S, Srinivasan S, Teitelbaum DH, Bitar KN. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology 141: 310–319, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raghavan S, Miyasaka EA, Hashish M, Somara S, Gilmont RR, Teitelbaum DH, Bitar KN. Successful implantation of physiologically functional bioengineered mouse internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 299: G430–G439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 126: S14–S22, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Rattan S, Benjamin P, Maxwell IVPJ. RhoA/ROCK-kinase: pathophysiologic and therapeutic implications in gastrointestinal smooth muscle tone and relaxation. Gastroenterology 138: 13–18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rattan S, Singh J. RhoA/ROCK pathway is the major molecular determinant of basal tone in intact human internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 302: G664–G675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sims SM, Chrones T, Preiksaitis HG. Calcium sensitization in human esophageal muscle: role for RhoA kinase in maintenance of lower esophageal sphincter tone. J Pharmacol Exp Ther 327: 178–186, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Singh J, Maxwell IVPJ, Rattan S. Immunocytochemical evidence for PDBu-induced activation of RhoA/ROCK in human internal anal sphincter smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 301: G317–G325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh J, Mehendiratta V, Del Galdo F, Jimenez SA, Cohen S, DiMarino AJ, Rattan S. Immunoglobulins from scleroderma patients inhibit the muscarinic receptor activation in internal anal sphincter smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 297: G1206–G1213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Somara S, Bitar KN. Direct association of calponin with specific domains of PKC-α. Am J Physiol Gastrointest Liver Physiol 295: G1246–G1254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Somara S, Gilmont RR, Dennis RG, Bitar KN. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology 137: 53–61, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci 119: 1769–1780, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Worth NF, Campbell GR, Campbell JH, Rolfe BE. Rho expression and activation in vascular smooth muscle cells. Cell Motil Cytoskeleton 59: 189–200, 2004 [DOI] [PubMed] [Google Scholar]